Tooth Decay: Genetic and Epigenetic Insights Driving the Development of Anti-Caries Vaccines
Abstract
1. Introduction
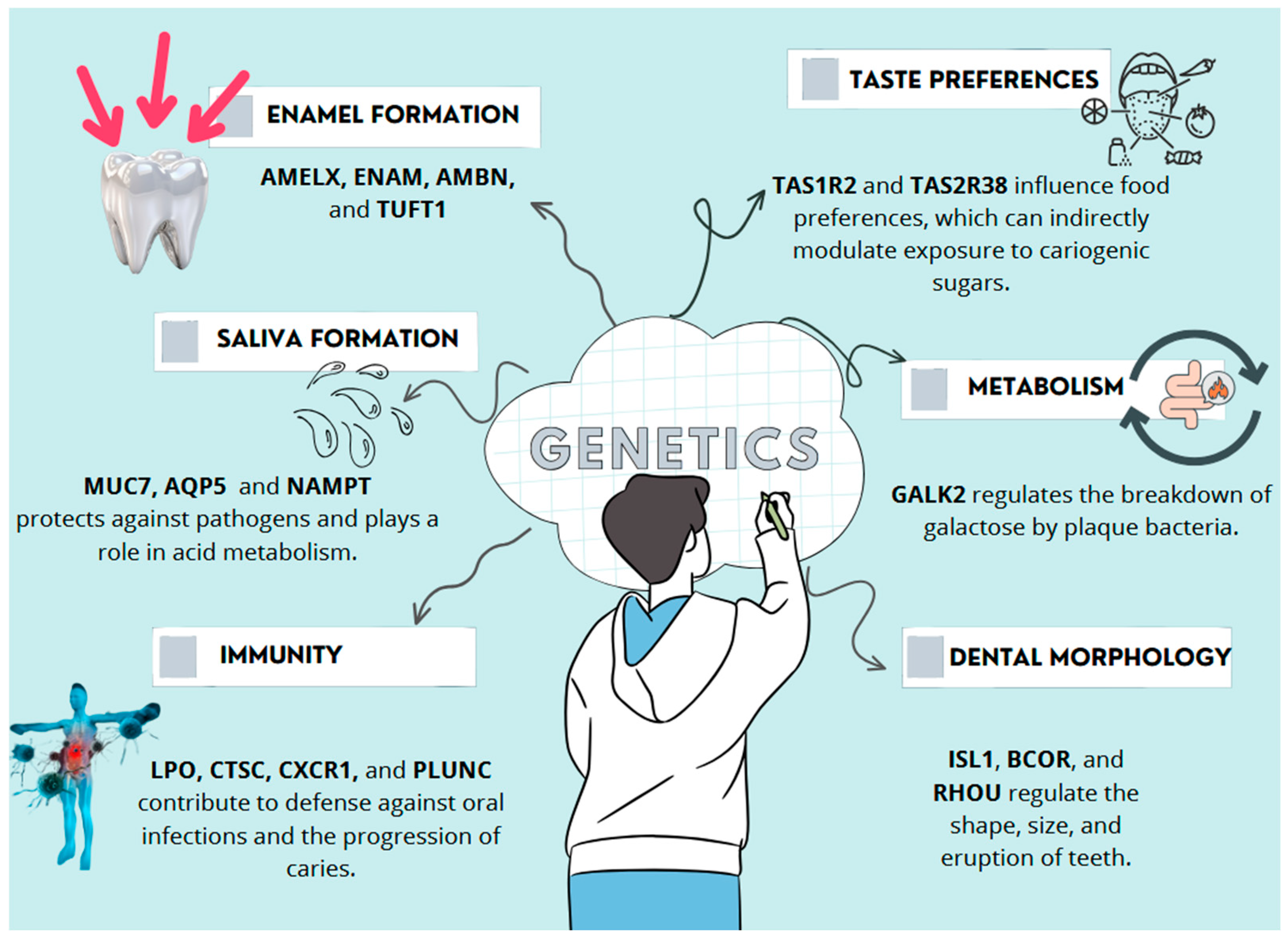
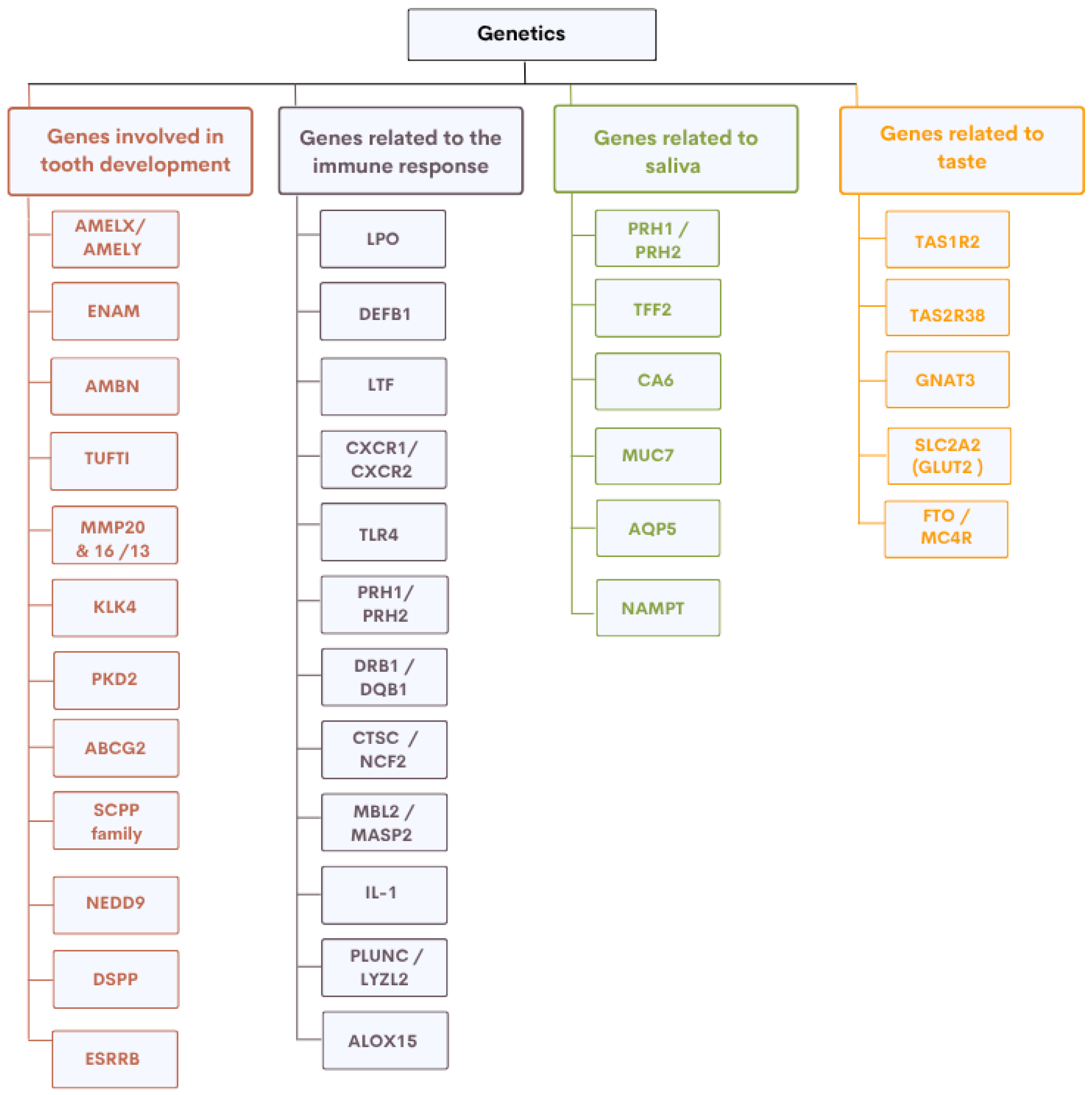
2. Genes Involved in Oral Health
2.1. Genes Involved in the Development of Dental Hard Tissues
2.2. Genes Involved in Saliva Composition and Function
2.3. Genes Involved in Oral Immune Response
2.4. Genes Influencing Taste Perception and Sugar Preference
2.5. From Candidate Genes to Genome-Wide Approaches
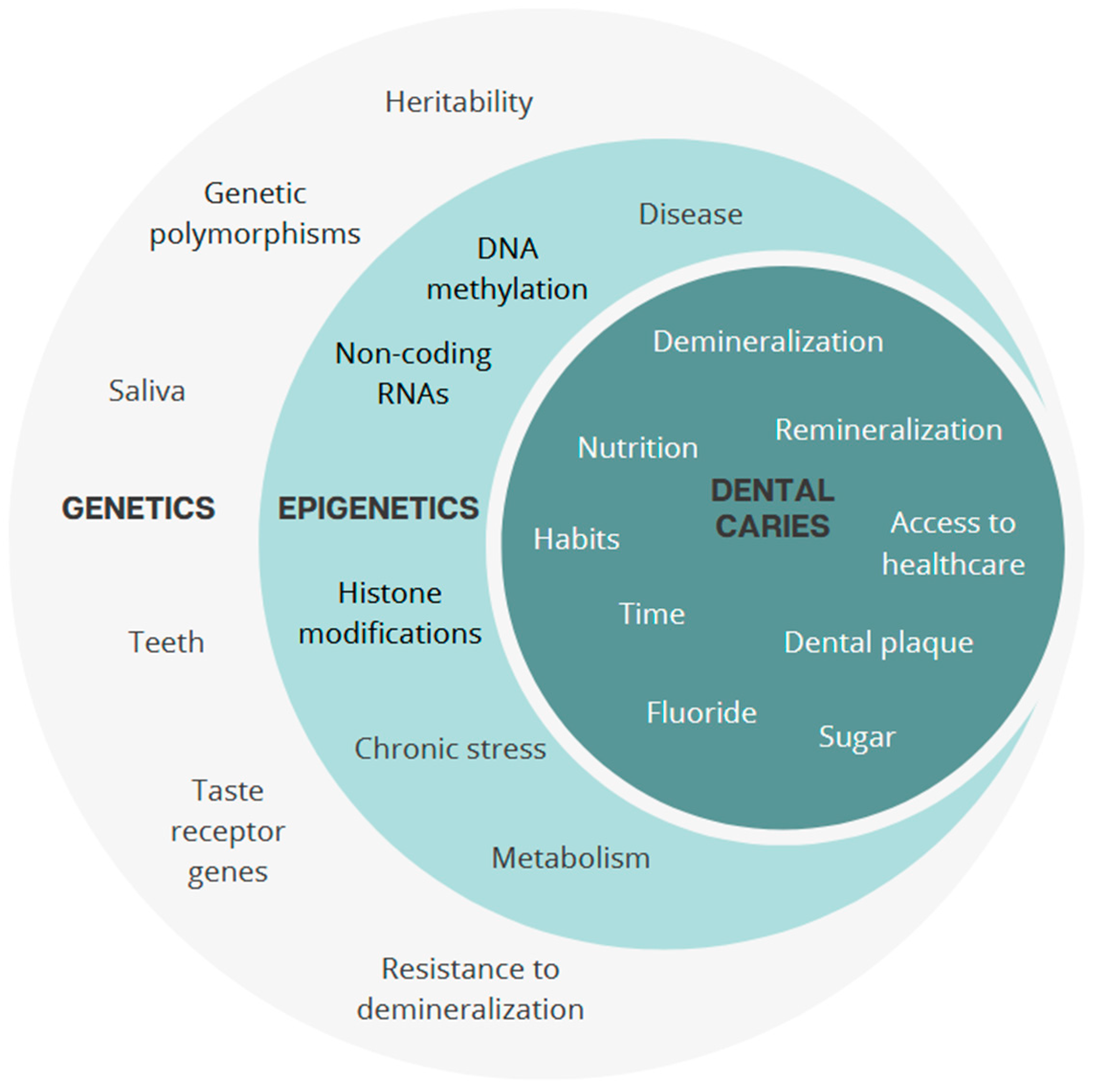
3. Epigenetics: A Dynamic Modulation
4. An Integrative View of Dental Caries: Genes, Epigenetics, and Environment
5. Towards an Anti-Caries Vaccine: Biological and Practical Challenges
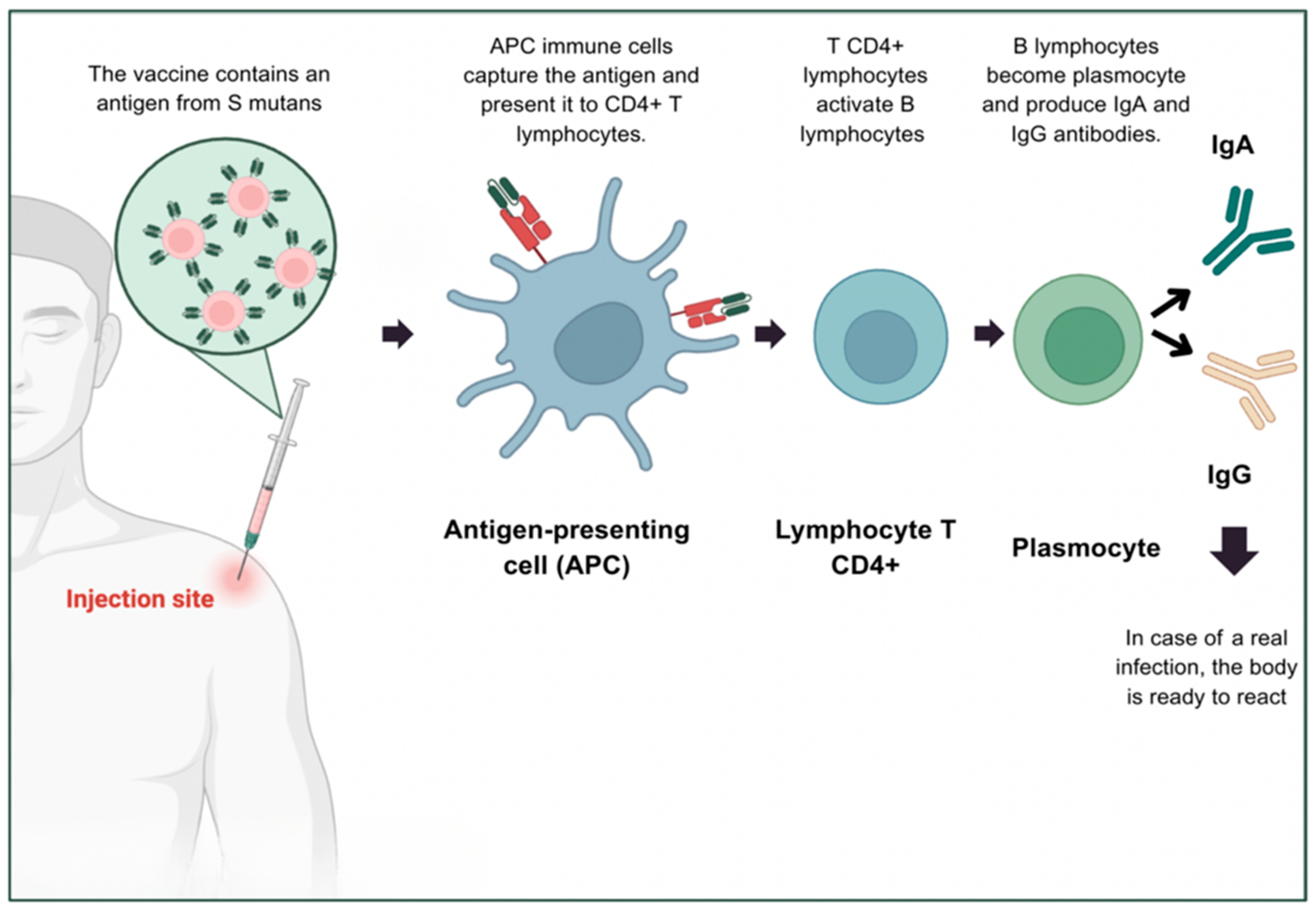
5.1. Immunological Principles of Vaccination Against Dental Caries
5.2. Vaccine Platforms and Antigen Targets
5.3. Feasibility and Limitations of Large-Scale Application
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Halasa, A.I.; Kalmanová, S.; Calkovský, B.; Juríček, R.; Malachovský, I.; Repiská, V.; Skerenová, M.; Janíčková, M. Genetic factors affecting susceptibility to dental caries. Bratisl. Med. J. 2024, 125, 635–647. [Google Scholar] [CrossRef]
- Opal, S.; Garg, S.; Jain, J.; Walia, I. Genetic factors affecting dental caries risk. Aust. Dent. J. 2015, 60, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Fries, N.; Haworth, S.; Shaffer, J.R.; Esberg, A.; Divaris, K.; Marazita, M.L.; Johansson, I. A polygenic score predicts caries experience in elderly Swedish adults. J. Dent. Res. 2024, 103, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Gullianne, B.R.; Gultom, F.P.; Auerkari, E.I. Genetic and epigenetic mechanisms in dental caries. AIP Conf. Proc. 2023, 2860, 020003. [Google Scholar] [CrossRef]
- Fernando, S.; Speicher, D.J.; Bakr, M.M.; Benton, M.C.; Lea, R.A.; Scuffham, P.A.; Mihala, G.; Johnson, N.W. Protocol for assessing maternal, environmental and epigenetic risk factors for dental caries in children. BMC Oral Health 2015, 15, 167. [Google Scholar] [CrossRef]
- Westerlund, A.; Khalifa, H.; Yousif, R.; Araujo, G.; Lundqvist, E.; Larsson, E.; Thrastardottir, R.; Akhlaghi, R.; Granciuc, V.; Svanberg, C.; et al. Personalized oral care (Precaries): An intervention study customized according to genetic cause and risk. medRxiv 2024. Preprint. [Google Scholar] [CrossRef]
