Uncovering the Epitranscriptome: A Review on mRNA Modifications and Emerging Frontiers
Abstract
1. Introduction
- RNA export: m5C can play a role in transporting mRNA from the nucleus to the cytoplasm.
- Translation: Both m5C and Ψ can influence the rate and fidelity of protein synthesis, potentially even leading to alternative protein products.
- mRNA stability: Ψ, for example, can enhance mRNA stability by affecting its structure and protecting it from degradation.
- Development and disease: Alterations in these modifications are linked to various physiological and pathological processes, including embryonic development and tumor formation.
2. Materials and Methods
3. Results
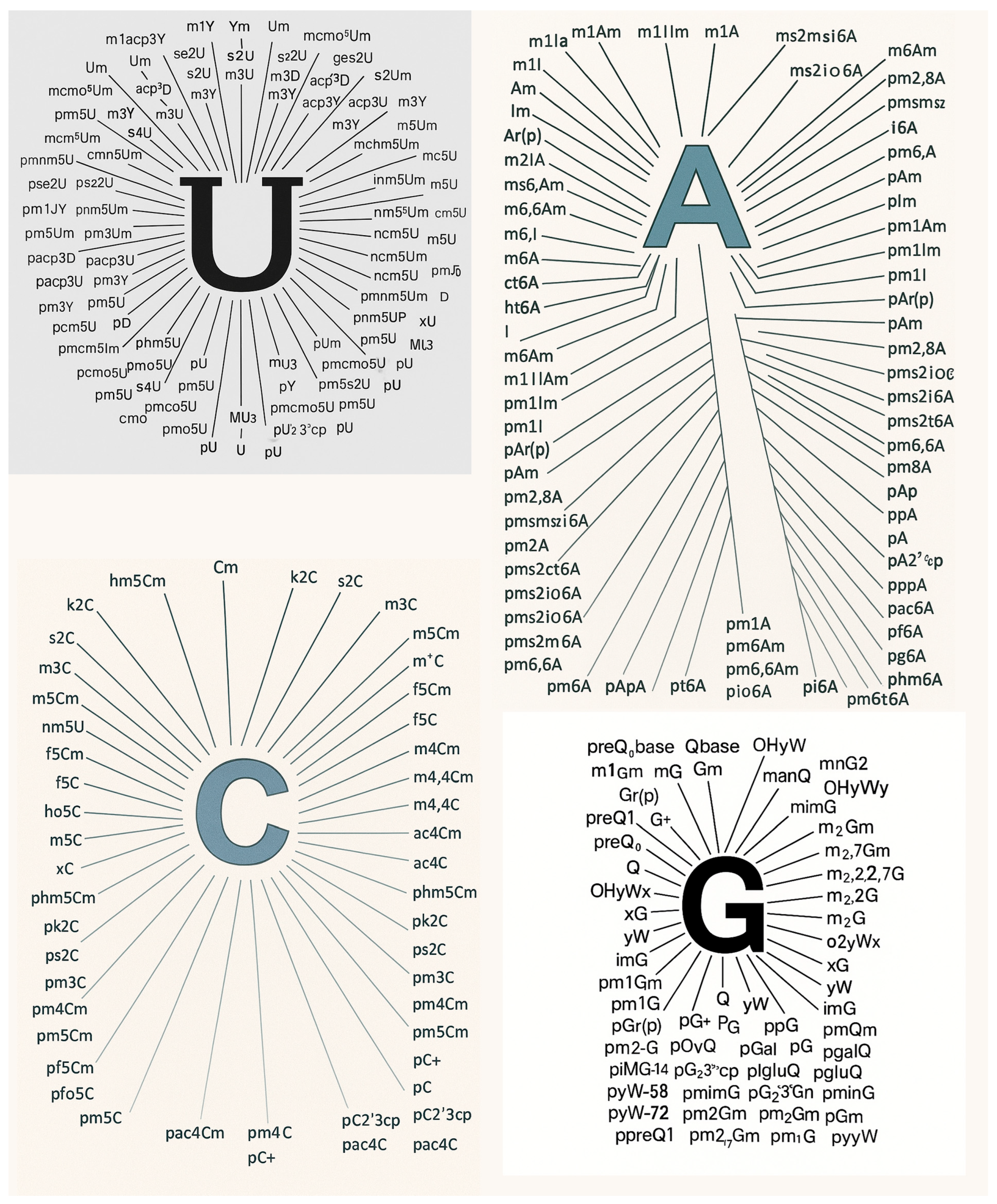
3.1. Ranking of mRNA Modifications by PubMed Prevalence and Research Emphasis
3.1.1. N6-Methyladenosine (m6A): A Central Regulatory Node
3.1.2. Pseudouridine (Ψ): Stability and Therapeutic Relevance
3.1.3. 5-Methylcytosine (m5C): An Emerging Modulator of mRNA Fate
3.1.4. Inosine (I): Codon Reprogramming and RNA Editing
3.1.5. N1-Methyladenosine (m1A): Translational Modulator
3.1.6. m6Am: Cap-Adjacent Modification
3.1.7. 5′ Cap Modifications (Cap0, Cap1, Cap2): Orchestrators of Immune Evasion and Translation Control
3.1.8. 5-Methyluridine (m5U): An Emerging Player in mRNA Regulation
3.1.9. 2′-O-Methyladenosine (Am): Emerging Regulatory Roles Beyond tRNA
3.1.10. N4-Acetylcytidine (ac4C): Enhanced Translation Fidelity and Stress Response Modulation
3.1.11. N7-Methylguanosine (m7G)
3.1.12. 2′-O-Methylguanosine (Gm): RNA Stability and Immune Evasion
3.1.13. 2′-O-Methylcytidine (Cm): RNA Stability, Translation, and Immune Modulation
3.1.14. 5-Hydroxymethylcytidine (hm5C): Potential Epitranscriptomic Regulator with Epigenetic Parallels
3.1.15. Comodified m6A/Ψ Sites: Combinatorial Control of RNA Fate
3.2. Positional Enrichment of mRNA Modifications
- ▪
- m6A: Highly enriched near stop codons and within 3′ UTRs. This spatial positioning facilitates regulated decay and translational control via reader proteins such as YTHDF2 [93].
