Muscle Aging Heterogeneity: Genetic and Structural Basis of Sarcopenia Resistance
Abstract
1. Heterogeneity in Sarcopenia Severity: Multifactorial Mechanisms Underlying Age-Related Muscle Decline
2. Genes Regulating Muscle Phenotype
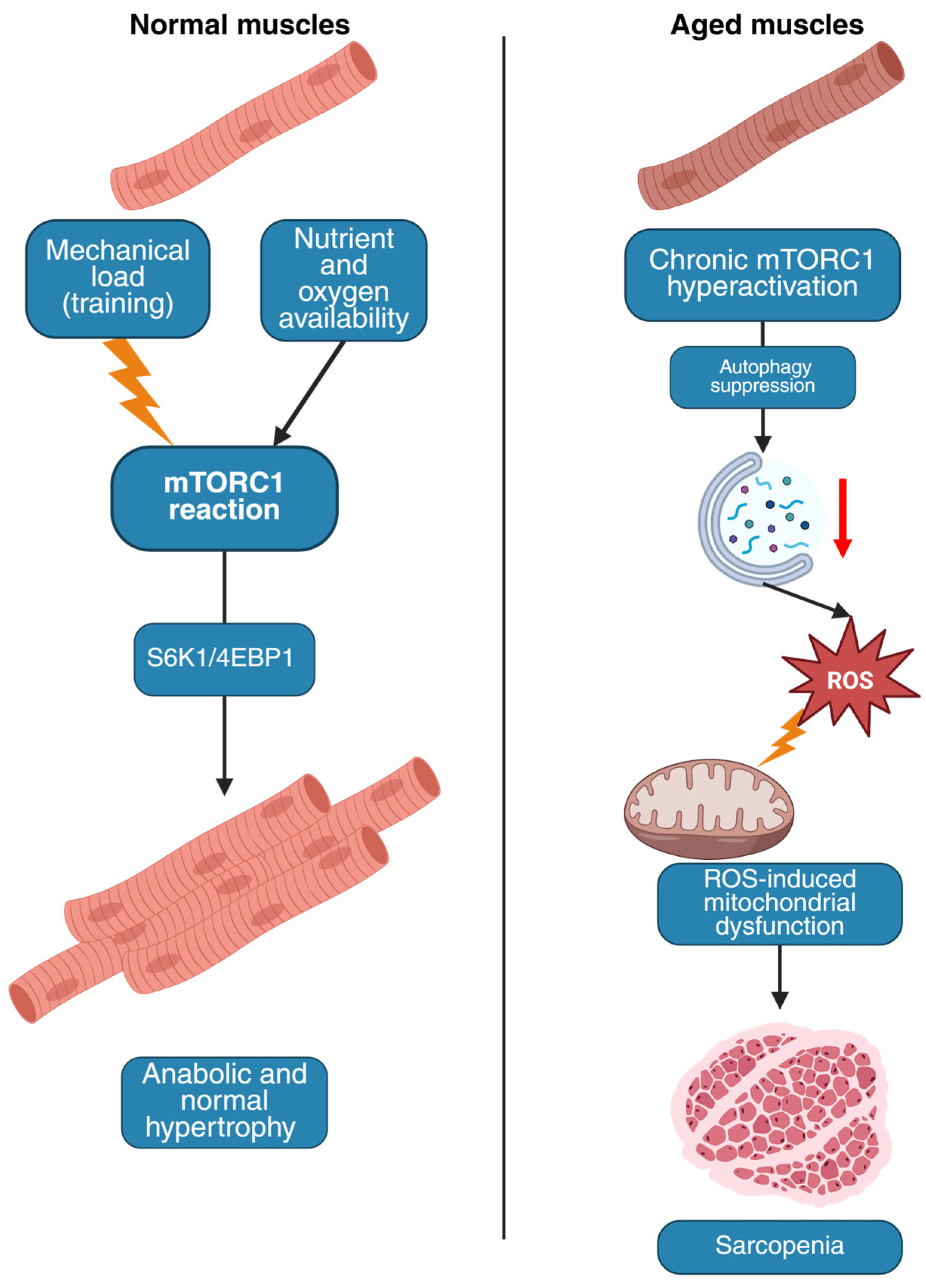
2.1. The mTOR Pathway
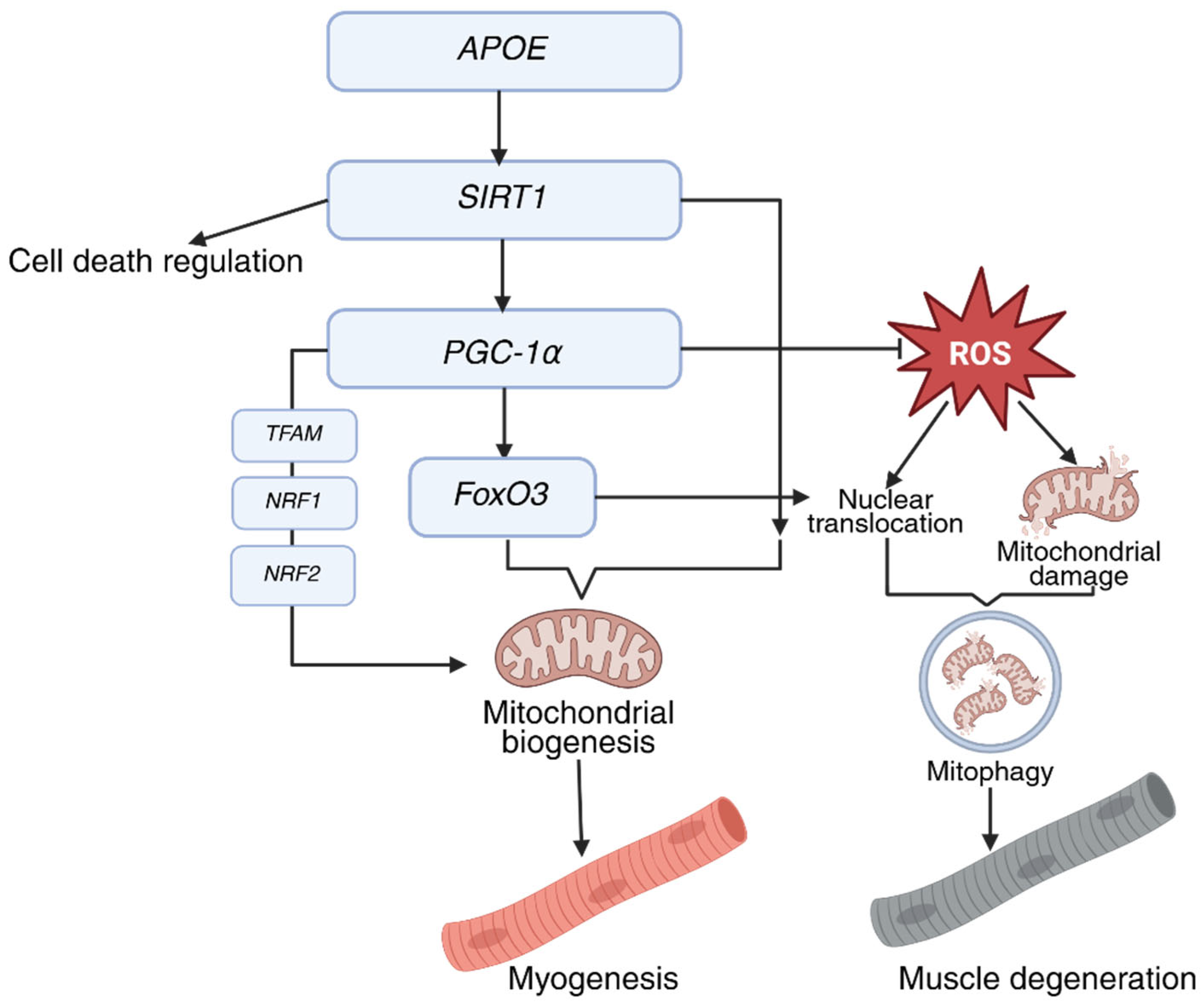
2.2. PGC-1α
2.3. ACTN3
2.4. MSTN
3. Genes of Centenarians That May Affect Resistance to Sarcopenia
4. Mechanisms of Resistance to Sarcopenia
- Muscles with slow-twitch fiber predominance:
- Soleus (maintains posture and walking);
- Diaphragm (continuously active during respiration);
- Spinal extensors (provide vertebral column stability).