- Cherukuri, G.; Veeramachaneni, C.; Rao, G.V.; Pacha, V.B.; Balla, S.B. Insight into the status of dental caries vaccination: A review. J. Conserv. Dent. 2020, 23, 544–549. [Google Scholar] [CrossRef]
- Alam, M.K.; Zheng, L.; Liu, R.; Papagerakis, S.; Papagerakis, P.; Geyer, C.R. Synthetic antigen-binding fragments (Fabs) against Streptococcus mutans and Streptococcus sobrinus inhibit caries formation. Sci. Rep. 2018, 8, 10173, Erratum in Sci. Rep. 2020, 10, 15040. https://doi.org/10.1038/s41598-020-71086-8. [Google Scholar] [CrossRef]
- de Souza Pereira, G.; Batista, M.T.; dos Santos, N.F.B.; Passos, H.M.; da Silva, D.A.; Ferreira, E.L.; de Souza Ferreira, L.C.; de Cássia Café Ferreira, R. Streptococcus mutans glutamate binding protein (GlnH) as antigen target for a mucosal anti-caries vaccine. Braz. J. Microbiol. 2022, 53, 1941–1949. [Google Scholar] [CrossRef]
- Yang, H.; Yan, Z.; Zhang, Z.; Realivazquez, A.; Ma, B.; Liu, Y. Anti-caries vaccine based on clinical cold-adapted influenza vaccine: A promising alternative for scientific and public-health protection against dental caries. Med. Hypotheses 2019, 126, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef]
- Goldberg, M. Genetic and structural alterations of enamel and dentin: Amelogenesis imperfecta, dentinogenesis imperfecta and dentin dysplasia. J. Dent. Health Oral Disord. Ther. 2019, 10, 260–266. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Sun, Y.; Yang, J.; Yu, Y. Association of genetic variants in enamel-formation genes with dental caries: A meta- and gene-cluster analysis. Saudi J. Biol. Sci. 2021, 28, 1645–1653. [Google Scholar] [CrossRef]
- Sharma, A.; Patil, S.S.; Muthu, M.S.; Venkatesan, V.; Kirubakaran, R.; Nuvvula, S.; Arockiam, S. Single nucleotide polymorphisms of enamel formation genes and early childhood caries: Systematic review, gene-based, gene cluster and meta-analysis. J. Indian Soc. Pedod. Prev. Dent. 2023, 41, 3–15. [Google Scholar] [CrossRef]
- Abbasoğlu, Z.; Tanboğa, İ.; Küchler, E.C.; Deeley, K.; Weber, M.; Kaspar, C.; Korachi, M.; Vieira, A.R. Early childhood caries is associated with genetic variants in enamel formation and immune response genes. Caries Res. 2015, 49, 70–77. [Google Scholar] [CrossRef]