- ▪
- ▪ Ψ: Broadly distributed across coding sequences and UTRs. Stress-induced Ψ sites often appear in transcripts involved in stress response and cancer [94].
- ▪
- m5C: Localized to coding regions and 3′ UTRs. m5C sites tend to enhance stability and promote nuclear export [35].
- ▪
- Inosine (I): Common in coding regions and 3′ UTRs, where it arises from ADAR-mediated A-to-I editing. Inosine affects codon identity, splicing, and miRNA targeting [95].
- ▪
- m1A: Found near start codons and 5′ UTRs. It can either promote or repress translation depending on its exact position and structural context [27].
- ▪
- m6Am: Located adjacent to the 5′ cap, m6Am increases mRNA stability and is deposited by the PCIF1 methyltransferase [96].
- ▪
- ac4C: Predominantly enriched in coding regions of highly translated genes, where it enhances translation fidelity [71].
3.3. Interpretation of Modification Ranking
3.4. Disease Relevance of Top RNA Modifications
| Rank 1 | Modification | Abbreviation | Enzyme(s) | Role | Positional Enrichment | ~N cited | Reference(s) |
|---|---|---|---|---|---|---|---|
| 1 | N6-methyladenosine | m6A | METTL3, METTL14, FTO, ALKBH5 | Splicing, translation, decay, export | Near stop codon and 3′ UTR | >7000 | [114,115,116] |
| 2 | Pseudouridine | Ψ | PUS1, PUS7 | Stability, decoding, stress response | Internal coding region | ~1000 | [117,118] |
| 3 | 5-methylcytidine | m5C | NSUN2, DNMT2 | Export, stability | 3′ UTR | ~800 | [119,120] |
| 4 | Inosine | I | ADAR1, ADAR2 | A-to-I editing, recoding | dsRNA regions | ~750 | [121] |
| 5 | N1-methyladenosine | m1A | TRMT6/TRMT61A | Translation initiation, structure | 5′ UTR near start codon | ~400 | [122,123] |
| 6 | N6,2′-O-dimethyladenosine | m6Am | PCIF1 | Cap-proximal stability | +1 position after 5′ cap | ~200 | [124] |
| 7 | 5′ cap modifications | Cap0, Cap1, Cap2 | RNGTT, RNMT, CMTR1, CMTR2 | Immune evasion, translation | 5′ cap | ~150–300 | [125,126] |
| 8 | 5-methyluridine | m5U | TRMT2A/B | tRNA-like stability role in mRNA | tRNA mimic sites | <100 | [127] |
| 9 | 2′-O-methyladenosine | Am | FTSJ1, CMTR1 | Cap stability and processing | Near 5′ cap | <100 | [126,128] |
| 10 | N4-acetylcytidine | ac4C | NAT10 | Translation, stress response | Internal coding region | <50 | [129] |
| 11 | N7-methylguanosine | m7G | RNGTT, RNMT, METTL1 | 5′ cap structure, nuclear export | 5′ cap | <50 | [125,130] |
| 12 | 2′-O-methylguanosine | Gm | CMTR2 | Cap and internal stability | 5′ cap | <30 | [125,126] |
| 13 | 2′-O-methylcytidine | Cm | FTSJ1 | Stability, cap modification | 5′ cap proximal | <30 | [62] |
| 14 | 5-hydroxymethylcytidine | hm5C | TET2 | Epigenetic-like regulation | 3′ UTR | <20 | [131] |
| 15 | m6A:Ψ comodified sites | m6A/Ψ | Multiple | Dynamic regulation, RNA structure | Near stop codon, 3′ UTR | <10 | [131] |
4. Discussion
5. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| m6A | N6-methyladenosine |
| Ψ | Pseudouridine |
| m5C | 5-methylcytidine |
| I | Inosine |
| m1A | N1-methyladenosine |
| m6Am | N6,2′-O-dimethyladenosine |
| Cap0, Cap1, Cap2 | 5′ cap modifications using m7G |
| m5U | 5-methyluridine |
| Am | 2′-O-methyladenosine |
| ac4C | N4-acetylcytidine |
| m7G | N7-methylguanosine |
| Gm | 2′-O-methylguanosine |
| Cm | 2′-O-methylcytidine |
| hm5C | 5-hydroxymethylcytidine |
| m6A/Ψ | m6A:Ψ comodified sites |
References
- Alasar, A.A.; Tuncel, O.; Gelmez, A.B.; Saglam, B.; Vatansever, I.E.; Akgul, B. Genomewide m(6)A Mapping Uncovers Dynamic Changes in the m(6)A Epitranscriptome of Cisplatin-Treated Apoptotic HeLa Cells. Cells 2022, 11, 3905. [Google Scholar] [CrossRef]
- Meyer, K.D.; Jaffrey, S.R. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 2014, 15, 313–326. [Google Scholar] [CrossRef]
- Pilala, K.M.; Panoutsopoulou, K.; Papadimitriou, M.A.; Soureas, K.; Scorilas, A.; Avgeris, M. Exploring the methyl-verse: Dynamic interplay of epigenome and m6A epitranscriptome. Mol. Ther. 2025, 33, 447–464. [Google Scholar] [CrossRef]
- Cappannini, A.; Ray, A.; Purta, E.; Mukherjee, S.; Boccaletto, P.; Moafinejad, S.N.; Lechner, A.; Barchet, C.; Klaholz, B.P.; Stefaniak, F.; et al. MODOMICS: A database of RNA modifications and related information. 2023 update. Nucleic Acids Res. 2024, 52, D239–D244. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- Tegowski, M.; Prater, A.K.; Holley, C.L.; Meyer, K.D. Single-cell m(6)A profiling in the mouse brain uncovers cell type-specific RNA methylomes and age-dependent differential methylation. Nat. Neurosci. 2024, 27, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, S.; Wu, Y.; Li, Y.; Kong, L.; Wu, R.; Zhao, M.; Liu, W.; Yu, H. Defining context-dependent m(6)A RNA methylomes in Arabidopsis. Dev. Cell 2024, 59, 2772–2786.e3. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wylder, A.C.; Pan, T. Simultaneous nanopore profiling of mRNA m(6)A and pseudouridine reveals translation coordination. Nat. Biotechnol. 2024, 42, 1831–1835. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Ye, C.; Zou, Z.; Jiang, B.; Yang, F.; He, K.; Ju, C.; Zhang, L.; Gao, B.; et al. Quantitative RNA pseudouridine maps reveal multilayered translation control through plant rRNA, tRNA and mRNA pseudouridylation. Nat. Plants 2025, 11, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Pan, K.; Fang, S.; Ye, L.; Tong, X.; Wang, Z.; Xue, X.; Zhang, H. Advances in mRNA 5-methylcytosine modifications: Detection, effectors, biological functions, and clinical relevance. Mol. Ther. Nucleic Acids 2021, 26, 575–593. [Google Scholar] [CrossRef]
