Genotype–Phenotype Correlation Insights in a Rare Case Presenting with Multiple Osteodysplastic Syndromes
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. DNA Sequence Analysis
2.3. Molecular Modeling and Molecular Dynamics Simulations
3. Results
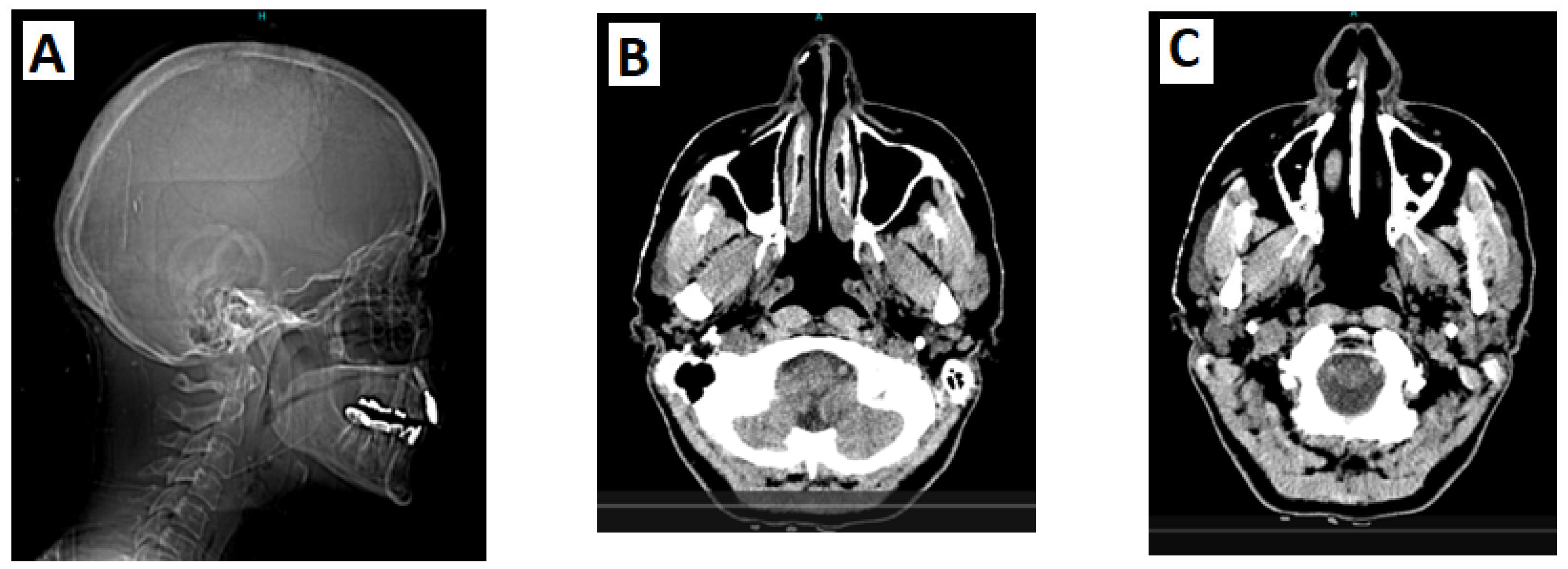
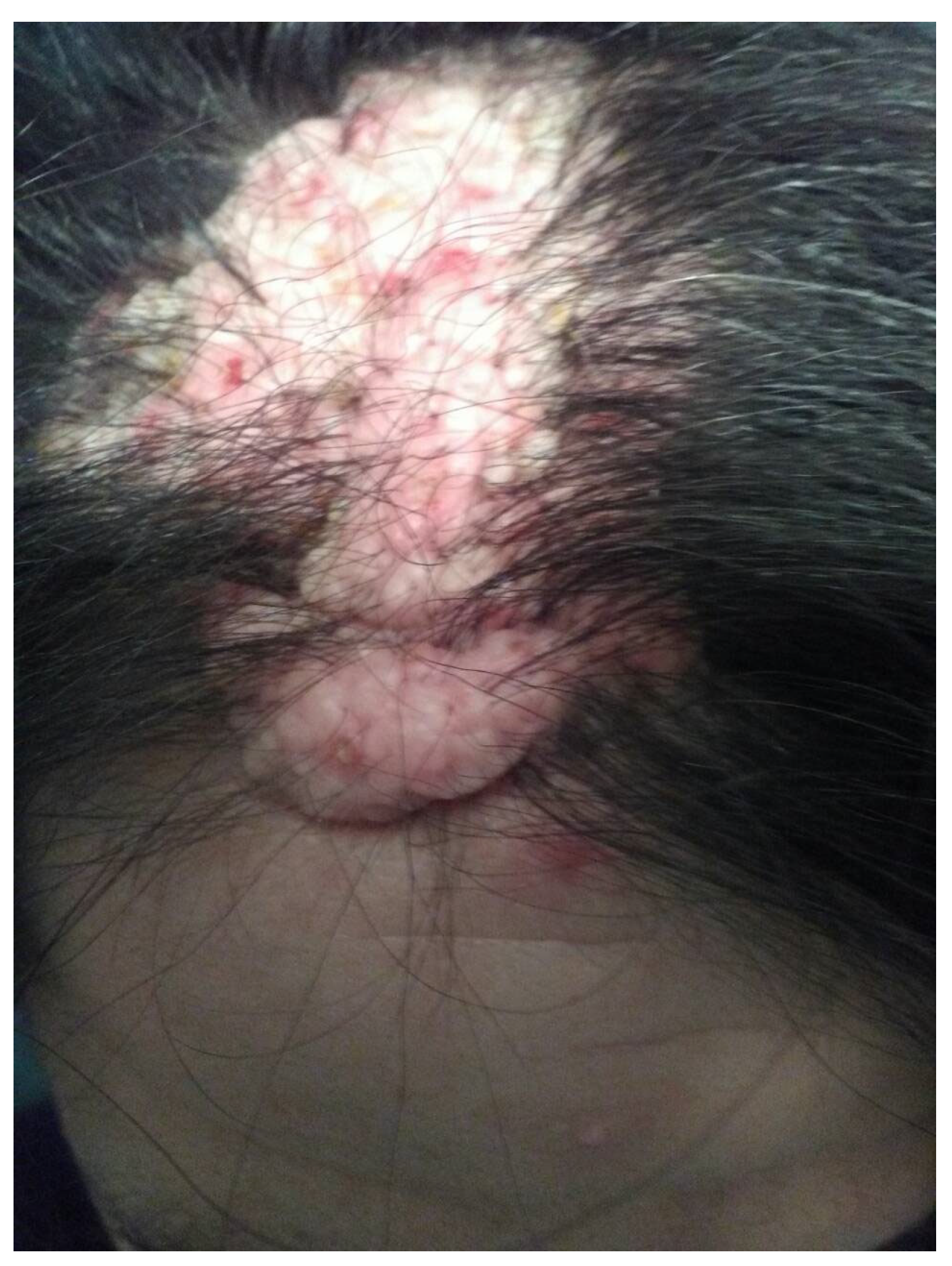
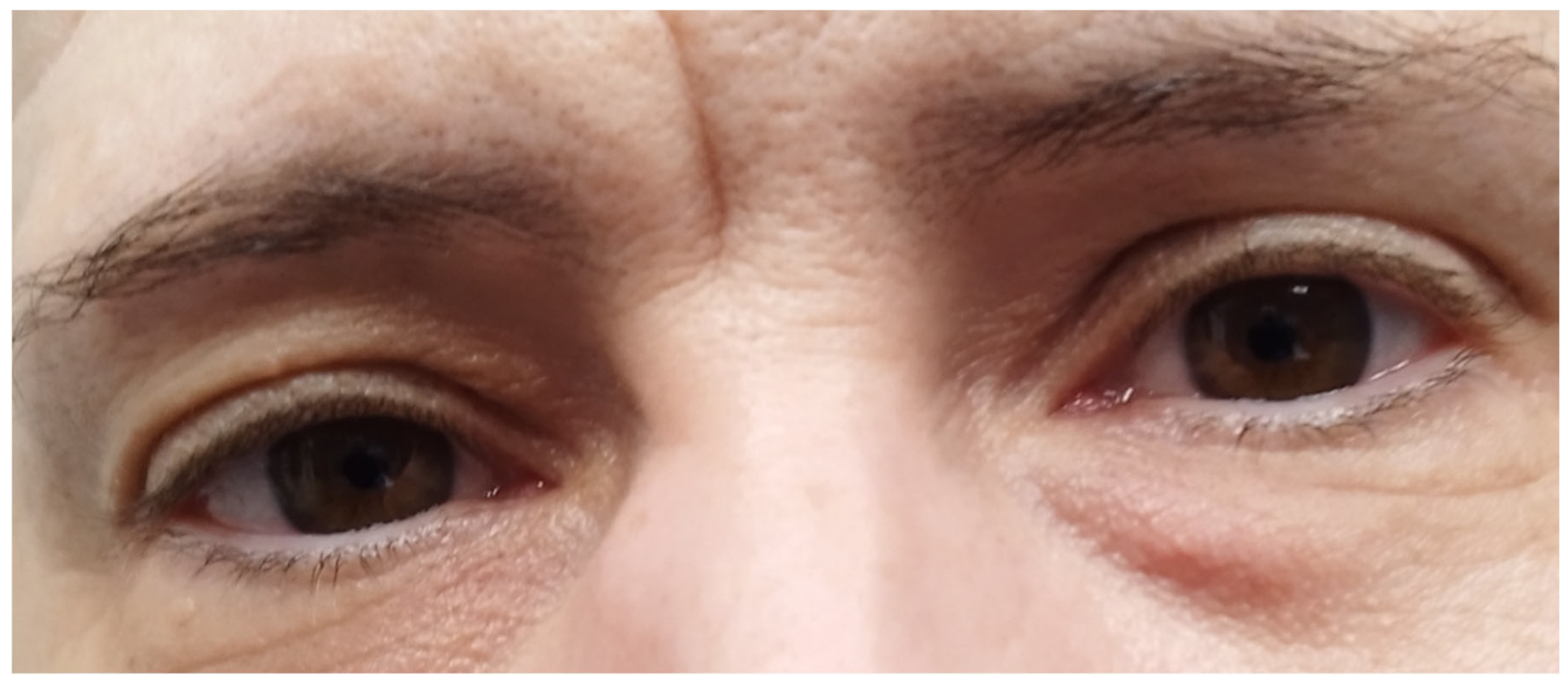
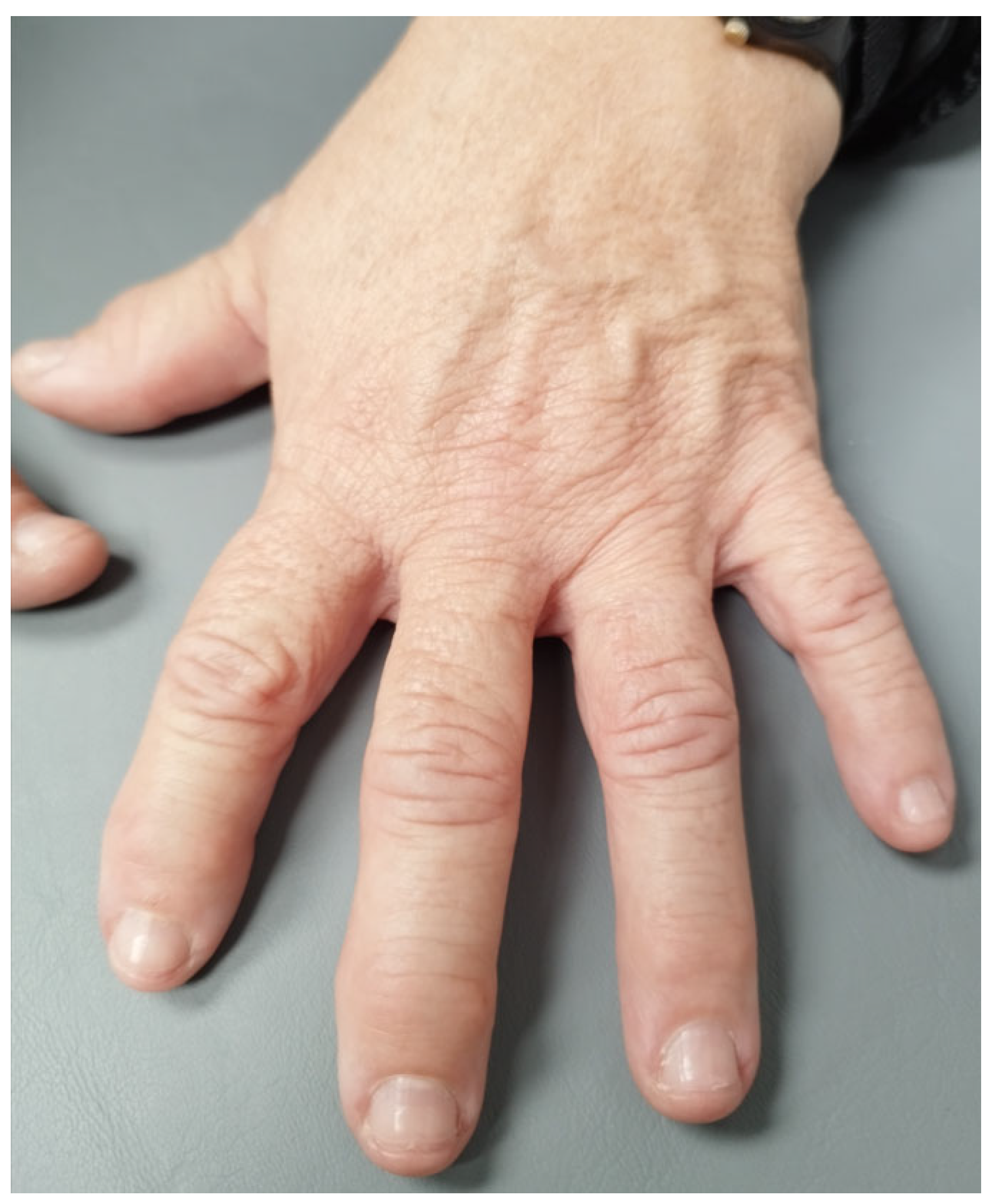
3.1. Case Report
3.2. Genetic Analysis
- (a)
- A single-nucleotide deletion c.1523+3G>- in intron 11 of gene CREB3L1 (NM_052854) on chromosome 11p11.2, predicted to cause abnormal splicing. Mutations in gene CREB3L1 are associated with autosomal recessive osteogenesis imperfecta type XVI (OMIM 616229);