- Nibali, L.; Di Iorio, A.; Tu, Y.K.; Vieira, A.R. Host genetics role in the pathogenesis of periodontal disease and caries. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S52–S78. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.; Bezamat, M.; Modesto, A.; Vieira, A.R. Biomarkers for lifetime caries-free status. J. Pers. Med. 2020, 11, 23. [Google Scholar] [CrossRef]
- Lewis, D.D.; Shaffer, J.R.; Feingold, E.; Cooper, M.; Vanyukov, M.M.; Maher, B.S.; Slayton, R.L.; Willing, M.C.; Reis, S.E.; McNeil, D.W.; et al. Genetic association of MMP10, MMP14, and MMP16 with dental caries. Int. J. Dent. 2017, 2017, 8465125. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.L.; Hsin, H.Y.; Kalay, E.; Brožková, D.S.; Shimizu, T.; Bayram, M.; Deeley, K.; Küchler, E.C.; Forella, J.; Ruff, T.D.; et al. Role of estrogen related receptor beta (ESRRB) in DFN35B hearing impairment and dental decay. BMC Med. Genet. 2014, 15, 81. [Google Scholar] [CrossRef]
- Verdelis, K.; Szabo-Rogers, H.L.; Xu, Y.; Chong, R.; Kang, R.; Cusack, B.J.; Jani, P.; Boskey, A.L.; Qin, C.; Beniash, E. Accelerated enamel mineralization in Dspp mutant mice. Matrix Biol. 2016, 52–54, 246–259. [Google Scholar] [CrossRef]
- Liang, T.; Xu, Q.; Zhang, H.; Wang, S.; Diekwisch, T.G.H.; Qin, C.; Lu, Y. Enamel defects associated with dentin sialophosphoprotein mutation in mice. Front. Physiol. 2021, 12, 724098. [Google Scholar] [CrossRef]
- Aruna, P.; Patil, S.; Muthu, M.S.; Venkatesan, V.; Arockiam, S.; Kirubakaran, R.; Nuvvula, S. Association between polymorphisms of immune response genes and early childhood caries: Systematic review, gene-based, gene cluster, and meta-analysis. J. Genet. Eng. Biotechnol. 2023, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Chisini, L.A.; Varella de Carvalho, R.; Dos Santos Costa, F.; Salvi, L.C.; Demarco, F.F.; Britto Correa, M. Genes and single nucleotide polymorphisms in the pathway of saliva and dental caries: A systematic review and meta-analysis. Biofouling 2023, 39, 8–23. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil factor family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: Changing the paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef]
