Abstract
Hereditary ataxias are a highly heterogenous group of diseases characterized by loss of coordination. In this study, we investigated a family of random-bred dogs, in which two siblings were affected by a slowly progressive ataxia. They presented with clinical signs of progressive cerebellar ataxia, hypermetria, and absent menace response. The MRI revealed generalized brain atrophy, reduced cortical demarcation, hypoplastic corpus callosum, and cerebellar folia thinning, highly suggestive of a neurodegenerative disorder. We sequenced the genomes of the two affected dogs and their unaffected parents. Filtering for protein-changing variants that had homozygous alternate genotypes in the affected dogs, heterozygous genotypes in the parents, and homozygous reference genotypes in 1576 control genomes yielded a single missense variant in the RAB24 gene, XM_038534663.1:c.239G>T or XP_038390591.1:p.(Gly80Val). Genotypes at this variant showed the expected co-segregation with the ataxia phenotype in the investigated family. The predicted amino acid affects the conserved RabF4 motif. Glycine-80 resides at the protein surface and the introduction of a hydrophobic isopropyl side chain of the mutant valine might impede solvent accessibility. Another missense variant in RAB24, p.Glu38Pro, was previously reported to cause a clinically similar form of cerebellar ataxia in Gordon Setters and Old English Sheepdogs. Taken together, the available data suggest that RAB24:p.Gly80Val represents the causal variant in the studied dogs. To the best of our knowledge, this is only the second report of a potentially pathogenic RAB24 variant in any species and further supports that RAB24 should be considered a candidate gene in human ataxia patients with unclear molecular etiology.
1. Introduction
Canine hereditary ataxias are a heterogeneous group of neurological diseases characterized by cerebellar or spinocerebellar dysfunction and resulting in uncoordinated movement as their hallmark [1]. Variants in more than 35 genes have been associated with canine ataxia. Based on the neuropathological alterations, five different subgroups have been proposed [1], which comprise cerebellar cortical degenerations [2,3,4,5,6], primary granule cell degenerations, spinocerebellar degenerations [7,8,9,10,11,12], cerebellar ataxia without substantial neurodegeneration [13,14,15], and multifocal degenerations with a predominant (spino)cerebellar component [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. The ataxia may represent the only clinical sign or may be part of a syndromic disease. While most canine hereditary ataxias represent neurodegenerative disorders due to defects in the genes required for the metabolism and homeostasis of cerebellar neurons, ataxia can also result from impaired brain development [37]. The developmental ataxias may be characterized by clinical phenotypes that remain stable or even show improvements over time [37].
Due to the enormous heterogeneity of inherited ataxias, it is normally not possible to reach a precise diagnosis based on clinical signs alone. To aid in the diagnosis, advanced diagnostic imaging, metabolic analyses, pathological examinations, and/or genetic investigations are required.
The present study was prompted by reports of two dog littermates presenting with progressive loss of coordination and ataxia. The aim of our study was to characterize the clinical phenotype together with an investigation of the underlying causative genetic variant.
2. Materials and Methods
2.1. Ethics Statement
All of the dogs in this study were privately owned. Blood samples were collected with the consent of the owners. The diagnostic workup of the affected dogs was performed in the context of veterinary care and did not constitute an animal experiment in the legal sense. The collection of blood samples from control animals was approved by the Cantonal Committee for Animal Experiments (Canton of Bern; permit BE94/2022). All animal experiments were performed in accordance with local laws and regulations.
2.2. Index Family
The index family consisted of five dogs, two unaffected parents and three male offspring, of which two were affected by ataxia. The sire was reported as an American Bulldog and the dam as an Old English Bulldog, both without official pedigree papers. Samples and information on the five dogs were donated by their owners.
2.3. Clinical Examination and Diagnostic Imaging
The clinical and neurological examinations were performed by a veterinarian (neurologist in training, FD), and the MRI interpretation was performed by a board-certified specialist in diagnostic imaging (Dipl.-ECVDI, JW). The MRI images were taken using a Vet-MR Grande instrument with 0.25 Tesla field strength (Esaote Vet, Köln, Germany).
2.4. DNA Isolation and Whole-Genome Sequencing
Genomic DNA was isolated from the EDTA blood from all five dogs on a Maxwell RSC48 instrument using the Maxwell RSC Whole Blood DNA Kit (Promega, Dübendorf, Switzerland). PCR-free libraries with ~400 bp insert size were prepared from genomic DNA of the two affected dogs and their parents. The libraries were sequenced with 2 × 150 bp paired-end chemistry at 22×–27× coverage on an Illumina NovaSeq 6000 instrument (Illumina, San Diego, CA, USA). The raw reads were mapped to the UU_Cfam_GSD_1.0/CanFam4 genome reference assembly (GCF_011100685.1). Subsequently, single-nucleotide variants and small indels were indicated as described before [38]. The sequence data were deposited in the European Nucleotide Archive, and the accession numbers are listed in Table S1. The functional effects of the called variants were predicted with the SnpEff version 4.3t software [39], together with NCBI annotation release 106 for the UU_Cfam_GSD_1.0 genome reference assembly to produce an annotated vcf-file.