- Muscles with fast-twitch fiber predominance:
- Gastrocnemius (responsible for explosive movements like jumping);
- Biceps brachii (used for short-term forceful actions);
- Eyelid muscles (enable rapid blinking).
- Hybrid muscles (e.g., tibialis anterior (supports dorsiflexion and inversion of the foot, contributing to walking, running, and postural balance)) typically contain a mixed fiber composition [88].
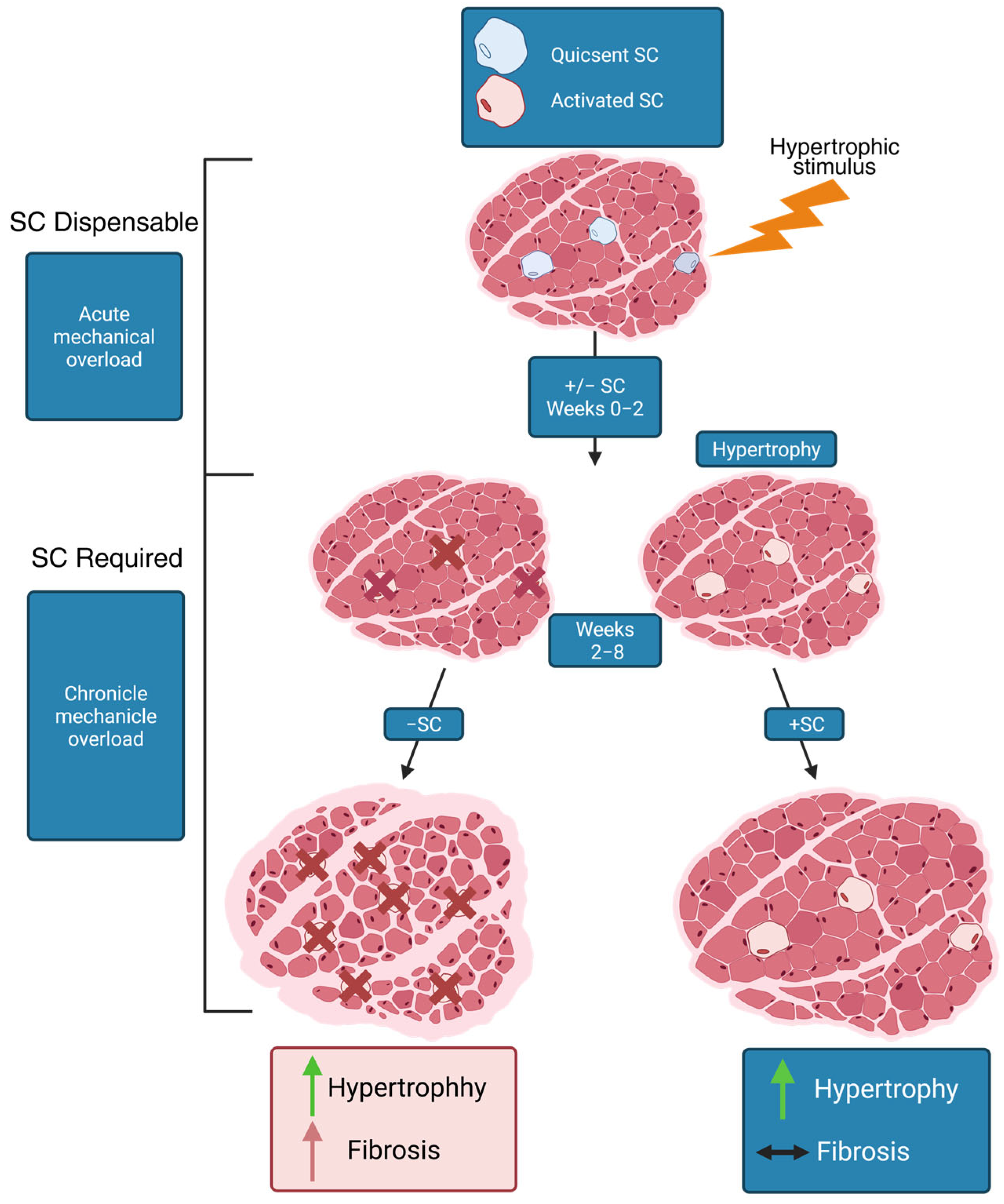
5. Activity of Satellite Cells in Age-Related Atrophy
6. Neuronal Factors
Adaptive Synaptic Architecture: Extraocular Muscles and Other Examples of Stable Innervation
7. Sarcopenia-Resistant Muscles
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Belenguer-Varea, A.; Avellana-Zaragoza, J.A.; Inglés, M.; Cunha-Pérez, C.; Cuesta-Peredo, D.; Borrás, C.; Viña, J.; Tarazona-Santabalbina, F.J. Effect of Familial Longevity on Frailty and Sarcopenia: A Case-Control Study. Int. J. Environ. Res. Public Health 2023, 20, 1534. [Google Scholar] [CrossRef]
- Naruse, M.; Trappe, S.; Trappe, T.A. Human skeletal muscle-specific atrophy with aging: A comprehensive review. J. Appl. Physiol. 2023, 134, 900–914. [Google Scholar] [CrossRef]
- Urzi, F.; Pokorny, B.; Buzan, E. Pilot Study on Genetic Associations with Age-Related Sarcopenia. Front. Genet. 2020, 11, 615238. [Google Scholar] [CrossRef]
- Simon, M.; Yang, J.; Gigas, J.; Earley, E.J.; Hillpot, E.; Zhang, L.; Zagorulya, M.; Tombline, G.; Gilbert, M.; Yuen, S.L.; et al. A rare human centenarian variant of SIRT6 enhances genome stability and interaction with Lamin A. EMBO J. 2022, 41, e110393. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, L.; Xu, W.; Liu, J.; Chen, Y.; Lin, W.; Lu, H.; Wang, B.; Luo, B.; Peng, G.; et al. Sirt6 Mono-ADP-Ribosylates YY1 to Promote Dystrophin Expression for Neuromuscular Transmission. Adv. Sci. 2024, 11, e2406390. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C. The role of mTORC1 in the regulation of skeletal muscle mass. Fac. Rev. 2022, 11, 32. [Google Scholar] [CrossRef]
- Drummond, M.J.; Conlee, R.K.; Mack, G.W.; Sudweeks, S.; Schaalje, G.B.; Parcell, A.C. Myogenic regulatory factor response to resistance exercise volume in skeletal muscle. Eur. J. Appl. Physiol. 2010, 108, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wei, Y.; Feng, Y.; Zhang, S.; Ma, N.; Wang, K.; Tan, P.; Zhao, Y.; Zhao, J.; Ma, X. Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 6184. [Google Scholar] [CrossRef] [PubMed]
- De Bandt, J.P. Leucine and Mammalian Target of Rapamycin-Dependent Activation of Muscle Protein Synthesis in Aging. J. Nutr. 2016, 146, 2616S–2624S. [Google Scholar] [CrossRef]
- Sirago, G.; Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Marzetti, E. Mammalian Target of Rapamycin (mTOR) Signaling at the Crossroad of Muscle Fiber Fate in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 13823. [Google Scholar] [CrossRef]
- Yoon, M.S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Uemichi, K.; Shirai, T.; Hanakita, H.; Takemasa, T. Effect of mechanistic/mammalian target of rapamycin complex 1 on mitochondrial dynamics during skeletal muscle hypertrophy. Physiol. Rep. 2021, 9, e14789. [Google Scholar] [CrossRef]
- Ang, S.J.; Crombie, E.M.; Dong, H.; Tan, K.T.; Hernando, A.; Yu, D.; Adamson, S.; Kim, S.; Withers, D.J.; Huang, H.; et al. Muscle 4EBP1 activation modifies the structure and function of the neuromuscular junction in mice. Nat. Commun. 2022, 13, 7792. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Romanino, K.; Cloëtta, D.; Lin, S.; Mascarenhas, J.B.; Oliveri, F.; Xia, J.; Casanova, E.; Costa, C.F.; Brink, M.; et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008, 8, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.J.; Börsch, A.; Lin, S.; Thürkauf, M.; Weihrauch, M.; Reinhard, J.R.; Delezie, J.; Battilana, F.; Wang, X.; Kaiser, M.S.; et al. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nat. Commun. 2020, 11, 4510. [Google Scholar] [CrossRef]
- Joseph, G.A.; Wang, S.X.; Jacobs, C.E.; Zhou, W.; Kimble, G.C.; Tse, H.W.; Eash, J.K.; Shavlakadze, T.; Glass, D.J. Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Mol. Cell. Biol. 2019, 39, e00141-19. [Google Scholar] [CrossRef]
- Castets, P.; Lin, S.; Rion, N.; Di Fulvio, S.; Romanino, K.; Guridi, M.; Frank, S.; Tintignac, L.A.; Sinnreich, M.; Rüegg, M.A. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metab. 2013, 17, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Guridi, M.; Tintignac, L.A.; Lin, S.; Kupr, B.; Castets, P.; Rüegg, M.A. Activation of mTORC1 in skeletal muscle regulates whole-body metabolism through FGF21. Sci. Signal. 2015, 8, ra113. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Inoki, K.; Brooks, S.V.; Okazawa, H.; Lee, M.; Wang, J.; Kim, M.; Kennedy, C.L.; Macpherson, P.C.D.; Ji, X.; et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 2019, 18, e12943. [Google Scholar] [CrossRef]