- Shimomura-Kuroki, J.; Nashida, T.; Miyagawa, Y.; Sekimoto, T. The role of genetic factors in the outbreak mechanism of dental caries. J. Clin. Pediatr. Dent. 2018, 42, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Gachova, D.; Lipovy, B.; Deissova, T.; Izakovicova Holla, L.; Danek, Z.; Borilova Linhartova, P. Polymorphisms in genes expressed during amelogenesis and their association with dental caries: A case-control study. Clin. Oral Investig. 2023, 27, 1681–1695. [Google Scholar] [CrossRef]
- Holla, L.I.; Linhartova, P.B.; Lucanova, S.; Kastovsky, J.; Musilova, K.; Bartosova, M.; Kukletova, M.; Kukla, L.; Dusek, L. GLUT2 and TAS1R2 polymorphisms and susceptibility to dental caries. Caries Res. 2015, 49, 417–424. [Google Scholar] [CrossRef]
- Chamoun, E.; Mutch, D.M.; Allen-Vercoe, E.; Buchholz, A.C.; Duncan, A.M.; Spriet, L.L.; Haines, J.; Ma, D.W.L.; on behalf of the Guelph Family Health Study. A review of the associations between single nucleotide polymorphisms in taste receptors, eating behaviors, and health. Crit. Rev. Food Sci. Nutr. 2018, 58, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.V.; Agnes, G.; Vitolo, M.R.; Mattevi, V.S.; Campagnolo, P.D.B.; Almeida, S. Evaluation of the association between the TAS1R2 and TAS1R3 variants and food intake and nutritional status in children. Genet. Mol. Biol. 2017, 40, 415–420. [Google Scholar] [CrossRef]
- Chisini, L.A.; Cademartori, M.G.; Conde, M.C.M.; Costa, F.D.S.; Salvi, L.C.; Tovo-Rodrigues, L.; Correa, M.B. Single nucleotide polymorphisms of taste genes and caries: A systematic review and meta-analysis. Acta Odontol. Scand. 2021, 79, 147–155. [Google Scholar] [CrossRef]
- Xi, R.; Zheng, X.; Tizzano, M. Role of taste receptors in innate immunity and oral health. J. Dent. Res. 2022, 101, 759–768. [Google Scholar] [CrossRef]
- Eriksson, L.; Esberg, A.; Haworth, S.; Holgerson, P.L.; Johansson, I. Allelic variation in taste genes is associated with taste and diet preferences and dental caries. Nutrients 2019, 11, 1491. [Google Scholar] [CrossRef]
- Janzi, S.; González-Padilla, E.; Najafi, K.; Ramne, S.; Ahlqvist, E.; Borné, Y.; Sonestedt, E. Single nucleotide polymorphisms in close proximity to the fibroblast growth factor 21 (FGF21) gene found to be associated with sugar intake in a Swedish population. Nutrients 2021, 13, 3954. [Google Scholar] [CrossRef]