- Wang, R.; Ding, L.; Lin, Y.; Luo, W.; Xu, Z.; Li, W.; Lu, Y.; Zhu, Z.; Lu, Z.; Li, F.; et al. The Quiet Giant: Identification, Effectors, Molecular Mechanism, Physiological and Pathological Function in mRNA 5-methylcytosine Modification. Int. J. Biol. Sci. 2024, 20, 6241–6254. [Google Scholar] [CrossRef]
- Chen, S.; Meng, J.; Zhang, Y. Quantitative profiling N1-methyladenosine (m1A) RNA methylation from Oxford nanopore direct RNA sequencing data. Methods 2024, 228, 30–37. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Gao, X.; Ru, Y.; Gu, X.; Hu, X. Research progress of N1-methyladenosine RNA modification in cancer. Cell Commun. Signal. 2024, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, H.G.; Beal, P.A. Structural and functional effects of inosine modification in mRNA. RNA 2024, 30, 512–520. [Google Scholar] [CrossRef]
- Poyau, A.; Vincent, L.; Berthomme, H.; Paul, C.; Nicolas, B.; Pujol, J.F.; Madjar, J.J. Identification and relative quantification of adenosine to inosine editing in serotonin 2c receptor mRNA by CE. Electrophoresis 2007, 28, 2843–2852. [Google Scholar] [CrossRef]
- Xia, L.; Yin, P. Upregulated m7G methyltransferase METTL1 is a potential biomarker and tumor promoter in skin cutaneous melanoma. Front. Immunol. 2025, 16, 1575219. [Google Scholar] [CrossRef]
- Xu, C.; Yu, X.H.; Wang, G.; Luo, W.; Chen, L.; Xia, X.D. The m(7)G methylation modification: An emerging player of cardiovascular diseases. Int. J. Biol. Macromol. 2025, 309, 142940. [Google Scholar] [CrossRef]
- Akichika, S.; Suzuki, T. Cap-specific m(6)Am modification: A transcriptional anti-terminator by sequestering PCF11 with implications for neuroblastoma therapy. Mol. Cell 2024, 84, 4051–4052. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F. CROWN-seq reveals m(6)Am landscapes and transcription start site diversity. Nat. Rev. Genet. 2025, 26, 509. [Google Scholar] [CrossRef] [PubMed]
- Dohnalkova, M.; Krasnykov, K.; Mendel, M.; Li, L.; Panasenko, O.; Fleury-Olela, F.; Vagbo, C.B.; Homolka, D.; Pillai, R.S. Essential roles of RNA cap-proximal ribose methylation in mammalian embryonic development and fertility. Cell Rep. 2023, 42, 112786. [Google Scholar] [CrossRef]
- Kishore, U.; Kufer, T.A. Editorial: Updates on RIG-I-like receptor-mediated innate immune responses. Front. Immunol. 2023, 14, 1153410. [Google Scholar] [CrossRef] [PubMed]
- Kouwaki, T.; Nishimura, T.; Wang, G.; Oshiumi, H. RIG-I-Like Receptor-Mediated Recognition of Viral Genomic RNA of Severe Acute Respiratory Syndrome Coronavirus-2 and Viral Escape From the Host Innate Immune Responses. Front. Immunol. 2021, 12, 700926. [Google Scholar] [CrossRef]
- Li, Y.; Song, S.; Li, C.; Yu, J. MeRIP-PF: An easy-to-use pipeline for high-resolution peak-finding in MeRIP-Seq data. Genom. Proteom. Bioinform. 2013, 11, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Hawley, B.R.; Jaffrey, S.R. Transcriptome-Wide Mapping of m(6) A and m(6) Am at Single-Nucleotide Resolution Using miCLIP. Curr. Protoc. Mol. Biol. 2019, 126, e88. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhao, X.; Zhu, C.; Ruan, J.; Chen, C. N6-Methyladenosine Modification of the Three Components “Writers”, “Erasers”, and “Readers” in Relation to Osteogenesis. Int. J. Mol. Sci. 2025, 26, 5620. [Google Scholar] [CrossRef]
- Wang, S.; Wan, L.; Zhang, M.; Yan, D.; Li, F. New Targets for Immune Inflammatory Response in Rheumatoid Arthritis: Focus on the Potential Significance of N6-Methyladenosine, Ferroptosis and Cuproptosis. J. Inflamm. Res. 2025, 18, 8085–8106. [Google Scholar] [CrossRef]
- Schwartz, S.; Bernstein, D.A.; Mumbach, M.R.; Jovanovic, M.; Herbst, R.H.; Leon-Ricardo, B.X.; Engreitz, J.M.; Guttman, M.; Satija, R.; Lander, E.S.; et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 2014, 159, 148–162. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cui, Q.; He, C.; Yi, C.; Shi, Y. Decoding pseudouridine: An emerging target for therapeutic development. Trends Pharmacol. Sci. 2022, 43, 522–535. [Google Scholar] [CrossRef]
- Morais, P.; Adachi, H.; Yu, Y.T. The Critical Contribution of Pseudouridine to mRNA COVID-19 Vaccines. Front. Cell Dev. Biol. 2021, 9, 789427. [Google Scholar] [CrossRef]
- Khong, A.; Matheny, T.; Jain, S.; Mitchell, S.F.; Wheeler, J.R.; Parker, R. The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol. Cell 2017, 68, 808–820.e5. [Google Scholar] [CrossRef]