- Crombie, E.M.; Kim, S.; Adamson, S.; Dong, H.; Lu, T.C.; Wu, Y.; Wu, Y.; Levy, Y.; Stimple, N.; Lam, W.M.R.; et al. Activation of eIF4E-binding-protein-1 rescues mTORC1-induced sarcopenia by expanding lysosomal degradation capacity. J. Cachexia Sarcopenia Muscle 2023, 14, 198–213. [Google Scholar] [CrossRef]
- Holland, S.H.; Carmona-Martinez, R.; O’Connor, K.; O’Neil, D.; Roos, A.; Spendiff, S.; Lochmüller, H. A Deficiency in Glutamine-Fructose-6-Phosphate Transaminase 1 (Gfpt1) in Skeletal Muscle Results in Reduced Glycosylation of the Delta Subunit of the Nicotinic Acetylcholine Receptor (AChRδ). Biomolecules 2024, 14, 1252. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. mTORC1 as the main gateway to autophagy. Essays Biochem. 2017, 61, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, W.; Li, G.; Cui, W. Weighing In on mTOR Complex 2 Signaling: The Expanding Role in Cell Metabolism. Oxidative Med. Cell. Longev. 2018, 2018, 7838647. [Google Scholar] [CrossRef]
- Oh, H.J.; Jin, H.; Lee, J.Y.; Lee, B.Y. Silk Peptide Ameliorates Sarcopenia Through the Regulation of Akt/mTOR/FoxO3a Signaling Pathways and the Inhibition of Low-Grade Chronic Inflammation in Aged Mice. Cells 2023, 12, 2257. [Google Scholar] [CrossRef]
- Kumagai, H.; Coelho, A.R.; Wan, J.; Mehta, H.H.; Yen, K.; Huang, A.; Zempo, H.; Fuku, N.; Maeda, S.; Oliveira, P.J.; et al. MOTS-c reduces myostatin and muscle atrophy signaling. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E680–E690. [Google Scholar] [CrossRef]
- Kumar, A.; Harris, T.E.; Keller, S.R.; Choi, K.M.; Magnuson, M.A.; Lawrence, J.C. Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances Basal glycogen synthase activity. Mol. Cell. Biol. 2008, 28, 61–70. [Google Scholar] [CrossRef]
- Bhat, N.; Narayanan, A.; Fathzadeh, M.; Kahn, M.; Zhang, D.; Goedeke, L.; Neogi, A.; Cardone, R.L.; Kibbey, R.G.; Fernandez-Hernando, C.; et al. Dyrk1b promotes hepatic lipogenesis by bypassing canonical insulin signaling and directly activating mTORC2 in mice. J. Clin. Investig. 2022, 132, e153724. [Google Scholar] [CrossRef]
- Ye, L.; Varamini, B.; Lamming, D.W.; Sabatini, D.M.; Baur, J.A. Rapamycin has a biphasic effect on insulin sensitivity in C2C12 myotubes due to sequential disruption of mTORC1 and mTORC2. Front. Genet. 2012, 3, 177. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, W.T. mTORC1 and 2 Adrenergic Regulation and Function in Brown Adipose Tissue. Physiology 2025, 40, 134–144. [Google Scholar] [CrossRef]
- Ogasawara, R.; Knudsen, J.R.; Li, J.; Ato, S.; Jensen, T.E. Rapamycin and mTORC2 inhibition synergistically reduce contraction-stimulated muscle protein synthesis. J. Physiol. 2020, 598, 5453–5466. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Bomhoff, G.L.; Geiger, P.C. Age-related differences in skeletal muscle insulin signaling: The role of stress kinases and heat shock proteins. J. Appl. Physiol. 2008, 105, 839–848. [Google Scholar] [CrossRef]
- Hatazawa, Y.; Tadaishi, M.; Nagaike, Y.; Morita, A.; Ogawa, Y.; Ezaki, O.; Takai-Igarashi, T.; Kitaura, Y.; Shimomura, Y.; Kamei, Y.; et al. PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS ONE 2014, 9, e91006. [Google Scholar] [CrossRef]
- Popov, D.V.; Lysenko, E.A.; Kuzmin, I.V.; Vinogradova, V.; Grigoriev, A.I. Regulation of PGC-1α Isoform Expression in Skeletal Muscles. Acta Nat. 2015, 7, 48–59. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef]
- Graham, R.B.; Wachowiak, M.P.; Gurd, B.J. The Assessment of Muscular Effort, Fatigue, and Physiological Adaptation Using EMG and Wavelet Analysis. PLoS ONE 2015, 10, e0135069. [Google Scholar] [CrossRef]
- Hatazawa, Y.; Senoo, N.; Tadaishi, M.; Ogawa, Y.; Ezaki, O.; Kamei, Y.; Miura, S. Metabolomic Analysis of the Skeletal Muscle of Mice Overexpressing PGC-1α. PLoS ONE 2015, 10, e0129084. [Google Scholar] [CrossRef]
- Agudelo, L.Z.; Ferreira, D.M.S.; Dadvar, S.; Cervenka, I.; Ketscher, L.; Izadi, M.; Zhengye, L.; Furrer, R.; Handschin, C.; Venckunas, T.; et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat. Commun. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Song, P.; Zhao, J.; Li, F.; Zhao, X.; Feng, J.; Su, Y.; Wang, B.; Zhao, J. Vitamin A regulates mitochondrial biogenesis and function through p38 MAPK-PGC-1α signaling pathway and alters the muscle fiber composition of sheep. J. Anim. Sci. Biotechnol. 2024, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Rao, Z.; Wu, J.; Ma, X.; Jiang, Z.; Xiao, W. Resistance Exercise Improves Glycolipid Metabolism and Mitochondrial Biogenesis in Skeletal Muscle of T2DM Mice via miR-30d-5p/SIRT1/PGC-1α Axis. Int. J. Mol. Sci. 2024, 25, 12416. [Google Scholar] [CrossRef] [PubMed]
- Summermatter, S.; Shui, G.; Maag, D.; Santos, G.; Wenk, M.R.; Handschin, C. PGC-1α improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes 2013, 62, 85–95. [Google Scholar] [CrossRef]
- Rowe, G.C.; El-Khoury, R.; Patten, I.S.; Rustin, P.; Arany, Z. PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS ONE 2012, 7, e41817. [Google Scholar] [CrossRef]