2.5. Variant Filtering
For the identification of candidate causal variants, we used a hard filtering approach on the annotated vcf-file with the following conditions: (1) genotypes in the two affected dogs had to be homozygous alternate (1/1), genotypes in the parents had to be heterozygous (0/1), and genotypes in 1576 control genomes from genetically diverse dogs had to be homozygous for the reference genotype (0/0). For the control genomes, we additionally allowed missing genotypes (./.). Accession numbers of the control genomes are listed in Table S1. In a second step, protein-changing variants were prioritized. We considered variants with a SnpEff predicted impact of “high” or “moderate” as protein-changing.
2.6. Targeted Genotyping
The RAB24:XM_038534663.1:c.239G>T candidate variant was independently confirmed and genotyped by direct Sanger sequencing of the PCR amplicons. Primers 5′-CCGGACGGTGACTTTAGGTA-3′ and 5′-CACGCAGAGAACAACCAAGA-3′ were used for the generation of a 393 bp amplicon containing the variant. PCR products were amplified from genomic DNA using AmpliTaq Gold 360 Master Mix (Thermo Fisher Scientific, Reinach, Switzerland). Direct Sanger sequencing of the PCR amplicons on an ABI 3730 DNA Analyzer (Thermo Fisher Scientific) was performed after treatment with exonuclease I and alkaline phosphatase. Sanger sequences were analyzed using the Sequencher 5.1 software (Gene Codes, Ann Arbor, MI, USA).
2.7. In Silico Pathogenicity Prediction and Structural Modeling
The online classification tools Polyphen-2 [40], PredictSNP [41], and MutPred2 [42] were utilized to predict the potential functional impact of the XP_038390591.1:p.Gly80Val missense variant. The Mol*3D viewer [43] from the RCSB Protein Data Bank was used to visualize the protein structure of the canine RAB24 protein (XP_038390591.1) as modeled with ColabFold [44].
3. Results
3.1. Clinical Phenotype
Two 5-month-old male random-bred (American Bulldog x Old English Bulldog) siblings presented with similar contemporaneous clinical signs of progressive gait abnormalities. Only case #1 was clinically examined in detail. The general clinical examination was within normal limits. The neurological examination showed normal consciousness and behavior. An examination of the cranial nerves identified an absent menace response bilaterally. The gait was abnormal with cerebellar ataxia with hypermetria. Intention tremor was also evident. Proprioception and spinal reflexes were within normal limits. Neuroanatomical localization was to the cerebellum, with differential diagnoses including degenerative, inflammatory, and hereditary conditions. Owner-provided videos of both cases illustrate the clinical phenotype (Video S1).
The brain MRI of case #1 revealed generalized brain atrophy, reduced cortical demarcation, hypoplastic corpus callosum, and cerebellar folia thinning (Figure 1). These findings were primarily consistent with a neurodegenerative disorder, potentially a storage disease, with secondary generalized atrophy of the cerebrum and cerebellum. Inflammatory/infectious or metabolic disorders were judged to be less likely.
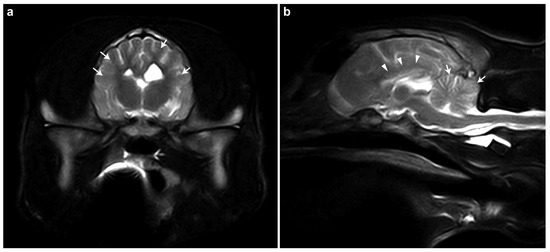
Figure 1.
MRI findings in case #1. (a) T2-weighted transverse MRI image of the brain. Generalized reduction in the demarcation between the cortical gray matter and the subcortical white matter within the region of the corona radiata can be seen (white arrows). (b) T2-weighted sagittal MRI image of the brain. The corpus callosum is markedly thinned and poorly delineated (white arrowheads), suggesting hypoplasia. Additionally, the cerebellar folia are diffusely attenuated (white arrows), indicating cerebellar atrophy or hypoplasia.
The complete blood test of case #1 was unremarkable. Cerebrospinal fluid (CSF) analysis revealed a cell-poor fluid (3 cells/µL, reference < 15) with no cytological evidence of inflammation, infection, or neoplastic cells. The biochemical parameters showed CSF total protein at 12 mg/dL (reference < 60 mg/dL), glucose at 80 mg/dL (reference 50−75 mg/dL), and lactate at 12.4 mg/dL (reference 11.0−19.0 mg/dL). Further specific tests for IgA, CrP, and TBE-Ab (IgG) in CSF, along with PCR for distemper, Anaplasma phagocytophilum, Neospora caninum, and Toxoplasma gondii, were all negative, supporting a non-inflammatory and non-infectious etiology for the neurological signs observed.