- Zhang, L.S.; Ye, C.; Ju, C.W.; Gao, B.; Feng, X.; Sun, H.L.; Wei, J.; Yang, F.; Dai, Q.; He, C. BID-seq for transcriptome-wide quantitative sequencing of mRNA pseudouridine at base resolution. Nat. Protoc. 2024, 19, 517–538. [Google Scholar] [CrossRef]
- Bohnsack, K.E.; Hobartner, C.; Bohnsack, M.T. Eukaryotic 5-methylcytosine (m(5)C) RNA Methyltransferases: Mechanisms, Cellular Functions, and Links to Disease. Genes 2019, 10, 102. [Google Scholar] [CrossRef]
- Li, M.; Tao, Z.; Zhao, Y.; Li, L.; Zheng, J.; Li, Z.; Chen, X. 5-methylcytosine RNA methyltransferases and their potential roles in cancer. J. Transl. Med. 2022, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Tuo, Z.; Chen, K.; Wu, R.; Wang, J.; Yu, Q.; Ye, L.; Miyamoto, A.; Yoo, K.H.; Zhang, C.; et al. Pan-cancer analysis of RNA 5-methylcytosine reader (ALYREF). Oncol. Res. 2024, 32, 503–515. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Sun, B.F.; Chen, Y.S.; Xu, J.W.; Lai, W.Y.; Li, A.; Wang, X.; Bhattarai, D.P.; Xiao, W.; et al. 5-methylcytosine promotes mRNA export—NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017, 27, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.L. RNA editing by adenosine deaminases that act on RNA. Annu. Rev. Biochem. 2002, 71, 817–846. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sinha, A.N.; Ray, A.; Lal, M.; Nayak, S.; Sharma, A.; Mehani, B.; Mukherjee, D.; Laddha, S.V.; Suri, A.; et al. A-to-I editing in human miRNAs is enriched in seed sequence, influenced by sequence contexts and significantly hypoedited in glioblastoma multiforme. Sci. Rep. 2017, 7, 2466. [Google Scholar] [CrossRef]
- Walkley, C.R.; Li, J.B. Rewriting the transcriptome: Adenosine-to-inosine RNA editing by ADARs. Genome Biol. 2017, 18, 205. [Google Scholar] [CrossRef]
- Goncharov, A.O.; Shender, V.O.; Kuznetsova, K.G.; Kliuchnikova, A.A.; Moshkovskii, S.A. Interplay between A-to-I Editing and Splicing of RNA: A Potential Point of Application for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 5240. [Google Scholar] [CrossRef]
- Rosenthal, J.J.; Seeburg, P.H. A-to-I RNA editing: Effects on proteins key to neural excitability. Neuron 2012, 74, 432–439. [Google Scholar] [CrossRef]
- Roth, S.H.; Levanon, E.Y.; Eisenberg, E. Genome-wide quantification of ADAR adenosine-to-inosine RNA editing activity. Nat. Methods 2019, 16, 1131–1138. [Google Scholar] [CrossRef]
- Yuting, K.; Ding, D.; Iizasa, H. Adenosine-to-Inosine RNA Editing Enzyme ADAR and microRNAs. Methods Mol. Biol. 2021, 2181, 83–95. [Google Scholar] [CrossRef]
- Dominissini, D.; Nachtergaele, S.; Moshitch-Moshkovitz, S.; Peer, E.; Kol, N.; Ben-Haim, M.S.; Dai, Q.; Di Segni, A.; Salmon-Divon, M.; Clark, W.C.; et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature 2016, 530, 441–446. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, X.; Hou, M.; Li, Q.; Li, X.; Lu, L.; Qi, E.; Wu, M.; Qi, L.; Jian, H.; et al. The landscape of m(1)A modification and its posttranscriptional regulatory functions in primary neurons. Elife 2023, 12, e85324. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, L.; Guo, H.; Li, L.; Chen, S.; Fan, Y.; Tian, J.; Xu, L.; Kong, X.; Xuan, A. m(1)A demethylase Alkbh3 regulates neurogenesis through m(1)A demethylation of Mmp15 mRNA. Cell Biosci. 2024, 14, 92. [Google Scholar] [CrossRef]
- James, C.C.; Smyth, J.W. Alternative mechanisms of translation initiation: An emerging dynamic regulator of the proteome in health and disease. Life Sci. 2018, 212, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Granulation of m1A-modified mRNAs protects their functionality through cellular stress. J. Mol. Cell Biol. 2021, 12, 821–822. [Google Scholar] [CrossRef]
- Kawarada, L.; Suzuki, T.; Ohira, T.; Hirata, S.; Miyauchi, K.; Suzuki, T. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017, 45, 7401–7415. [Google Scholar] [CrossRef] [PubMed]
- Monshaugen, I.; Luna, L.; Rhodes, J.; Kristiansen, F.I.S.; Lang, A.; Boe, S.O.; Dutta, A.; Su, Z.; Klungland, A.; Ougland, R. Depletion of the m1A writer TRMT6/TRMT61A reduces proliferation and resistance against cellular stress in bladder cancer. Front. Oncol. 2023, 13, 1334112. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, C.; Jian, H.; Hou, M.; Lou, Y.; Kang, Y.; Wang, W.; Lv, Y.; Shang, S.; Wang, C.; et al. N(1)-Methyladenosine modification of mRNA regulates neuronal gene expression and oxygen glucose deprivation/reoxygenation induction. Cell Death Discov. 2023, 9, 159. [Google Scholar] [CrossRef]
- Deng, Y.; Tan, Z.; Cai, S.; Feng, Y.; Tang, Z.; Li, J.; He, H.; Wu, Z.; Liu, R.; Huang, H.; et al. N1-methyladenosine RNA methylation patterns are associated with an increased risk to biochemical recurrence in prostate cancer and serve as a potential novel biomarker for patient stratification. Int. Immunopharmacol. 2024, 143, 113404. [Google Scholar] [CrossRef]
- Mauer, J.; Luo, X.; Blanjoie, A.; Jiao, X.; Grozhik, A.V.; Patil, D.P.; Linder, B.; Pickering, B.F.; Vasseur, J.J.; Chen, Q.; et al. Reversible methylation of m(6)A(m) in the 5′ cap controls mRNA stability. Nature 2017, 541, 371–375. [Google Scholar] [CrossRef]