- Silva, M.J.; Colón, N.M.; Chen, J.M.; Modesto, D.J.; Starr, R.; Sanders, M.C.; Burgner, D.P.; Lucas, J.; Kilpatrick, N.M.; Hopper, J.L.; et al. DNA methylation in childhood dental caries and hypomineralization. J. Dent. 2022, 117, 103913. [Google Scholar] [CrossRef]
- Valente, A.; Vieira, L.; Silva, M.J.; Ventura, C. The effect of nanomaterials on DNA methylation: A review. Nanomaterials 2023, 13, 1880. [Google Scholar] [CrossRef]
- Bernabé, E.; MacRitchie, H.; Longbottom, C.; Pitts, N.B.; Sabbah, W. Birth weight, breastfeeding, maternal smoking and caries trajectories. J. Dent. Res. 2017, 96, 171–178. [Google Scholar] [CrossRef]
- Zhong, Y.; Tang, Q.; Tan, B.; Huang, R. Correlation between maternal smoking during pregnancy and dental caries in children: A systematic review and meta-analysis. Front. Oral Health 2021, 2, 673449. [Google Scholar] [CrossRef] [PubMed]
- Joubert, B.R.; Håberg, S.E.; Bell, D.A.; Nilsen, R.M.; Vollset, S.E.; Midttun, Ø.; Ueland, P.M.; Wu, M.C.; Nystad, W.; Peddada, S.D.; et al. Maternal smoking and DNA methylation in newborns: In utero effect or epigenetic inheritance? Cancer Epidemiol. Biomark. Prev. 2014, 23, 1007–1017. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Yang, X.; Zhang, Y.; Liang, Y.; Qiu, X. Elucidating epigenetic mechanisms governing odontogenic differentiation in dental pulp stem cells: An in-depth exploration. Front. Cell Dev. Biol. 2024, 12, 1394582. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Bollati, V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009, 21, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Marsit, C.J. Influence of environmental exposure on human epigenetic regulation. J. Exp. Biol. 2015, 218, 71–79. [Google Scholar] [CrossRef]
- Tian, H.; She, Z.; Gao, X.; Wang, W.; Tian, H. MicroRNA-31 regulates dental epithelial cell proliferation by targeting Satb2. Biochem. Biophys. Res. Commun. 2020, 532, 321–328. [Google Scholar] [CrossRef]
- Sweat, M.; Sweat, Y.; Yu, W.; Su, D.; Leonard, R.J.; Eliason, S.L.; Amendt, B.A. The miR-200 family is required for ectodermal organ development through the regulation of the epithelial stem cell niche. Stem Cells 2021, 39, 761–775. [Google Scholar] [CrossRef]