3.2. Genetic Analysis
We sequenced the genomes of both of the affected dogs and their parents and compared the sequence data to 1576 genetically diverse control genomes in a search for plausible causative variants. Assuming recessive inheritance, we searched for variants that had homozygous mutant (alternate) genotypes in the two affected dogs, heterozygous genotypes in both parents, and homozygous wildtype (reference) genotypes in the controls. A total of 13 variants in the entire genome fulfilled these search criteria, and only one of them was predicted to be protein-changing (Table 1 and Table S2).

Table 1.
Overview of variant filtering results. The genomes of the two affected littermates and their parents were analyzed together with 1576 control genomes. Private variants have the genotype 1/1 in case #1 or case #2 and the genotype 0/0 or ./. in the 1576 control genomes.
The identified single-candidate variant was located on chromosome 4, NC_049225.1:g.36,943,081G>T. It is a missense variant in the RAB24 gene, XM_038534663.1:c.239G>T, predicted to change a glycine into a valine residue at the protein level, XP_038390591.1:p.(Gly80Val). The variant and correct co-segregation of the genotypes in the family were verified by Sanger sequencing. Both of the affected dogs were homozygous mutant, their parents were heterozygous, and the unaffected littermate was homozygous for the wildtype allele (Figure 2).
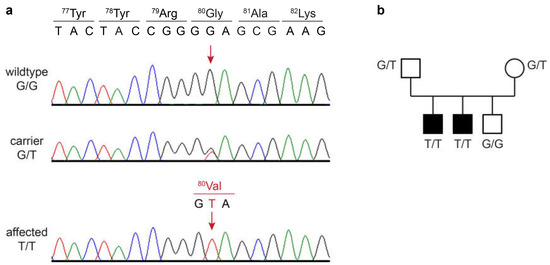
Figure 2.
Details of the RAB24:c.239G>T variant. (a) Sanger electropherograms of a wildtype dog, a heterozygous carrier, and an affected dog. The position of the variant is shown with red arrows. The reading frame and amino acid translations of the wildtype and mutant sequence are indicated. (b) Genotype–phenotype co-segregation in the family. Affected dogs are represented with filled symbols. Genotypes at the RAB24:c.239G>T variant are indicated.
The predicted Gly80Val amino acid substitution affects the RabF4 motif [45]. This is a highly conserved YYRGA sequence motif at the surface of the RAB24 protein (Figure 3). The mutant valine introduces a hydrophobic isopropyl side chain into the surrounding solvent, which might interfere with the normal contacts to water molecules.
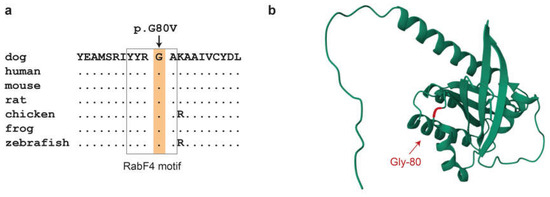
Figure 3.
In silico analyses of the RAB24:p.Gly80Val variant. (a) Evolutionary conservation. The glycine at position 80 is strictly conserved in all vertebrates. Gly-80 is part of the RabF4 motif that is a conserved feature distinguishing Rab family members from other members of the Ras superfamily [45]. Accession numbers of the sequences used for the alignment are dog, XP_038390591.1; human, NP_001026847.1; mouse, NP_033026.1; rat, NP_001015023.2; chicken, NP_001264642.1; Western clawed frog, NP_001016171.1; zebrafish, NP_001025141.1. (b) Three-dimensional structure model of the canine RAB24 protein. Gly-80 is in a solvent exposed loop connecting the α2 helix with the β4 sheet.
In silico pathogenicity prediction tools classified the p.Gly80Val variant as potentially deleterious. MutPred2 gave a score of 0.890, which is above the 5% pathogenicity threshold of 0.8. PredictSNP classified the p.Gly80Val variant as deleterious with 87% probability. Polyphen-2 classified the variant as probably damaging with a score of 0.982.
4. Discussion
In this study, we investigated two dog siblings affected by hereditary ataxia. Using a powerful quartet whole-genome sequencing approach, we identified RAB24:p.Gly80Val as the candidate causal variant for this phenotype.
The constellation of clinical signs in young dogs (less than a year of age), particularly the cerebellar ataxia, hypermetria, intention tremor, and absent menace response, strongly pointed towards a cerebellar involvement. The MRI findings of generalized mild brain atrophy, reduced cortical demarcation, hypoplastic corpus callosum, thinning of cerebral gyri, and prominent sulci were highly suspicious of a metabolic or degenerative encephalopathy. A lysosomal storage disease with secondary generalized atrophy of both the cerebrum and cerebellum was suspected. Atrophy of the corpus callosum has been reported in animals with neuronal ceroid lipofuscinosis (NCL) and gangliosidosis [45], although it is not specific to these conditions and can also occur in other conditions [46].
The CSF analysis was overall unremarkable and suggestive of a non-inflammatory/infectious process. The mildly elevated glucose in CSF could be a minor variation or non-specific finding given the lack of other inflammatory markers.