- Sugita, A.; Kano, R.; Ishiguro, H.; Yanagisawa, N.; Kuruma, S.; Wani, S.; Tanaka, A.; Tabuchi, Y.; Ohkuma, Y.; Hirose, Y. Cap-Specific m(6)Am Methyltransferase PCIF1/CAPAM Regulates mRNA Stability of RAB23 and CNOT6 through the m(6)A Methyltransferase Activity. Cells 2024, 13, 1689. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, K.; Zhang, X.; Liu, J.; Zhang, M.; Meng, H.; Yi, C. m(6)Am-seq reveals the dynamic m(6)Am methylation in the human transcriptome. Nat. Commun. 2021, 12, 4778. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, Z.; Yao, X.; Pang, S.; Liu, M.; Jiang, W.; Jiang, J.; Zhang, Q. Capping Enzyme mRNA-cap/RNGTT Regulates Hedgehog Pathway Activity by Antagonizing Protein Kinase A. Sci. Rep. 2017, 7, 2891. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.A.; Petit, A.P.; Mendoza Martinez, C.; Bellany, F.; Lin, D.; Niven, S.; Swift, R.; Eadsforth, T.; Fyfe, P.; Paul, M.; et al. Characterisation of RNA guanine-7 methyltransferase (RNMT) using a small molecule approach. Biochem. J. 2025, 482, 209–224. [Google Scholar] [CrossRef]
- Liang, S.; Almohammed, R.; Cowling, V.H. The RNA cap methyltransferases RNMT and CMTR1 co-ordinate gene expression during neural differentiation. Biochem. Soc. Trans. 2023, 51, 1131–1141. [Google Scholar] [CrossRef]
- Li, Z.; Mao, J.; Huang, D.; Song, B.; Meng, J. RNADSN: Transfer-Learning 5-Methyluridine (m(5)U) Modification on mRNAs from Common Features of tRNA. Int. J. Mol. Sci. 2022, 23, 13493. [Google Scholar] [CrossRef]
- Ao, C.; Ye, X.; Sakurai, T.; Zou, Q.; Yu, L. m5U-SVM: Identification of RNA 5-methyluridine modification sites based on multi-view features of physicochemical features and distributed representation. BMC Biol. 2023, 21, 93. [Google Scholar] [CrossRef]
- Noor, S.; Naseem, A.; Awan, H.H.; Aslam, W.; Khan, S.; AlQahtani, S.A.; Ahmad, N. Deep-m5U: A deep learning-based approach for RNA 5-methyluridine modification prediction using optimized feature integration. BMC Bioinform. 2024, 25, 360. [Google Scholar] [CrossRef]
- Witzenberger, M.; Burczyk, S.; Settele, D.; Mayer, W.; Welp, L.M.; Heiss, M.; Wagner, M.; Monecke, T.; Janowski, R.; Carell, T.; et al. Human TRMT2A methylates tRNA and contributes to translation fidelity. Nucleic Acids Res. 2023, 51, 8691–8710. [Google Scholar] [CrossRef] [PubMed]
- Freude, K.; Hoffmann, K.; Jensen, L.R.; Delatycki, M.B.; des Portes, V.; Moser, B.; Hamel, B.; van Bokhoven, H.; Moraine, C.; Fryns, J.P.; et al. Mutations in the FTSJ1 gene coding for a novel S-adenosylmethionine-binding protein cause nonsyndromic X-linked mental retardation. Am. J. Hum. Genet. 2004, 75, 305–309. [Google Scholar] [CrossRef]
- Boulias, K.; Greer, E.L. Biological roles of adenine methylation in RNA. Nat. Rev. Genet. 2023, 24, 143–160. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.N.; Xu, B.S.; Liu, Y.P.; Zhou, M.; Long, T.; Li, H.; Dong, H.; Nie, Y.; Chen, P.R.; et al. Intellectual disability-associated gene ftsj1 is responsible for 2′-O-methylation of specific tRNAs. EMBO Rep. 2020, 21, e50095. [Google Scholar] [CrossRef]
- Nagayoshi, Y.; Chujo, T.; Hirata, S.; Nakatsuka, H.; Chen, C.W.; Takakura, M.; Miyauchi, K.; Ikeuchi, Y.; Carlyle, B.C.; Kitchen, R.R.; et al. Loss of Ftsj1 perturbs codon-specific translation efficiency in the brain and is associated with X-linked intellectual disability. Sci. Adv. 2021, 7, eabf3072. [Google Scholar] [CrossRef]
- Galloway, A.; Cowling, V.H. mRNA cap regulation in mammalian cell function and fate. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 270–279. [Google Scholar] [CrossRef]
- Engel, M.; Eggert, C.; Kaplick, P.M.; Eder, M.; Roh, S.; Tietze, L.; Namendorf, C.; Arloth, J.; Weber, P.; Rex-Haffner, M.; et al. The Role of m(6)A/m-RNA Methylation in Stress Response Regulation. Neuron 2018, 99, 389–403.e9. [Google Scholar] [CrossRef]
- Boo, S.H.; Kim, Y.K. The emerging role of RNA modifications in the regulation of mRNA stability. Exp. Mol. Med. 2020, 52, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Marchand, V.; Motorin, Y.; Lafontaine, D.L.J. Identification of sites of 2′-O-methylation vulnerability in human ribosomal RNAs by systematic mapping. Sci. Rep. 2017, 7, 11490. [Google Scholar] [CrossRef] [PubMed]
- Galeone, V.; Dabernig-Heinz, J.; Lohde, M.; Brandt, C.; Kohler, C.; Wagner, G.E.; Holzer, M. Decoding bacterial methylomes in four public health-relevant microbial species: Nanopore sequencing enables reproducible analysis of DNA modifications. BMC Genom. 2025, 26, 394. [Google Scholar] [CrossRef]
- Jin, G.; Xu, M.; Zou, M.; Duan, S. The Processing, Gene Regulation, Biological Functions, and Clinical Relevance of N4-Acetylcytidine on RNA: A Systematic Review. Mol. Ther. Nucleic Acids 2020, 20, 13–24. [Google Scholar] [CrossRef]