- Islam, H.; Hood, D.A.; Gurd, B.J. Looking beyond PGC-1α: Emerging regulators of exercise-induced skeletal muscle mitochondrial biogenesis and their activation by dietary compounds. Appl. Physiol. Nutr. Metab. 2020, 45, 11–23. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. ACTN3, Morbidity, and Healthy Aging. Front. Genet. 2018, 9, 15. [Google Scholar] [CrossRef]
- Blanchard, A.; Ohanian, V.; Critchley, D. The structure and function of alpha-actinin. J. Muscle Res. Cell Motil. 1989, 10, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Gasman, B. Disentangling the genetics of sarcopenia: Prioritization of NUDT3 and KLF5 as genes for lean mass & HLA-DQB1-AS1 for hand grip strength with the associated enhancing SNPs & a scoring system. BMC Med. Genet. 2020, 21, 40. [Google Scholar] [CrossRef]
- Seto, J.T.; Roeszler, K.N.; Meehan, L.R.; Wood, H.D.; Tiong, C.; Bek, L.; Lee, S.F.; Shah, M.; Quinlan, K.G.R.; Gregorevic, P.; et al. ACTN3 genotype influences skeletal muscle mass regulation and response to dexamethasone. Sci. Adv. 2021, 7, eabg0088. [Google Scholar] [CrossRef]
- Cho, J.; Lee, I.; Kang, H. ACTN3 Gene and Susceptibility to Sarcopenia and Osteoporotic Status in Older Korean Adults. Biomed. Res. Int. 2017, 2017, 4239648. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, D.; Taniguchi, Y.; Kiuchi, Y.; Akaida, S.; Makizako, H. Association of alpha-actinin-3 genotype with muscle mass and physical function in community-dwelling older adults. Eur. Geriatr. Med. 2025, 16, 15–22. [Google Scholar] [CrossRef]
- Seto, J.T.; Chan, S.; Turner, N.; MacArthur, D.G.; Raftery, J.M.; Berman, Y.D.; Quinlan, K.G.; Cooney, G.J.; Head, S.; Yang, N.; et al. The effect of α-actinin-3 deficiency on muscle aging. Exp. Gerontol. 2011, 46, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Romero-Blanco, C.; Artiga González, M.J.; Gómez-Cabello, A.; Vila-Maldonado, S.; Casajús, J.A.; Ara, I.; Aznar, S. ACTN3 R577X polymorphism related to sarcopenia and physical fitness in active older women. Climacteric 2021, 24, 89–94. [Google Scholar] [CrossRef]
- Norman, B.; Esbjörnsson, M.; Rundqvist, H.; Österlund, T.; Glenmark, B.; Jansson, E. ACTN3 genotype and modulation of skeletal muscle response to exercise in human subjects. J. Appl. Physiol. 2014, 116, 1197–1203. [Google Scholar] [CrossRef]
- Spiller, M.P.; Kambadur, R.; Jeanplong, F.; Thomas, M.; Martyn, J.K.; Bass, J.J.; Sharma, M. The myostatin gene is a downstream target gene of basic helix-loop-helix transcription factor MyoD. Mol. Cell. Biol. 2002, 22, 7066–7082. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhao, Y.P.; Zhao, Y.; Deng, S.L.; Yu, K. Regulation of Myostatin on the Growth and Development of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.E.; Bhasin, S.; Artaza, J.; Byhower, F.; Azam, M.; Willard, D.H.; Kull, F.C.; Gonzalez-Cadavid, N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E221–E228. [Google Scholar] [CrossRef]
- Rodgers, B.D.; Wiedeback, B.D.; Hoversten, K.E.; Jackson, M.F.; Walker, R.G.; Thompson, T.B. Myostatin stimulates, not inihibits, C2C12 myoblast proliferation. Endocrinology 2014, 155, 670–675. [Google Scholar] [CrossRef]
- Braun, P.; Alawi, M.; Saygi, C.; Pantel, K.; Wagers, A.J. Expression profiling by high-throughput sequencing reveals GADD45, SMAD7, EGR-1 and HOXA3 activation in Myostatin (MSTN) and GDF11 treated myoblasts. Genet. Mol. Biol. 2024, 47, e20230304. [Google Scholar] [CrossRef] [PubMed]
- Grobet, L.; Martin, L.J.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J.; et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, S.A.J.; Schönfelder, M.; Becker, L.; Yaghoob Nezhad, F.; Hrabě de Angelis, M.; Wackerhage, H. Genes Whose Gain or Loss-Of-Function Increases Skeletal Muscle Mass in Mice: A Systematic Literature Review. Front. Physiol. 2018, 9, 553. [Google Scholar] [CrossRef]
- Lee, J.; Tompkins, Y.; Kim, D.H.; Kim, W.K.; Lee, K. Increased sizes and improved qualities of tibia bones by myostatin mutation in Japanese quail. Front. Physiol. 2022, 13, 1085935. [Google Scholar] [CrossRef]
- Siriett, V.; Platt, L.; Salerno, M.S.; Ling, N.; Kambadur, R.; Sharma, M. Prolonged absence of myostatin reduces sarcopenia. J. Cell. Physiol. 2006, 209, 866–873. [Google Scholar] [CrossRef]
- Fuku, N.; Alis, R.; Yvert, T.; Zempo, H.; Naito, H.; Abe, Y.; Arai, Y.; Murakami, H.; Miyachi, M.; Pareja-Galeano, H.; et al. Muscle-Related Polymorphisms (MSTN rs1805086 and ACTN3 rs1815739) Are Not Associated with Exceptional Longevity in Japanese Centenarians. PLoS ONE 2016, 11, e0166605. [Google Scholar] [CrossRef]
- da Silva, A.P.; Matos, A.; Ribeiro, R.; Gil, Â.; Valente, A.; Bicho, M.; Gorjão-Clara, J. Sarcopenia and osteoporosis in Portuguese centenarians. Eur. J. Clin. Nutr. 2017, 71, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.T.; Nguyen, B.T.; Huynh, D.T.; Nguyen, B.L.T.; Tran, P.N.; Van Vo, T.; Bui, H.T.; Thai, T.T. Community-based prevalence and associated factors of sarcopenia in the Vietnamese elderly. Sci. Rep. 2024, 14, 17. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, H.; Liu, X.; Wang, X.; Liu, Q.; Zhao, Y.; Nie, Y.; Huang, D.; Fu, S. Calf circumference was negatively associated with all-cause mortality among the Chinese centenarians: A prospective study with a 5-year follow-up. Aging Clin. Exp. Res. 2024, 36, 199. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J.; Willcox, D.C.; Donlon, T.A.; Willcox, B.J. FOXO3: A Major Gene for Human Longevity—A Mini-Review. Gerontology 2015, 61, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zuo, Y.; Yu, Y.; Sun, L.; Yu, Z.; Ma, S.; Zhao, Q.; Sun, G.; Hu, H.; Li, J.; et al. Single-nucleus profiling unveils a geroprotective role of the FOXO3 in primate skeletal muscle aging. Protein Cell 2023, 14, 497–512. [Google Scholar] [CrossRef]