Based on the clinical and MRI findings, a neurodegenerative process was the main differential diagnosis. Histopathology, typically the next step in confirming a diagnosis of neurodegenerative disorder, could not be performed as both dogs were still alive. According to the owners, the affected dogs had a good quality of life, albeit being coordinatively impaired. This underscores the importance of genetic testing in living patients for the investigation of neurodegenerative disorders. Based on the findings in Gordon Setters and Old English Sheepdogs with RAB24-related ataxia that have been studied for more than 40 years [4,47,48,49,50,51], the most likely course of disease in the affected dogs from this study will be slowly progressive.
The function of the RAB24 protein has only partially been clarified. It is highly conserved in all vertebrates and comprises 213 amino acids in dogs and humans, of which 211 are identical. RAB24 belongs to the Rab family within the Ras superfamily of small GTPases [52,53]. While most Ras proteins have a GTPase activity, RAB24 is considered an atypical Rab GTPase, as it lacks a conserved glutamine in the catalytic domain and therefore has only weak or absent GTPase activity and is mostly found in a GTP-bound state [53]. RAB24 is involved in intracellular transport and autophagy [54,55]. A more recent study reported a function in mitochondrial dynamics in the liver [56]. To the best of our knowledge, ataxic Gordon Setters and Old English Sheepdogs, homozygous for the mutant allele at RAB24:p.Gln38Pro, represent the only spontaneous RAB24 mutant with a well-defined clinical phenotype [4]. So far, no human patients with RAB24 loss of function variants have been reported.
The RAB24:p.Gly80Val variant detected in this study affects a highly conserved RabF4 sequence motif (YYRGA) [52]. The functional impact of this variant is predicted as deleterious or damaging by three different software tools. Based on three-dimensional structure modeling, it is conceivable that the introduction of the hydrophobic valine at the protein surface causes alterations in water solubility that might affect RAB24 function.
For a formal assessment of pathogenicity, we applied the ACMG/AMP guidelines for human variants [57]. We considered three criteria, one strong and two supporting, as evidence for pathogenicity: The homozygous mutant genotype was only seen in the two affected dogs, whereas 1576 genetically diverse dogs did not carry the mutant allele (PS4). The genotypes at RAB24:p.Gly80Val showed the expected co-segregation with disease in the family (PP1), and multiple lines of computational evidence supported pathogenicity (PP3). With these criteria, the variant would be classified as likely pathogenic.
While RAB24:p.Gly80Val represents a highly plausible candidate causal variant, we have to caution that our analyses had some limitations. We did not perform any functional confirmation experiments to prove that the mutant RAB24 is inactive. Furthermore, we relied on short-read sequencing data and only investigated small variants. We did not consider large structural variants. Gaps in the genome reference assembly and/or incorrect genome annotation might also have caused us to miss the true causal variant. Therefore, our assessment of RAB24:p.Gly80Val being likely causal should be considered preliminary and requires further confirmation before it can be taken as definitively proven. Functional studies on the effect of the p.Gly80Val substitution on the RAB24 protein are required to clarify the exact molecular pathogenesis. However, while we acknowledge the limitations of our study, the remarkable similarity in clinical phenotype between RAB24 mutant ataxic Gordon Setters and Old English Sheepdogs [4] with the dogs investigated in our study strongly supports RAB24:p.Gly80Val as a candidate causal variant.
We found the mutant RAB24 allele only in the index family consisting of non-pedigree dogs with owner-reported American Bulldog and Old English Bulldog origin, respectively. Further research is warranted to determine whether the mutant allele exclusively occurs in non-pedigree dogs or whether it is also segregating in purebred dogs.
5. Conclusions
We characterized the clinical phenotype of two dog littermates with an autosomal recessive hereditary ataxia. The genetic investigation identified RAB24:c.239G>T, a single-nucleotide variant resulting in the p.Gly80Val missense change as a most likely causative defect. To the best of our knowledge, this is only the second RAB24 mutant with a neurological phenotype reported in any species. Therefore, our findings corroborate earlier findings on the previously reported other canine RAB24 mutant. RAB24 should be considered a candidate gene for unexplained forms of hereditary ataxia in human patients. Knowledge of the canine RAB24 variant enables genetic testing to avoid the unintentional mating of carriers and production of further affected dogs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes16080934/s1, Table S1: Accession numbers of 1580 dog genome sequences; Table S2: Private protein-changing variants in the two affected dogs; Video S1: Clinical presentation of the two affected dogs.
Author Contributions
Conceptualization, T.L.; data curation, V.J.; investigation, C.S., J.W., F.D. and T.L.; writing—original draft, C.S., J.W., J.G. and T.L.; writing—review and editing, C.S., J.W., J.G., F.D., V.J. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The diagnostic examinations of the affected dogs were conducted during the clinical workup; this did not constitute an experimental study, and therefore it did not require official or institutional ethical approval. The collection of blood samples from unaffected dogs was approved by the Cantonal Committee for Animal Experiments (Canton of Bern; permit BE94/2022).