- Qiu, L.; Jing, Q.; Li, Y.; Han, J. RNA modification: Mechanisms and therapeutic targets. Mol. Biomed. 2023, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Schiffers, S.; Oberdoerffer, S. ac4C: A fragile modification with stabilizing functions in RNA metabolism. RNA 2024, 30, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, T.; Zhang, D.; Yang, P.; Zhang, C.; Peng, W.; Jin, K.; Wang, L.; Zhou, J.; Peng, C.; et al. Acetyltransferase NAT10 regulates the Wnt/beta-catenin signaling pathway to promote colorectal cancer progression via ac(4)C acetylation of KIF23 mRNA. J. Exp. Clin. Cancer Res. 2022, 41, 345. [Google Scholar] [CrossRef] [PubMed]
- Kudrin, P.; Singh, A.; Meierhofer, D.; Kusnierczyk, A.; Orom, U.A.V. N4-acetylcytidine (ac4C) promotes mRNA localization to stress granules. EMBO Rep. 2024, 25, 1814–1834. [Google Scholar] [CrossRef]
- Liu, W.C.; Wei, Y.H.; Chen, J.F.; Xing, X.L.; Jia, H.X.; Yang, X.Y.; Huang, Y.J.; Liu, X.M.; Xiao, K.; Guo, X.D.; et al. Inhibition of tumor-intrinsic NAT10 enhances antitumor immunity by triggering type I interferon response via MYC/CDK2/DNMT1 pathway. Nat. Commun. 2025, 16, 5154. [Google Scholar] [CrossRef]
- Liu, C.; Dou, X.; Zhao, Y.; Zhang, L.; Zhang, L.; Dai, Q.; Liu, J.; Wu, T.; Xiao, Y.; He, C. IGF2BP3 promotes mRNA degradation through internal m(7)G modification. Nat. Commun. 2024, 15, 7421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.I.; Pecot, C.V.; Holley, C.L. 2′-O-methylation (Nm) in RNA: Progress, challenges, and future directions. RNA 2024, 30, 570–582. [Google Scholar] [CrossRef]
- Pyronnet, S.; Dostie, J.; Sonenberg, N. Suppression of cap-dependent translation in mitosis. Genes Dev. 2001, 15, 2083–2093. [Google Scholar] [CrossRef]
- Freund, I.; Eigenbrod, T.; Helm, M.; Dalpke, A.H. RNA Modifications Modulate Activation of Innate Toll-Like Receptors. Genes 2019, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.; Fatima, H.; Ahmad, S.; Rehman, A.; Safdar, F. The interplay between epitranscriptomic RNA modifications and neurodegenerative disorders: Mechanistic insights and potential therapeutic strategies. Ibrain 2024, 10, 395–426. [Google Scholar] [CrossRef]
- Gumienny, R.; Jedlinski, D.J.; Schmidt, A.; Gypas, F.; Martin, G.; Vina-Vilaseca, A.; Zavolan, M. High-throughput identification of C/D box snoRNA targets with CLIP and RiboMeth-seq. Nucleic Acids Res. 2017, 45, 2341–2353. [Google Scholar] [CrossRef]
- Krogh, N.; Birkedal, U.; Nielsen, H. RiboMeth-seq: Profiling of 2′-O-Me in RNA. Methods Mol. Biol. 2017, 1562, 189–209. [Google Scholar] [CrossRef]
- Tardu, M.; Jones, J.D.; Kennedy, R.T.; Lin, Q.; Koutmou, K.S. Identification and Quantification of Modified Nucleosides in Saccharomyces cerevisiae mRNAs. ACS Chem. Biol. 2019, 14, 1403–1409. [Google Scholar] [CrossRef]
- Li, Y.; Yi, Y.; Gao, X.; Wang, X.; Zhao, D.; Wang, R.; Zhang, L.S.; Gao, B.; Zhang, Y.; Zhang, L.; et al. 2′-O-methylation at internal sites on mRNA promotes mRNA stability. Mol. Cell 2024, 84, 2320–2336.e6. [Google Scholar] [CrossRef] [PubMed]
- Zust, R.; Cervantes-Barragan, L.; Habjan, M.; Maier, R.; Neuman, B.W.; Ziebuhr, J.; Szretter, K.J.; Baker, S.C.; Barchet, W.; Diamond, M.S.; et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011, 12, 137–143. [Google Scholar] [CrossRef]
- Parker, M.T.; Knop, K.; Sherwood, A.V.; Schurch, N.J.; Mackinnon, K.; Gould, P.D.; Hall, A.J.; Barton, G.J.; Simpson, G.G. Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m(6)A modification. Elife 2020, 9, e49658. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Chen, J.; Guo, Z.; Liu, Y.; Hua, H. Exploiting RIG-I-like receptor pathway for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Trixl, L.; Lusser, A. The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. Wiley Interdiscip. Rev. RNA 2019, 10, e1510. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, J. RNA 5-methylcytosine modification and its emerging role as an epitranscriptomic mark. RNA Biol. 2021, 18, 117–127. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, X.; Zhou, Y.; Shi, Z.; Xie, X.; Zhang, X.; Gao, L.; Fu, A.; Liu, C.; He, B.; et al. Base-resolution m(5)C profiling across the mammalian transcriptome by bisulfite-free enzyme-assisted chemical labeling approach. Mol. Cell 2024, 84, 2984–3000.e8. [Google Scholar] [CrossRef] [PubMed]
- Cerneckis, J.; Ming, G.L.; Song, H.; He, C.; Shi, Y. The rise of epitranscriptomics: Recent developments and future directions. Trends Pharmacol. Sci. 2024, 45, 24–38. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Nombela, P.; Miguel-Lopez, B.; Blanco, S. The role of m(6)A, m(5)C and Psi RNA modifications in cancer: Novel therapeutic opportunities. Mol. Cancer 2021, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Licht, K.; Hartl, M.; Amman, F.; Anrather, D.; Janisiw, M.P.; Jantsch, M.F. Inosine induces context-dependent recoding and translational stalling. Nucleic Acids Res. 2019, 47, 3–14. [Google Scholar] [CrossRef]
- Sendinc, E.; Valle-Garcia, D.; Dhall, A.; Chen, H.; Henriques, T.; Navarrete-Perea, J.; Sheng, W.; Gygi, S.P.; Adelman, K.; Shi, Y. PCIF1 Catalyzes m6Am mRNA Methylation to Regulate Gene Expression. Mol. Cell 2019, 75, 620–630.e9. [Google Scholar] [CrossRef]