- Oliveira, D.S.B.; Segato, R.A.B.; Oliveira, S.; Dutra, A.L.T.; Santos, A.S.D.; Praxedes, A.D.N.; Belém, L.C.; Antunes, L.A.; Lips, A.; Nelson-Filho, P.; et al. Association between genetic polymorphisms in DEFB1 and microRNA-202 with caries in two groups of Brazilian children. Arch. Oral Biol. 2018, 92, 1–7. [Google Scholar] [CrossRef]
- Muñoz-Carrillo, J.L.; Vázquez-Alcaraz, S.J.; Vargas-Barbosa, J.M.; Ramos-Gracia, L.G.; Alvarez-Barreto, I.; Medina-Quiroz, A.; Díaz-Huerta, K.K. The role of microRNAs in pulp inflammation. Cells 2021, 10, 2142. [Google Scholar] [CrossRef]
- Wiegand, C.; Heusser, P.; Klinger, C.; Cysarz, D.; Büssing, A.; Ostermann, T.; Savelsbergh, A. Stress-associated changes in salivary microRNAs can be detected in response to the Trier Social Stress Test: An exploratory study. Sci. Rep. 2018, 8, 7112. [Google Scholar] [CrossRef]
- Kuppan, A.; Rodrigues, S.; Samuel, V.; Ramakrishnan, M.; Halawany, H.S.; Abraham, N.B.; Jacob, V.; Anil, S. Prevalence and heritability of early childhood caries among monozygotic and dizygotic twins. Twin Res. Hum. Genet. 2017, 20, 43–52. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, M.; Li, J.; Li, Y.; Teng, F.; Jiang, H.; Du, M. Comparative analysis of the microbial profiles in supragingival plaque samples obtained from twins with discordant caries phenotypes and their mothers. Front. Cell Infect. Microbiol. 2018, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, Y.; Xie, L.; Li, Y.; Jiang, H.; Du, M. Pyrosequencing of plaque microflora in twin children with discordant caries phenotypes. PLoS ONE 2015, 10, e0141310. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Espinoza, J.L.; Harkins, D.M.; Leong, P.; Saffery, R.; Bockmann, M.; Torralba, M.; Kuelbs, C.; Kodukula, R.; Inman, J.; et al. Host genetic control of the oral microbiome in health and disease. Cell Host Microbe 2017, 22, 269–278.e3. [Google Scholar] [CrossRef]
- Spatafora, G.; Li, Y.; He, X.; Cowan, A.; Tanner, A.C.R. The evolving microbiome of dental caries. Microorganisms 2024, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, C.; Herpoldt, K.L.; Freire, M. Is the oral microbiome a source to enhance mucosal immunity against infectious diseases? npj Vaccines 2021, 6, 80. [Google Scholar] [CrossRef]
- Wig, M.; Kumar, A.; Chaluvaiah, M.B.; Yadav, V.; Mendiratta, M.; Aggarwal, A. Dental caries vaccine: An overview. Int. J. Appl. Dent. Sci. 2021, 7, 461–465. [Google Scholar] [CrossRef]