Informed Consent Statement
Written consent for the use of samples and data for research purposes was obtained from the owners of the dogs in this study.
Data Availability Statement
The accession numbers of the sequence data that are reported in this study are listed in Table S1.
Acknowledgments
We would like to thank the dog owners for donating samples and information. Isabella Aebi and Carmen Rodriguez are acknowledged for their expert technical assistance. We thank the Next-Generation Sequencing Platform for performing the WGS experiments and the Interfaculty Bioinformatics for providing high-performance computing infrastructure. We acknowledge the DBVDC consortium, the Dog10K genomes project, and all researchers who deposited dog or wolf whole-genome sequencing data into public databases.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stee, K.; Van Poucke, M.; Lowrie, M.; Van Ham, L.; Peelman, L.; Olby, N.; Bhatti, S.F.M. Phenotypic and genetic aspects of hereditary ataxia in dogs. J. Vet. Intern. Med. 2023, 37, 1306–1322. [Google Scholar] [CrossRef]
- Forman, O.P.; De Risio, L.; Stewart, J.; Mellersh, C.S.; Beltran, E. Genome-wide mRNA sequencing of a single canine cerebellar cortical degeneration case leads to the identification of a disease associated SPTBN2 mutation. BMC Genet. 2012, 13, 55. [Google Scholar] [CrossRef]
- Kyöstilä, K.; Cizinauskas, S.; Seppälä, E.H.; Suhonen, E.; Jeserevics, J.; Sukura, A.; Syrjä, P.; Lohi, H. A SEL1L mutation links a canine progressive early-onset cerebellar ataxia to the endoplasmic reticulum-associated protein degradation (ERAD) machinery. PLoS Genet. 2012, 8, e1002759. [Google Scholar] [CrossRef] [PubMed]
- Agler, C.; Nielsen, D.M.; Urkasemsin, G.; Singleton, A.; Tonomura, N.; Sigurdsson, S.; Tang, R.; Linder, K.; Arepalli, S.; Hernandez, D.; et al. Canine hereditary ataxia in Old English Sheepdogs and Gordon Setters is associated with a defect in the autophagy gene encoding RAB24. PLoS Genet. 2014, 10, e1003991. [Google Scholar] [CrossRef]
- Fenn, J.; Boursnell, M.; Hitti, R.J.; Jenkins, C.A.; Terry, R.L.; Priestnall, S.L.; Kenny, P.J.; Mellersh, C.S.; Forman, O.P. Genome sequencing reveals a splice donor site mutation in the SNX14 gene associated with a novel cerebellar cortical degeneration in the Hungarian Vizsla dog breed. BMC Genet. 2016, 17, 123. [Google Scholar] [CrossRef]
- Christen, M.; Zdora, I.; Leschnik, M.; Jagannathan, V.; Puff, C.; Hünerfauth, E.; Volk, H.A.; Baumgärtner, W.; Koch, T.C.; Schäfer, W.; et al. RALGAPA1 deletion in Belgian Shepherd Dogs with cerebellar ataxia. Genes 2023, 14, 1520. [Google Scholar] [CrossRef]
- Forman, O.P.; De Risio, L.; Mellersh, C.S. Missense mutation in CAPN1 is associated with spinocerebellar ataxia in the Parson Russell Terrier dog breed. PLoS ONE 2013, 8, e64627. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, D.; O’Brien, D.P.; Coates, J.R.; Johnson, G.S.; Johnson, G.C.; Mhlanga-Mutangadura, T.; Hansen, L.; Taylor, J.F.; Schnabel, R.D. A homozygous KCNJ10 mutation in Jack Russell Terriers and related breeds with spinocerebellar ataxia with myokymia, seizures, or both. J. Vet. Intern. Med. 2014, 28, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Mauri, N.; Kleiter, M.; Leschnik, M.; Högler, S.; Dietschi, E.; Wiedmer, M.; Dietrich, J.; Henke, D.; Steffen, F.; Schuller, S.; et al. A missense variant in KCNJ10 in Belgian Shepherd Dogs affected by spongy degeneration with cerebellar ataxia (SDCA1). G3 Genes Genomes Genet. 2017, 7, 663–669. [Google Scholar] [CrossRef]
- Van Poucke, M.; Stee, K.; Bhatti, S.F.M.; Vanhaesebrouck, A.; Bosseler, L.; Peelman, L.J.; Van Ham, L. The novel homozygous KCNJ10 c.986T>C (p.(Leu329Pro)) variant is pathogenic for the SeSAME/EAST homologue in Malinois dogs. Eur. J. Hum. Genet. 2017, 25, 222–226. [Google Scholar] [CrossRef][Green Version]