- Yang, J.; Song, M.; Bu, Y.; Zhao, H.; Liu, C.; Zhang, T.; Zhang, C.; Xu, S.; Ma, C. Machine learning-augmented m6A-Seq analysis without a reference genome. Brief. Bioinform. 2025, 26, bbaf235. [Google Scholar] [CrossRef]
- Carlile, T.M.; Rojas-Duran, M.F.; Gilbert, W.V. Pseudo-Seq: Genome-Wide Detection of Pseudouridine Modifications in RNA. Methods Enzymol. 2015, 560, 219–245. [Google Scholar] [CrossRef]
- Suzuki, T.; Ueda, H.; Okada, S.; Sakurai, M. Transcriptome-wide identification of adenosine-to-inosine editing using the ICE-seq method. Nat. Protoc. 2015, 10, 715–732. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, Q. Translating the m(6)A epitranscriptome for prostate cancer. Nat. Rev. Urol. 2025, 22, 6. [Google Scholar] [CrossRef]
- Feng, B.; Chen, Y.; Tu, H.; Zhang, J.; Tong, L.; Lyu, X.; Irving, A.T.; Chen, D. Transcriptomic Analysis of the m6A Reader YTHDF2 in the Maintenance and Differentiation of Human Embryonic Stem Cells. Stem. Cells 2025, 43, sxaf032. [Google Scholar] [CrossRef]
- Widagdo, J.; Anggono, V. The m6A-epitranscriptomic signature in neurobiology: From neurodevelopment to brain plasticity. J. Neurochem. 2018, 147, 137–152. [Google Scholar] [CrossRef]
- Liu, H.; Tan, S.; Zhao, Z.; Tang, X.; Li, Z.; Qi, J. METTL3/YTDHF1 Stabilizes MTCH2 mRNA to Regulate Ferroptosis in Glioma Cells. Front. Biosci. 2025, 30, 25718. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Huang, K.; Yang, L.; Yang, X.; Luo, A.; Cai, M.; Wu, X.; Liu, X.; Yan, Y.; et al. Novel Associations Between METTL3 Gene Polymorphisms and Pediatric Acute Lymphoblastic Leukemia: A Five-Center Case-Control Study. Front. Oncol. 2021, 11, 635251. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Z.; Wang, Z.; Yi, X.; Wu, J. m6A methyltransferase METTL3 promotes glucose metabolism hub gene expression and induces metabolic dysfunction-associated steatotic liver disease (MASLD). BMC Genom. 2025, 26, 188. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Moheb, L.; Mertel, S.; Gonsior, M.; Nouri-Vahid, L.; Kahrizi, K.; Cirak, S.; Wieczorek, D.; Motazacker, M.M.; Esmaeeli-Nieh, S.; Cremer, K.; et al. Mutations in NSUN2 cause autosomal-recessive intellectual disability. Am. J. Hum. Genet. 2012, 90, 847–855. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Q.; Qin, Z.; Yi, S.; Luo, J. A novel variant in NSUN2 causes intellectual disability in a Chinese family. BMC Med. Genom. 2024, 17, 95. [Google Scholar] [CrossRef]
- Wang, Z.; Mierxiati, A.; Zhu, W.; Li, T.; Xu, H.; Wan, F.; Ye, D. FOXA1-dependent NSUN2 facilitates the advancement of prostate cancer by preserving TRIM28 mRNA stability in a m5C-dependent manner. NPJ Precis. Oncol. 2025, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Okada, S.; Sakurai, M. Adenosine-to-inosine RNA editing in neurological development and disease. RNA Biol. 2021, 18, 999–1013. [Google Scholar] [CrossRef]
- Cui, N.; Qian, Q.; Zhou, Y.; Zhang, H.; Zhang, H.; Wang, B.; Li, Y.; Wang, Q.; Lian, M.; You, Z.; et al. Extracellular Inosine Induces Anergy in B Cells to Alleviate Autoimmune Hepatitis. Cell Mol. Gastroenterol. Hepatol. 2025, 101539. [Google Scholar] [CrossRef]
- Alriquet, M.; Calloni, G.; Martinez-Limon, A.; Delli Ponti, R.; Hanspach, G.; Hengesbach, M.; Tartaglia, G.G.; Vabulas, R.M. The protective role of m1A during stress-induced granulation. J. Mol. Cell Biol. 2021, 12, 870–880. [Google Scholar] [CrossRef]
- Ren, M.; Fan, B.; Cao, G.; Zong, R.; Feng, L.; Sun, H. Exploration and validation of a combined Hypoxia and m6A/m5C/m1A regulated gene signature for prognosis prediction of liver cancer. BMC Genom. 2023, 24, 776. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Zhao, Y.; Chen, H.; Wang, C.; Wu, A.; Guo, X.; Huang, Y.; Wang, Q.; Hao, L.; et al. NAT10 inhibition alleviates astrocyte autophagy by impeding ac4C acetylation of Timp1 mRNA in ischemic stroke. Acta Pharm. Sin. B 2025, 15, 2575–2592. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vagbo, C.B.; Shi, Y.; Wang, W.L.; Song, S.H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Patton, J.R.; Bykhovskaya, Y.; Mengesha, E.; Bertolotto, C.; Fischel-Ghodsian, N. Mitochondrial myopathy and sideroblastic anemia (MLASA): Missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation. J. Biol. Chem. 2005, 280, 19823–19828. [Google Scholar] [CrossRef]
- de Brouwer, A.P.M.; Abou Jamra, R.; Kortel, N.; Soyris, C.; Polla, D.L.; Safra, M.; Zisso, A.; Powell, C.A.; Rebelo-Guiomar, P.; Dinges, N.; et al. Variants in PUS7 Cause Intellectual Disability with Speech Delay, Microcephaly, Short Stature, and Aggressive Behavior. Am. J. Hum. Genet. 2018, 103, 1045–1052. [Google Scholar] [CrossRef]
- Moon, H.J.; Redman, K.L. Trm4 and Nsun2 RNA:m5C methyltransferases form metabolite-dependent, covalent adducts with previously methylated RNA. Biochemistry 2014, 53, 7132–7144. [Google Scholar] [CrossRef]