- Gellhaus, B.; Böker, K.O.; Schilling, A.F.; Saul, D. Therapeutic Consequences of Targeting the IGF-1/PI3K/AKT/FOXO3 Axis in Sarcopenia: A Narrative Review. Cells 2023, 12, 2787. [Google Scholar] [CrossRef]
- Donlon, T.A.; Willcox, B.J.; Morris, B.J. FOXO3 cell resilience gene neighborhood. Aging 2017, 9, 2467–2468. [Google Scholar] [CrossRef][Green Version]
- Gellhaus, B.; Böker, K.O.; Gsaenger, M.; Rodenwaldt, E.; Hüser, M.A.; Schilling, A.F.; Saul, D. Foxo3 Knockdown Mediates Decline of Myod1 and Myog Reducing Myoblast Conversion to Myotubes. Cells 2023, 12, 2167. [Google Scholar] [CrossRef]
- Garatachea, N.; Marín, P.J.; Santos-Lozano, A.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A. The ApoE gene is related with exceptional longevity: A systematic review and meta-analysis. Rejuvenation Res. 2015, 18, 3–13. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Louhija, J.; Geroldi, C.; Trabucchi, M. Longevity and the epsilon2 allele of apolipoprotein E: The Finnish Centenarians Study. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M75–M78. [Google Scholar] [CrossRef]
- Sebastiani, P.; Monti, S.; Morris, M.; Gurinovich, A.; Toshiko, T.; Andersen, S.L.; Sweigart, B.; Ferrucci, L.; Jennings, L.L.; Glass, D.J.; et al. A serum protein signature of APOE genotypes in centenarians. Aging Cell 2019, 18, e13023. [Google Scholar] [CrossRef]
- Abondio, P.; Bruno, F.; Luiselli, D. Apolipoprotein E (APOE) Haplotypes in Healthy Subjects from Worldwide Macroareas: A Population Genetics Perspective for Cardiovascular Disease, Neurodegeneration, and Dementia. Curr. Issues Mol. Biol. 2023, 45, 2817–2831. [Google Scholar] [CrossRef]
- Suhail, H.; Soundararajan, C.C.; Vivekanandhan, S.; Singh, S.; Behari, M. Apolipoprotein-E genotypes and myasthenia gravis. Neurol. India 2010, 58, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Hadar, A.; Milanesi, E.; Walczak, M.; Puzianowska-Kuźnicka, M.; Kuźnicki, J.; Squassina, A.; Niola, P.; Chillotti, C.; Attems, J.; Gozes, I.; et al. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s Disease. Sci. Rep. 2018, 8, 8465. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Igarashi, M.; Nasamu, A.S.; Knezevic, J.; Guarente, L. Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of duchenne muscular dystrophy. PLoS Genet. 2014, 10, e1004490. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.Y.; Wong, J.K.S.; Cui, Z.Y.; Lin, Y.Y.; Lee, S.D. Angiotensin II Receptor Blocker Irbesartan Enhanced SIRT1 longevity Signaling Replaces the Mitochondrial Biogenetic Survival Pathway to Attenuate Hypertension-Induced Heart Apoptosis. J. Cardiovasc. Dev. Dis. 2022, 9, 266. [Google Scholar] [CrossRef]
- Lai, C.H.; Ho, T.J.; Kuo, W.W.; Day, C.H.; Pai, P.Y.; Chung, L.C.; Liao, P.H.; Lin, F.H.; Wu, E.T.; Huang, C.Y. Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age 2014, 36, 9706. [Google Scholar] [CrossRef]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: Regulation of aging via NF-kappaB acetylation. Bioessays 2008, 30, 939–942. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Smerdu, V.; Karsch-Mizrachi, I.; Campione, M.; Leinwand, L.; Schiaffino, S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am. J. Physiol. 1994, 267, C1723–C1728. [Google Scholar] [CrossRef]
- Curry, J.W.; Hohl, R.; Noakes, T.D.; Kohn, T.A. High oxidative capacity and type IIx fibre content in springbok and fallow deer skeletal muscle suggest fast sprinters with a resistance to fatigue. J. Exp. Biol. 2012, 215, 3997–4005. [Google Scholar] [CrossRef]
- Dong, H.; Tsai, S.Y. Mitochondrial Properties in Skeletal Muscle Fiber. Cells 2023, 12, 2183. [Google Scholar] [CrossRef]
- Shadiow, J.; Miranda, E.R.; Perkins, R.K.; Mazo, C.E.; Lin, Z.; Lewis, K.N.; Mey, J.T.; Solomon, T.P.J.; Haus, J.M. Exercise-induced changes to the fiber type-specific redox state in human skeletal muscle are associated with aerobic capacity. J. Appl. Physiol. 2023, 135, 508–518. [Google Scholar] [CrossRef]
- Schluessel, S.; Zhang, W.; Nowotny, H.; Bidlingmaier, M.; Hintze, S.; Kunz, S.; Martini, S.; Mehaffey, S.; Meinke, P.; Neuerburg, C.; et al. 11-beta-hydroxysteroid dehydrogenase type 1 (HSD11B1) gene expression in muscle is linked to reduced skeletal muscle index in sarcopenic patients. Aging Clin. Exp. Res. 2023, 35, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- McCuller, C.; Jessu, R.; Callahan, A.L. Physiology, Skeletal Muscle. Updated 30 July 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537139 (accessed on 18 January 2025).