- Van Poucke, M.; Stee, K.; Sonck, L.; Stock, E.; Bosseler, L.; Van Dorpe, J.; Van Nieuwerburgh, F.; Deforce, D.; Peelman, L.J.; Van Ham, L.; et al. Truncating SLC12A6 variants cause different clinical phenotypes in humans and dogs. Eur. J. Hum. Genet. 2019, 27, 1561–1568. [Google Scholar] [CrossRef]
- Letko, A.; Dietschi, E.; Nieburg, M.; Jagannathan, V.; Gurtner, C.; Oevermann, A.; Drögemüller, C. A missense variant in SCN8A in Alpine Dachsbracke Dogs affected by spinocerebellar ataxia. Genes 2019, 10, 362. [Google Scholar] [CrossRef]
- Zeng, R.; Farias, F.H.; Johnson, G.S.; McKay, S.D.; Schnabel, R.D.; Decker, J.E.; Taylor, J.F.; Mann, C.S.; Katz, M.L.; Johnson, G.C.; et al. A truncated retrotransposon disrupts the GRM1 coding sequence in Coton de Tulear dogs with Bandera’s neonatal ataxia. J. Vet. Intern. Med. 2011, 25, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Forman, O.P.; De Risio, L.; Matiasek, K.; Platt, S.; Mellersh, C. Spinocerebellar ataxia in the Italian Spinone dog is associated with an intronic GAA repeat expansion in ITPR1. Mamm. Genome 2015, 26, 108–117. [Google Scholar] [CrossRef][Green Version]
- Jenkins, C.A.; Kalmar, L.; Matiasek, K.; Mari, L.; Kyöstilä, K.; Lohi, H.; Schofield, E.C.; Mellersh, C.S.; De Risio, L.; Ricketts, S.L. Characterisation of canine KCNIP4: A novel gene for cerebellar ataxia identified by whole-genome sequencing two affected Norwegian Buhund dogs. PLoS Genet. 2020, 16, e1008527. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zeng, B.; Shibuya, H.; Johnson, G.S.; Alroy, J.; Pastores, G.M.; Raghavan, S.; Kolodny, E.H. Isolation and characterization of the normal canine beta-galactosidase gene and its mutation in a dog model of GM1-gangliosidosis. J. Inherit. Metab. Dis. 2000, 23, 593–606. [Google Scholar] [CrossRef]
- Kreutzer, R.; Leeb, T.; Müller, G.; Moritz, A.; Baumgärtner, W. A duplication in the canine beta-galactosidase gene GLB1 causes exon skipping and GM1-gangliosidosis in Alaskan huskies. Genetics 2005, 170, 1857–1861. [Google Scholar] [CrossRef]
- Melville, S.A.; Wilson, C.L.; Chiang, C.S.; Studdert, V.P.; Lingaas, F.; Wilton, A.N. A mutation in canine CLN5 causes neuronal ceroid lipofuscinosis in Border collie dogs. Genomics 2005, 86, 287–294. [Google Scholar] [CrossRef]
- Katz, M.L.; Khan, S.; Awano, T.; Shahid, S.A.; Siakotos, A.N.; Johnson, G.S. A mutation in the CLN8 gene in English Setter dogs with neuronal ceroid-lipofuscinosis. Biochem. Biophys. Res. Commun. 2005, 327, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Awano, T.; Katz, M.L.; O’Brien, D.P.; Taylor, J.F.; Evans, J.; Khan, S.; Sohar, I.; Lobel, P.; Johnson, G.S. A mutation in the cathepsin D gene (CTSD) in American Bulldogs with neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2006, 87, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Awano, T.; Katz, M.L.; O’Brien, D.P.; Sohar, I.; Lobel, P.; Coates, J.R.; Khan, S.; Johnson, G.C.; Giger, U.; Johnson, G.S. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2006, 89, 254–260. [Google Scholar] [CrossRef]
- Abitbol, M.; Thibaud, J.L.; Olby, N.J.; Hitte, C.; Puech, J.P.; Maurer, M.; Pilot-Storck, F.; Hédan, B.; Dréano, S.; Brahimi, S.; et al. A canine arylsulfatase G (ARSG) mutation leading to a sulfatase deficiency is associated with neuronal ceroid lipofuscinosis. Proc. Natl. Acad. Sci. USA 2010, 107, 14775–14780. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Guo, J.; Bullock, G.; Johnson, G.S.; Katz, M.L. Canine multiple system degeneration associated with sequence variants in SERAC1. Genes 2024, 15, 1378. [Google Scholar] [CrossRef]
- Katz, M.L.; Farias, F.H.; Sanders, D.N.; Zeng, R.; Khan, S.; Johnson, G.S.; O’Brien, D.P. A missense mutation in canine CLN6 in an Australian shepherd with neuronal ceroid lipofuscinosis. J. Biomed. Biotechnol. 2011, 2011, 198042. [Google Scholar] [CrossRef]