- Schaefer, M.; Hagemann, S.; Hanna, K.; Lyko, F. Azacytidine inhibits RNA methylation at DNMT2 target sites in human cancer cell lines. Cancer Res. 2009, 69, 8127–8132. [Google Scholar] [CrossRef]
- Gerber, A.; Grosjean, H.; Melcher, T.; Keller, W. Tad1p, a yeast tRNA-specific adenosine deaminase, is related to the mammalian pre-mRNA editing enzymes ADAR1 and ADAR2. EMBO J. 1998, 17, 4780–4789. [Google Scholar] [CrossRef]
- Wang, B.; Niu, L.; Wang, Z.; Zhao, Z. RNA m1A Methyltransferase TRMT6 Predicts Poorer Prognosis and Promotes Malignant Behavior in Glioma. Front. Mol. Biosci. 2021, 8, 692130. [Google Scholar] [CrossRef]
- Chujo, T.; Suzuki, T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA 2012, 18, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Akichika, S.; Hirano, S.; Shichino, Y.; Suzuki, T.; Nishimasu, H.; Ishitani, R.; Sugita, A.; Hirose, Y.; Iwasaki, S.; Nureki, O.; et al. Cap-specific terminal N (6)-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science 2019, 363, eaav0080. [Google Scholar] [CrossRef]
- Pillutla, R.C.; Shimamoto, A.; Furuichi, Y.; Shatkin, A.J. Human mRNA capping enzyme (RNGTT) and cap methyltransferase (RNMT) map to 6q16 and 18p11.22-p11.23, respectively. Genomics 1998, 54, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Belanger, F.; Stepinski, J.; Darzynkiewicz, E.; Pelletier, J. Characterization of hMTr1, a human Cap1 2′-O-ribose methyltransferase. J. Biol. Chem. 2010, 285, 33037–33044. [Google Scholar] [CrossRef]
- Carter, J.M.; Emmett, W.; Mozos, I.R.; Kotter, A.; Helm, M.; Ule, J.; Hussain, S. FICC-Seq: A method for enzyme-specified profiling of methyl-5-uridine in cellular RNA. Nucleic Acids Res. 2019, 47, e113. [Google Scholar] [CrossRef]
- Guy, M.P.; Podyma, B.M.; Preston, M.A.; Shaheen, H.H.; Krivos, K.L.; Limbach, P.A.; Hopper, A.K.; Phizicky, E.M. Yeast Trm7 interacts with distinct proteins for critical modifications of the tRNAPhe anticodon loop. RNA 2012, 18, 1921–1933. [Google Scholar] [CrossRef]
- Liu, H.; Ling, Y.; Gong, Y.; Sun, Y.; Hou, L.; Zhang, B. DNA damage induces N-acetyltransferase NAT10 gene expression through transcriptional activation. Mol. Cell Biochem. 2007, 300, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bahr, A.; Hankeln, T.; Fiedler, T.; Hegemann, J.; Schmidt, E.R. Molecular analysis of METTL1, a novel human methyltransferase-like gene with a high degree of phylogenetic conservation. Genomics 1999, 57, 424–428. [Google Scholar] [CrossRef]
- Fan, W.; Liu, P.; Tan, L.; Lv, H.; Zhou, H.; Tao, Z.; Xu, Y. Tet2 modulates M2 macrophage polarization via mRNA 5-methylcytosine in allergic rhinitis. Int. Immunopharmacol. 2024, 143, 113495. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Du, Y.; Wang, C.; Zhang, Y.; Liu, H. Evolutionary Origins and Adaptive Significance of A-to-I RNA Editing in Animals and Fungi. Bioessays 2025, 47, e202400220. [Google Scholar] [CrossRef]
- Potuznik, J.F.; Cahova, H. If the 5′ cap fits (wear it)—Non-canonical RNA capping. RNA Biol. 2024, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pajdzik, K.; Lyu, R.; Dou, X.; Ye, C.; Zhang, L.S.; Dai, Q.; He, C. Chemical manipulation of m(1)A mediates its detection in human tRNA. RNA 2024, 30, 548–559. [Google Scholar] [CrossRef]
- Guo, G.; Wang, H.; Shi, X.; Ye, L.; Yan, K.; Chen, Z.; Zhang, H.; Jin, Z.; Xue, X. Disease Activity-Associated Alteration of mRNA m(5) C Methylation in CD4(+) T Cells of Systemic Lupus Erythematosus. Front. Cell Dev. Biol. 2020, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Mizugaki, M.; Ishida, N. Detection of elevated amounts of urinary pseudouridine in cancer patients by use of a monoclonal antibody. Clin. Chim. Acta 1989, 181, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sinha, M. Targeting intracellular mRNA m(6)A-modifiers in advancing immunotherapeutics. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Ranga, S.; Yadav, R.; Chauhan, M.; Chhabra, R.; Ahuja, P.; Balhara, N. Modifications of RNA in cancer: A comprehensive review. Mol. Biol. Rep. 2025, 52, 321. [Google Scholar] [CrossRef]
- Spangenberg, J.; Mundnich, S.; Busch, A.; Pastore, S.; Wierczeiko, A.; Goettsch, W.; Dietrich, V.; Pryszcz, L.P.; Cruciani, S.; Novoa, E.M.; et al. The RMaP challenge of predicting RNA modifications by nanopore sequencing. Commun. Chem. 2025, 8, 115. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruden, D.M. Uncovering the Epitranscriptome: A Review on mRNA Modifications and Emerging Frontiers. Genes 2025, 16, 951. https://doi.org/10.3390/genes16080951
Ruden DM. Uncovering the Epitranscriptome: A Review on mRNA Modifications and Emerging Frontiers. Genes. 2025; 16(8):951. https://doi.org/10.3390/genes16080951
Chicago/Turabian StyleRuden, Douglas M. 2025. "Uncovering the Epitranscriptome: A Review on mRNA Modifications and Emerging Frontiers" Genes 16, no. 8: 951. https://doi.org/10.3390/genes16080951
APA StyleRuden, D. M. (2025). Uncovering the Epitranscriptome: A Review on mRNA Modifications and Emerging Frontiers. Genes, 16(8), 951. https://doi.org/10.3390/genes16080951