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chan, P. Skeletal Muscle Metabolic Alternation Develops Sarcopenia. Aging Dis. 2022, 13, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.; Chen, M.M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- McIntosh, M.C.; Sexton, C.L.; Godwin, J.S.; Ruple, B.A.; Michel, J.M.; Plotkin, D.L.; Ziegenfuss, T.N.; Lopez, H.L.; Smith, R.; Dwaraka, V.B.; et al. Different Resistance Exercise Loading Paradigms Similarly Affect Skeletal Muscle Gene Expression Patterns of Myostatin-Related Targets and mTORC1 Signaling Markers. Cells 2023, 12, 898. [Google Scholar] [CrossRef]
- Goodman, C.A. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev. Physiol. Biochem. Pharmacol. 2014, 166, 43–95. [Google Scholar] [CrossRef]
- Kukurugya, M.A.; Rosset, S.; Titov, D.V. The Warburg Effect is the result of faster ATP production by glycolysis than respiration. Proc. Natl. Acad. Sci. USA 2024, 121, e2409509121. [Google Scholar] [CrossRef]
- Juel, C. Lactate-proton cotransport in skeletal muscle. Physiol. Rev. 1997, 77, 321–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xie, N.; Ye, H.; Miao, J.; Xia, B.; Yang, Y.; Peng, H.; Xu, S.; Wu, T.; Tao, C.; et al. Glucose restriction enhances oxidative fiber formation: A multi-omic signal network involving AMPK and CaMK2. iScience 2024, 27, 108590. [Google Scholar] [CrossRef] [PubMed]
- Koo-Ng, R.; Falkous, G.; Reilly, M.; Peters, T.J.; Mantle, D.; Preedy, V.R. Carbonyl levels in type I and II fiber-rich muscles and their response to chronic ethanol feeding in vivo and hydroxyl and superoxide radicals in vitro. Alcohol. Clin. Exp. Res. 2000, 24, 1862–1868. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Fiebig, R.; Chandwaney, R.; Ji, L.L. Aging and exercise training in skeletal muscle: Responses of glutathione and antioxidant enzyme systems. Am. J. Physiol. 1994, 267, R439–R445. [Google Scholar] [CrossRef]
- Zhang, F.M.; Wu, H.F.; Wang, K.F.; Yu, D.Y.; Zhang, X.Z.; Ren, Q.; Chen, W.Z.; Lin, F.; Yu, Z.; Zhuang, C.L. Transcriptome profiling of fast/glycolytic and slow/oxidative muscle fibers in aging and obesity. Cell Death Dis. 2024, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Frank, D.; Lippl, S.; Kuhn, C.; Kögler, H.; Barrientos, T.; Rohr, C.; Will, R.; Müller, O.J.; Weiler, H.; et al. Calsarcin-2 deficiency increases exercise capacity in mice through calcineurin/NFAT activation. J. Clin. Investig. 2008, 118, 3598–3608. [Google Scholar] [CrossRef]
- D’Andrea, M.; Pisaniello, A.; Serra, C.; Senni, M.I.; Castaldi, L.; Molinaro, M.; Bouché, M. Protein kinase C theta co-operates with calcineurin in the activation of slow muscle genes in cultured myogenic cells. J. Cell. Physiol. 2006, 207, 379–388. [Google Scholar] [CrossRef]
- Ağaşcıoğlu, E.; Çolak, R.; Çakatay, U. Redox status biomarkers in the fast-twitch extensor digitorum longus resulting from the hypoxic exercise. Nagoya J. Med. Sci. 2022, 84, 433–447. [Google Scholar] [CrossRef]
- Chen, C.N.; Thompson, L.V. Interplay between aging and unloading on oxidative stress in fast-twitch muscles. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 793–802. [Google Scholar] [CrossRef]
- Rakus, D.; Gizak, A.; Deshmukh, A.; Wiśniewski, J.R. Absolute quantitative profiling of the key metabolic pathways in slow and fast skeletal muscle. J. Proteome Res. 2015, 14, 1400–1411. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Shi, X.; Yuan, W.; Liu, G.; Wang, C. Comparative Transcriptome Analysis of Slow-Twitch and Fast-Twitch Muscles in Dezhou Donkeys. Genes 2022, 13, 1610. [Google Scholar] [CrossRef]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef]
- Okamoto, T.; Torii, S.; Machida, S. Differential gene expression of muscle-specific ubiquitin ligase MAFbx/Atrogin-1 and MuRF1 in response to immobilization-induced atrophy of slow-twitch and fast-twitch muscles. J. Physiol. Sci. 2011, 61, 537–546. [Google Scholar] [CrossRef]
- Bronisz-Budzyńska, I.; Kozakowska, M.; Pietraszek-Gremplewicz, K.; Madej, M.; Józkowicz, A.; Łoboda, A.; Dulak, J. NRF2 Regulates Viability, Proliferation, Resistance to Oxidative Stress, and Differentiation of Murine Myoblasts and Muscle Satellite Cells. Cells 2022, 11, 3321. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Arpke, R.W.; Shams, A.S.; Collins, B.C.; Larson, A.A.; Lu, N.; Lowe, D.A.; Kyba, M. Preservation of satellite cell number and regenerative potential with age reveals locomotory muscle bias. Skelet. Muscle 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–547. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Koopman, R.; Schaart, G.; Meijer, K.; Savelberg, H.H.; van Loon, L.J. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E151–E157. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Drummond, M.J.; Glynn, E.L.; Dickinson, J.M.; Gundermann, D.M.; Timmerman, K.L.; Walker, D.K.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet. Muscle 2011, 1, 11. [Google Scholar] [CrossRef]
- Murach, K.A.; Fry, C.S.; Kirby, T.J.; Jackson, J.R.; Lee, J.D.; White, S.H.; Dupont-Versteegden, E.E.; McCarthy, J.J.; Peterson, C.A. Starring or Supporting Role? Satellite Cells and Skeletal Muscle Fiber Size Regulation. Physiology 2018, 33, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Datzkiw, D.; Rudnicki, M.A. Satellite cells in ageing: Use it or lose it. Open Biol. 2020, 10, 200048. [Google Scholar] [CrossRef]