- Kyöstilä, K.; Syrjä, P.; Jagannathan, V.; Chandrasekar, G.; Jokinen, T.S.; Seppälä, E.H.; Becker, D.; Drögemüller, M.; Dietschi, E.; Drögemüller, C.; et al. A missense change in the ATG4D gene links aberrant autophagy to a neurodegenerative vacuolar storage disease. PLoS Genet. 2015, 11, e1005169. [Google Scholar] [CrossRef]
- Guo, J.; O’Brien, D.P.; Mhlanga-Mutangadura, T.; Olby, N.J.; Taylor, J.F.; Schnabel, R.D.; Katz, M.L.; Johnson, G.S. A rare homozygous MFSD8 single-base-pair deletion and frameshift in the whole genome sequence of a Chinese Crested dog with neuronal ceroid lipofuscinosis. BMC Vet. Res. 2015, 10, 960. [Google Scholar] [CrossRef]
- Wiedmer, M.; Oevermann, A.; Borer-Germann, S.E.; Gorgas, D.; Shelton, G.D.; Drögemüller, M.; Jagannathan, V.; Henke, D.; Leeb, T. A RAB3GAP1 SINE insertion in Alaskan Huskies with polyneuropathy, ocular abnormalities, and neuronal vacuolation (POANV) resembling human Warburg Micro Syndrome 1 (WARBM1). Genes Genomes Genet. 2015, 6, 255–262. [Google Scholar] [CrossRef]
- Mauri, N.; Kleiter, M.; Dietschi, E.; Leschnik, M.; Högler, S.; Wiedmer, M.; Dietrich, J.; Henke, D.; Steffen, F.; Schuller, S.; et al. A SINE Insertion in ATP1B2 in Belgian Shepherd Dogs affected by spongy degeneration with cerebellar ataxia (SDCA2). G3 Genes Genomes Genet. 2017, 7, 2729–2737. [Google Scholar] [CrossRef]
- Lucot, K.L.; Dickinson, P.J.; Finno, C.J.; Mansour, T.A.; Letko, A.; Minor, K.M.; Mickelson, J.R.; Drögemüller, C.; Brown, C.T.; Bannasch, D.L. A missense mutation in the vacuolar protein sorting 11 (VPS11) gene is associated with neuroaxonal dystrophy in Rottweiler dogs. G3 Genes Genomes Genet. 2018, 8, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Högler, S.; Kleiter, M.; Leschnik, M.; Weber, C.; Thaller, D.; Jagannathan, V.; Leeb, T. Deletion of the SELENOP gene leads to CNS atrophy with cerebellar ataxia in dogs. PLoS Genet. 2021, 17, e1009716. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, M.; Jagannathan, V.; Laurent, N.; Noblet, E.; Dutil, G.F.; Troupel, T.; de Dufaure de Citres, C.; Gache, V.; Blot, S.; Escriou, C.; et al. A PNPLA8 frameshift variant in Australian shepherd dogs with hereditary ataxia. Anim. Genet. 2022, 53, 709–712. [Google Scholar] [CrossRef]
- Christen, M.; Rupp, S.; Van Soens, I.; Bhatti, S.F.M.; Matiasek, K.; von Klopmann, T.; Jagannathan, V.; Madden, I.; Batcher, K.; Bannasch, D.; et al. SLC25A12 missense variant in Nova Scotia Duck Tolling Retrievers affected by cerebellar degeneration-myositis complex (CDMC). Genes 2022, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Gutierrez-Quintana, R.; Vandenberghe, H.; Kaczmarska, A.; Penderis, J.; José-López, R.; Rupp, A.; Griffiths, I.R.; Jagannathan, V.; Leeb, T. Mitochondrial fission factor (MFF) frameshift variant in Bullmastiffs with mitochondrial fission encephalopathy. Anim. Genet. 2022, 53, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Gregor, A.; Gutierrez-Quintana, R.; Bongers, J.; Rupp, A.; Penderis, J.; Shelton, G.D.; Jagannathan, V.; Zweier, C.; Leeb, T. NDUFS7 variant in dogs with Leigh syndrome and its functional validation in a Drosophila melanogaster model. Sci. Rep. 2024, 14, 2975. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Oevermann, A.; Rupp, S.; Vaz, F.M.; Wever, E.J.M.; Braus, B.K.; Jagannathan, V.; Kehl, A.; Hytönen, M.K.; Lohi, H.; et al. PCYT2 deficiency in Saarlooswolfdogs with progressive retinal, central, and peripheral neurodegeneration. Mol. Genet. Metab. 2024, 141, 108149. [Google Scholar] [CrossRef]
- Cook, S.R.; Schwarz, C.; Guevar, J.; Assenmacher, C.A.; Sheehy, M.; Fanzone, N.; Church, M.E.; Murgiano, L.; Casal, M.L.; Jagannathan, V.; et al. Canine RNF170 single base deletion in a naturally occurring model for human neuroaxonal dystrophy. Mov. Disord. 2024, 39, 2049–2057. [Google Scholar] [CrossRef]