- Srivastava, R.; Tangade, P.; Priyadarshi, S. The future of preventive dentistry: Caries vaccine on the horizon. Int. Dent. J. Stud. Res. 2023, 11, 44–49. [Google Scholar] [CrossRef]
- Yu, Y.B.; Liu, Y.; Liang, H.; Dong, X.; Yang, X.Y.; Li, S.; Guo, Z.; Khursigara, C.M. A nanoparticle-based anticaries vaccine enhances the persistent immune response to inhibit Streptococcus mutans and prevent caries. Microbiol. Spectr. 2023, 11, e0432822. [Google Scholar] [CrossRef]
- Liu, D.; Ma, X.; Ji, Y.; Chen, R.; Zhou, S.; Yao, H.; Zhang, Z.; Ye, M.; Xu, Z.; Du, M. Bioresponsive nanotherapy for preventing dental caries by inhibiting multispecies cariogenic biofilms. Bioact. Mater. 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Liu, B.; Li, M.; Li, X.; Yang, J.; Yan, H. An optimized caries model of Streptococcus mutans in rats and its application for evaluating prophylactic vaccines. Hum. Vaccines Immunother. 2024, 20, 2345943. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.L.; Batista, M.T.; Cavalcante, R.C.; Pegos, V.R.; Passos, H.M.; Silva, D.A.; Balan, A.; Ferreira, L.; Ferreira, R. Sublingual immunization with the phosphate-binding-protein (PstS) reduces oral colonization by Streptococcus mutans. Mol. Oral Microbiol. 2016, 31, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, N.; Smales, C.M.; Schillberg, S.; Fischer, R.; Schiermeyer, A. Developments in the production of mucosal antibodies in plants. Biotechnol. Adv. 2016, 34, 77–87. [Google Scholar] [CrossRef]
- Hager, K.J.; Pérez Marc, G.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F.; et al. Efficacy and safety of a recombinant plant-based adjuvanted Covid-19 vaccine. N. Engl. J. Med. 2022, 386, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hu, Y.; Yang, M.; Liu, H.; Jiang, G. Enhanced immune response to a dual-promoter anti-caries DNA vaccine orally delivered by attenuated Salmonella typhimurium. Immunobiology 2017, 222, 730–737. [Google Scholar] [CrossRef]
- Yan, Y.H.; Yu, F.; Zeng, C.; Cao, L.H.; Zhang, Z.; Xu, Q.A. CCL17 combined with CCL19 as a nasal adjuvant enhances the immunogenicity of an anti-caries DNA vaccine in rodents. Acta Pharmacol. Sin. 2016, 37, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.; Hikmat, B.Y.; Wycoff, K.; Vine, N.D.; Chargelegue, D.; Yu, L.; Hein, M.B.; Lehner, T. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 1998, 4, 601–606. [Google Scholar] [CrossRef]