- Lee, A.S.; Anderson, J.E.; Joya, J.E.; Head, S.I.; Pather, N.; Kee, A.J.; Gunning, P.W.; Hardeman, E.C. Aged skeletal muscle retains the ability to fully regenerate functional architecture. Bioarchitecture 2013, 3, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Titova, A.; Nikolaev, S.; Bilyalov, A.; Filatov, N.; Brovkin, S.; Shestakov, D.; Khatkov, I.; Pismennaya, E.; Bondarev, V.; Antyuxina, M.; et al. Extreme Tolerance of Extraocular Muscles to Diseases and Aging: Why and How. Int. J. Mol. Sci. 2024, 25, 4985. [Google Scholar] [CrossRef]
- Verma, M.; Fitzpatrick, K.; McLoon, L.K. Extraocular Muscle Repair and Regeneration. Curr. Ophthalmol. Rep. 2017, 5, 207–215. [Google Scholar] [CrossRef]
- Carrero-Rojas, G.; Benítez-Temiño, B.; Pastor, A.M.; Davis López de Carrizosa, M.A. Muscle Progenitors Derived from Extraocular Muscles Express Higher Levels of Neurotrophins and their Receptors than other Cranial and Limb Muscles. Cells 2020, 9, 747. [Google Scholar] [CrossRef]
- Blumer, R.; Maurer-Gesek, B.; Gesslbauer, B.; Blumer, M.; Pechriggl, E.; Davis-López de Carrizosa, M.A.; Horn, A.K.; May, P.J.; Streicher, J.; de la Cruz, R.R.; et al. Palisade Endings Are a Constant Feature in the Extraocular Muscles of Frontal-Eyed, but Not Lateral-Eyed, Animals. Investig. Ophthalmol. Vis. Sci. 2016, 57, 320–331. [Google Scholar] [CrossRef]
- Lienbacher, K.; Mustari, M.; Hess, B.; Büttner-Ennever, J.; Horn, A.K. Is there any sense in the Palisade endings of eye muscles. Ann. N. Y. Acad. Sci. 2011, 1233, 1–7. [Google Scholar] [CrossRef][Green Version]
- Rudolf, R.; Kettelhut, I.C.; Navegantes, L.C.C. Sympathetic innervation in skeletal muscle and its role at the neuromuscular junction. J. Muscle Res. Cell Motil. 2024, 45, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.A.; Hammond, K.G.; Bickel, C.S.; Windham, S.T.; Tuggle, S.C.; Bamman, M.M. Effects of aging and Parkinson’s disease on motor unit remodeling: Influence of resistance exercise training. J. Appl. Physiol. 2018, 124, 888–898. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Dirks, M.L.; Snijders, T.; Prompers, J.J.; Beelen, M.; Jonkers, R.A.; Thijssen, D.H.; Hopman, M.T.; Van Loon, L.J. Reduced satellite cell numbers with spinal cord injury and aging in humans. Med. Sci. Sports Exerc. 2012, 44, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.J.; Vukovic, J.; Dunlop, S.; Grounds, M.D.; Shavlakadze, T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS ONE 2011, 6, e28090. [Google Scholar] [CrossRef]
- Drey, M.; Sieber, C.C.; Bauer, J.M.; Uter, W.; Dahinden, P.; Fariello, R.G.; Vrijbloed, J.W.; Zech, A.; Freiberger, E.; Pfeifer, K.; et al. C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp. Gerontol. 2013, 48, 76–80. [Google Scholar] [CrossRef]
- Ikemoto-Uezumi, M.; Zhou, H.; Kurosawa, T.; Yoshimoto, Y.; Toyoda, M.; Kanazawa, N.; Nakazawa, T.; Morita, M.; Tsuchida, K.; Uezumi, A. Increased MFG-E8 at neuromuscular junctions is an exacerbating factor for sarcopenia-associated denervation. Aging Cell 2022, 21, e13536. [Google Scholar] [CrossRef]
- Kung, T.A.; Cederna, P.S.; van der Meulen, J.H.; Urbanchek, M.G.; Kuzon, W.M.; Faulkner, J.A. Motor unit changes seen with skeletal muscle sarcopenia in oldest old rats. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 657–665. [Google Scholar] [CrossRef][Green Version]
- Liu, J.X.; Pedrosa Domellöf, F. Complex Correlations Between Desmin Content, Myofiber Types, and Innervation Patterns in the Human Extraocular Muscles. Investig. Ophthalmol. Vis. Sci. 2020, 61, 15. [Google Scholar] [CrossRef] [PubMed]
- Blumer, R.; Carrero-Rojas, G.; Calvo, P.M.; Streicher, J.; de la Cruz, R.R.; Pastor, A.M. Proprioceptors in extraocular muscles. Exp. Physiol. 2024, 109, 17–26. [Google Scholar] [CrossRef]
- Neuhuber, W.L.; Eichhorn, U.; Wörl, J. Enteric co-innervation of striated muscle fibers in the esophagus: Just a “hangover”. Anat. Rec. 2001, 262, 41–46. [Google Scholar] [CrossRef]
- Hisa, Y.; Tadaki, N.; Uno, T.; Koike, S.; Tanaka, M.; Okamura, H.; Ibata, Y. Nitrergic innervation of the rat larynx measured by nitric oxide synthase immunohistochemistry and NADPH-diaphorase histochemistry. Ann. Otol. Rhinol. Laryngol. 1996, 105, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Tracicaru, R.V.; Bräuer, L.; Döllinger, M.; Schicht, M.; Tillmann, B.; Hînganu, D.; Hristian, L.; Hînganu, M.V.; Paulsen, F. Morphological Evidence for a Unique Neuromuscular Functional Unit of the Human Vocalis Muscle. Int. J. Mol. Sci. 2024, 25, 11916. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Sanders, I. Human tongue neuroanatomy: Nerve supply and motor endplates. Clin. Anat. 2010, 23, 777–791. [Google Scholar] [CrossRef]
- Hardy, E.J.O.; Inns, T.B.; Hatt, J.; Doleman, B.; Bass, J.J.; Atherton, P.J.; Lund, J.N.; Phillips, B.E. The time course of disuse muscle atrophy of the lower limb in health and disease. J. Cachexia Sarcopenia Muscle 2022, 13, 2616–2629. [Google Scholar] [CrossRef] [PubMed]
- Magnus, C.R.; Barss, T.S.; Lanovaz, J.L.; Farthing, J.P. Effects of cross-education on the muscle after a period of unilateral limb immobilization using a shoulder sling and swathe. J. Appl. Physiol. 2010, 109, 1887–1894. [Google Scholar] [CrossRef]
- Phillips, S.M.; McGlory, C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J. Physiol. 2014, 592, 5341–5343. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Hardy, E.J.O.; Inns, T.B.; Wilkinson, D.J.; Piasecki, M.; Morris, R.H.; Spicer, A.; Sale, C.; Smith, K.; Atherton, P.J.; et al. Atrophy Resistant vs. Atrophy Susceptible Skeletal Muscles: “aRaS” as a Novel Experimental Paradigm to Study the Mechanisms of Human Disuse Atrophy. Front. Physiol. 2021, 12, 653060. [Google Scholar] [CrossRef]
- Dalton, B.H.; McNeil, C.J.; Doherty, T.J.; Rice, C.L. Age-related reductions in the estimated numbers of motor units are minimal in the human soleus. Muscle Nerve 2008, 38, 1108–1115. [Google Scholar] [CrossRef]