- Gerber, M.; Fischer, A.; Jagannathan, V.; Drögemüller, M.; Drögemüller, C.; Schmidt, M.J.; Bernardino, F.; Manz, E.; Matiasek, K.; Rentmeister, K.; et al. A deletion in the VLDLR gene in Eurasier dogs with cerebellar hypoplasia resembling a Dandy-Walker-like malformation (DWLM). PLoS ONE 2015, 10, e0108917. [Google Scholar] [CrossRef]
- Jagannathan, V.; Drögemüller, C.; Leeb, T.; Aguirre, G.; André, C.; Bannasch, D.; Becker, D.; Davis, B.; Ekenstedt, K.; Faller, K.; et al. A Comprehensive Biomedical Variant Catalogue Based on Whole Genome Sequences of 582 Dogs and Eight Wolves. Anim. Genet. 2019, 50, 695–704. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Bendl, J.; Stourac, J.; Salanda, O.; Pavelka, A.; Wieben, E.D.; Zendulka, J.; Brezovsky, J.; Damborsky, J. PredictSNP: Robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput. Biol. 2014, 10, e1003440. [Google Scholar] [CrossRef] [PubMed]
- Pejaver, V.; Urresti, J.; Lugo-Martinez, J.; Pagel, K.A.; Lin, G.N.; Nam, H.; Mort, M.; Cooper, D.N.; Sebat, J.; Iakoucheva, L.M.; et al. Inferring the molecular and phenotypic impact of amino acid variants with MutPred2. Nat. Comm. 2020, 11, 5918. [Google Scholar] [CrossRef]
- Mol* 3D Viewer. Available online: https://www.rcsb.org/3d-view (accessed on 5 May 2025).
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Hasegawa, D.; Tamura, S.; Nakamoto, Y.; Matsuki, N.; Takahashi, K.; Fujita, M.; Uchida, K.; Yamato, O. Magnetic resonance findings of the corpus callosum in canine and feline lysosomal storage diseases. PLoS ONE 2013, 8, e83455. [Google Scholar] [CrossRef]
- Gonçalves, R.; Volk, H.; Smith, P.M.; Penderis, J.; Garosi, L.; MacKillop, E.; de Stefani, A.; Cherubini, G.; McConnell, J.F. Corpus callosal abnormalities in dogs. J. Vet. Intern. Med. 2014, 28, 1275–1279. [Google Scholar] [CrossRef]
- De Lahunta, A.; Fenner, W.R.; Indrieri, P.J.; Mellick, P.W.; Gardner, S.; Bell, J.S. Hereditary cerebellar cortical abiotrophy in the Gordon Setter. J. Am. Vet. Med. Assoc. 1980, 177, 538–541. [Google Scholar] [CrossRef]
- Steinberg, H.S.; Troncoso, J.C.; Cork, L.C.; Price, D.L. Clinical features of inherited cerebellar degeneration in Gordon Setters. J. Am. Vet. Med. Assoc. 1981, 179, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Tiemeyer, M.J.; Singer, H.S.; Troncoso, J.C.; Cork, L.C.; Coyle, J.T.; Price, D.L. Synaptic neurochemical alterations associated with neuronal degeneration in an inherited cerebellar ataxia of Gordon Setters. J. Neuropathol. Exp. Neurol. 1984, 43, 580–591. [Google Scholar] [CrossRef]
- Troncoso, J.C.; Cork, L.C.; Price, D.L. Canine inherited ataxia: Ultrastructural observations. J. Neuropathol. Exp. Neurol. 1985, 44, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, K.S.; Van Winkle, T.; Bell, J.S.; de Lahunta, A. Cerebellar degeneration in Old English Sheepdogs. J. Am. Vet. Med. Assoc. 2000, 217, 1162–1165. [Google Scholar] [CrossRef]
- Pereira-Leal, J.B.; Seabra, M.C. The mammalian Rab family of small GTPases: Definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 2000, 301, 1077–1087. [Google Scholar] [CrossRef]
- Erdman, R.A.; Shellenberger, K.E.; Overmeyer, J.H.; Maltese, W.A. Rab24 is an atypical member of the rab GTPase family. Deficient GTPase activity, GDP dissociation inhibitor interaction, and prenylation of Rab24 expressed in cultured cells. J. Biol. Chem. 2000, 275, 3848–3856. [Google Scholar] [CrossRef]
- Munafo, D.B.; Colombo, M.I. Induction of autophagy causes dramatic changes in the subcellular distribution of GFP-Rab24. Traffic 2002, 3, 472–482. [Google Scholar] [CrossRef]
- Ylä-Anttila, P.; Eskelinen, E.L. Roles for RAB24 in autophagy and disease. Small GTPases 2018, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Seitz, S.; Kwon, Y.; Hartleben, G.; Jülg, J.; Sekar, R.; Krahmer, N.; Najafi, B.; Loft, A.; Gancheva, S.; Stemmer, K.; et al. Hepatic Rab24 controls blood glucose homeostasis via improving mitochondrial plasticity. Nat. Metab. 2019, 1, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).