- Chan, A.K.Y.; Tamrakar, M.; Jiang, C.M.; Lo, E.C.M.; Leung, K.C.M.; Chu, C.H. A systematic review on caries status of older adults. Int. J. Environ. Res. Public Health 2021, 18, 10662. [Google Scholar] [CrossRef]
- Vasireddy, D.; Sathiyakumar, T.; Mondal, S.; Sur, S. Socioeconomic factors associated with the risk and prevalence of dental caries and dental treatment trends in children: A cross-sectional analysis of National Survey of Children’s Health (NSCH) data, 2016–2019. Cureus 2021, 13, e19184. [Google Scholar] [CrossRef]
- Pellegrino, P.; Falvella, F.S.; Cheli, S.; Perrotta, C.; Clementi, E.; Radice, S. The role of toll-like receptor 4 polymorphisms in vaccine immune response. Pharmacogenomics J. 2016, 16, 96–101. [Google Scholar] [CrossRef]
- Saleh, A.; Auwalu, S.; Rufa’i, A.A. The implications of genomics and epigenetics in vaccine response for personalised vaccination. Int. J. Innov. Sci. Res. Technol. 2024, 9, 2837. [Google Scholar] [CrossRef]
- Contreras, S.; Mayta-Tovalino, F.; Munive-Degregori, A.; Mendoza, R.; Barja-Ore, J.; Mauricio-Vilchez, C. Anticaries vaccine as a promising alternative for protection against dental caries: A literature review. J. Int. Oral Health 2023, 15, 34–42. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouaita, I.; Peixoto, A.; Mascarenhas, P.; Manso, C. Tooth Decay: Genetic and Epigenetic Insights Driving the Development of Anti-Caries Vaccines. Genes 2025, 16, 952. https://doi.org/10.3390/genes16080952
Bouaita I, Peixoto A, Mascarenhas P, Manso C. Tooth Decay: Genetic and Epigenetic Insights Driving the Development of Anti-Caries Vaccines. Genes. 2025; 16(8):952. https://doi.org/10.3390/genes16080952
Chicago/Turabian StyleBouaita, Inès, André Peixoto, Paulo Mascarenhas, and Cristina Manso. 2025. "Tooth Decay: Genetic and Epigenetic Insights Driving the Development of Anti-Caries Vaccines" Genes 16, no. 8: 952. https://doi.org/10.3390/genes16080952
APA StyleBouaita, I., Peixoto, A., Mascarenhas, P., & Manso, C. (2025). Tooth Decay: Genetic and Epigenetic Insights Driving the Development of Anti-Caries Vaccines. Genes, 16(8), 952. https://doi.org/10.3390/genes16080952