- Bordoni, B.; Morabito, B.; Simonelli, M. Ageing of the Diaphragm Muscle. Cureus 2020, 12, e6645. [Google Scholar] [CrossRef]
- Gamboa, J.L.; Andrade, F.H. Mitochondrial content and distribution changes specific to mouse diaphragm after chronic normobaric hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R575–R583. [Google Scholar] [CrossRef]
- Gamboa, J.L.; Andrade, F.H. Muscle endurance and mitochondrial function after chronic normobaric hypoxia: Contrast of respiratory and limb muscles. Pflug. Arch. 2012, 463, 327–338. [Google Scholar] [CrossRef]
- McMorrow, C.; Fredsted, A.; Carberry, J.; O’Connell, R.A.; Bradford, A.; Jones, J.F.; O’Halloran, K.D. Chronic hypoxia increases rat diaphragm muscle endurance and sodium-potassium ATPase pump content. Eur. Respir. J. 2011, 37, 1474–1481. [Google Scholar] [CrossRef]
- Monemi, M.; Eriksson, P.O.; Kadi, F.; Butler-Browne, G.S.; Thornell, L.E. Opposite changes in myosin heavy chain composition of human masseter and biceps brachii muscles during aging. J. Muscle Res. Cell Motil. 1999, 20, 351–361. [Google Scholar] [CrossRef]
- Daboul, A.; Schwahn, C.; Bülow, R.; Kiliaridis, S.; Kocher, T.; Klinke, T.; Mundt, T.; Mourad, S.; Völzke, H.; Habes, M.; et al. Influence of Age and Tooth Loss on Masticatory Muscles Characteristics: A Population Based MR Imaging Study. J. Nutr. Health Aging 2018, 22, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Kawata, S.; Seki, S.; Nishiura, A.; Kitaoka, Y.; Iwamori, K.; Fukada, S.I.; Kogo, M.; Tanaka, S. Preservation of masseter muscle until the end stage in the SOD1G93A mouse model for ALS. Sci. Rep. 2024, 14, 24279. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hoshino, T.; Ogawa, Y.; Sugahara, K.; Katakura, A. Aging-Related Metabolome Analysis of the Masseter Muscle in Senescence-Accelerated Mouse-Prone 8. Int. J. Mol. Sci. 2024, 25, 9684. [Google Scholar] [CrossRef] [PubMed]
- Agten, A.; Stevens, S.; Verbrugghe, J.; Eijnde, B.O.; Timmermans, A.; Vandenabeele, F. The lumbar multifidus is characterised by larger type I muscle fibres compared to the erector spinae. Anat. Cell Biol. 2020, 53, 143–150. [Google Scholar] [CrossRef]
- Agha, O.; Mueller-Immergluck, A.; Liu, M.; Zhang, H.; Theologis, A.A.; Clark, A.; Kim, H.T.; Liu, X.; Feeley, B.T.; Bailey, J.F. Intervertebral disc herniation effects on multifidus muscle composition and resident stem cell populations. JOR Spine 2020, 3, e1091. [Google Scholar] [CrossRef]
- Moiseeva, V.; Cisneros, A.; Sica, V.; Deryagin, O.; Lai, Y.; Jung, S.; Andrés, E.; An, J.; Segalés, J.; Ortet, L.; et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 2023, 613, 169–178. [Google Scholar] [CrossRef]
- Dowling, P.; Gargan, S.; Zweyer, M.; Henry, M.; Meleady, P.; Swandulla, D.; Ohlendieck, K. Proteomic reference map for sarcopenia research: Mass spectrometric identification of key muscle proteins of organelles, cellular signaling, bioenergetic metabolism and molecular chaperoning. Eur. J. Transl. Myol. 2024, 34, 12565. [Google Scholar] [CrossRef]
| Muscle Group | Muscle | Atrophy Rate (%/Year) (Human) [2] | Fiber Type Composition (I/II%) Type I/Type II% (Human) [2] | Inactivity (%Atrophy Rate) (Human) [134,135] |
|---|---|---|---|---|
| Elbow extensors | Triceps brachii | 0.38 * | 36/64 | 5.2 ± 2.7—28 d |
| Biceps brachii | 0.38 * | 46/54 | 2.8 ± 1.1—28 d | |
| Brachioradialis | 0.38 * | 44/56 | n/a | |
| Paraspinals | Erector spinae | 0.47 * | 61/39 | n/a |
| Lumbar multifidus | 0.47 * | 59/41 | n/a | |
| Psoas | Psoas major/Iliopsoas | 0.58 | 45/55 | 2.78—14 d 4.86—28 d 2.78—42 d |
| Hip adductors | Adductor magnus | 0.27 * | 55/45 | |
| Hamstrings | Biceps femoris short and long heads | short head 0.23 long head 0.28 | 54/46 | 1.7–2.38—3 d * 4.0–5.9—7 d * 9.3—10 d * 9.3—20 d * |
| Semimembranosus | 0.40 | 49/51 | ||
| Semitendinosus | 0.39 | 48/52 | ||
| Quadriceps | Rectus femoris | 0.66 | 38/62 | 3.5–4.1—14 d 5.1–7.4—56 d |
| Vastus lateralis | 0.59 | 46/54 | 4.7–6.7—14 d * 5.6–15.9—56 d * | |
| Vastus intermedius | 0.49 | 51/49 | ||
| Vastus medialis | 0.48 | 53/47 | ||
| Dorsiflexors | Tibialis anterior | 0.19 * | 74/26 | 0.7–9.2—14 d 0.8–7.7—28 d |
| Triceps surae | Gastrocnemius | 0.41 | 59/41 | Lateral: 2.4–7.7—14 d 14.4–16.5—56 d Medial: 3.0–9.4—14 d 20.4–22.3—56 d |
| Soleus | 0.13 | 81/19 | 3.0–9.4—14 d 20.4–22.3—56 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titova, A.; Bilyalov, A.; Filatov, N.; Perepechenov, S.; Kupriyanova, D.; Brovkin, S.; Shestakov, D.; Bodunova, N.; Gusev, O. Muscle Aging Heterogeneity: Genetic and Structural Basis of Sarcopenia Resistance. Genes 2025, 16, 948. https://doi.org/10.3390/genes16080948
Titova A, Bilyalov A, Filatov N, Perepechenov S, Kupriyanova D, Brovkin S, Shestakov D, Bodunova N, Gusev O. Muscle Aging Heterogeneity: Genetic and Structural Basis of Sarcopenia Resistance. Genes. 2025; 16(8):948. https://doi.org/10.3390/genes16080948
Chicago/Turabian StyleTitova, Angelina, Airat Bilyalov, Nikita Filatov, Stepan Perepechenov, Darya Kupriyanova, Sergei Brovkin, Dmitrii Shestakov, Natalia Bodunova, and Oleg Gusev. 2025. "Muscle Aging Heterogeneity: Genetic and Structural Basis of Sarcopenia Resistance" Genes 16, no. 8: 948. https://doi.org/10.3390/genes16080948
APA StyleTitova, A., Bilyalov, A., Filatov, N., Perepechenov, S., Kupriyanova, D., Brovkin, S., Shestakov, D., Bodunova, N., & Gusev, O. (2025). Muscle Aging Heterogeneity: Genetic and Structural Basis of Sarcopenia Resistance. Genes, 16(8), 948. https://doi.org/10.3390/genes16080948