- (b)
- A single-nucleotide substitution c.397+10T>C in intron 3 of gene SLCO2A1 (NM_005630) on chromosome 3q22.1-q22.2, predicted to cause abnormal splicing. Mutations in gene SLCO2A1 are associated with autosomal recessive primary hypertrophic osteoarthropathy (OMIM 614441);
- (c)
- A single-nucleotide substitution c.958C>A in exon 6 of gene SFRP4 (NM_003014) on chromosome 7p14.1, predicted to cause a missense substitution of proline into threonine in codon 320. Mutations in gene SFRP4 are associated with autosomal recessive metaphyseal dysplasia Pyle type (OMIM 265900).
- (a)
- A single-nucleotide substitution c.1913G>A in exon 17 of gene LRP5 (NM_001291902) on chromosome 11q13.4, predicted to cause a missense substitution of arginine into histidine in codon 638. Mutations in gene LRP5 are associated with autosomal dominant endosteal hyperostosis, also known as osteosclerosis (OMIM 144750), a disorder that is compatible with the patient’s phenotype. Other allelic mutations in gene LRP5 cause disorders with clinical characteristics not found in the patient, such as bone mineral density variability type 1 (OMIM 601884), osteopetrosis type 1 (OMIM 607634), exudative vitreoretinopathy type 4 (OMIM 601813), osteoporosis-pseudoglioma syndrome (OMIM 259770), and polycystic liver disease type 4 with or without kidney cysts (OMIM 617875).
- (b)
- A single-nucleotide substitution c.3184A>G in exon 14 of gene LRP6 (NM_002336) on chromosome 12p13.2, which was predicted to cause a missense substitution of isoleucine into valine in codon 1062. This variation has been associated with osteoporosis and an increased risk of fractures [11]. Other allelic mutations in the LRP6 gene have been reported to cause autosomal dominant tooth agenesis type 7 (OMIM 616724) and autosomal dominant coronary artery disease type 2 (OMIM 610947).
3.3. Molecular Dynamics Simulations of Detected Pathological Variants
4. Discussion
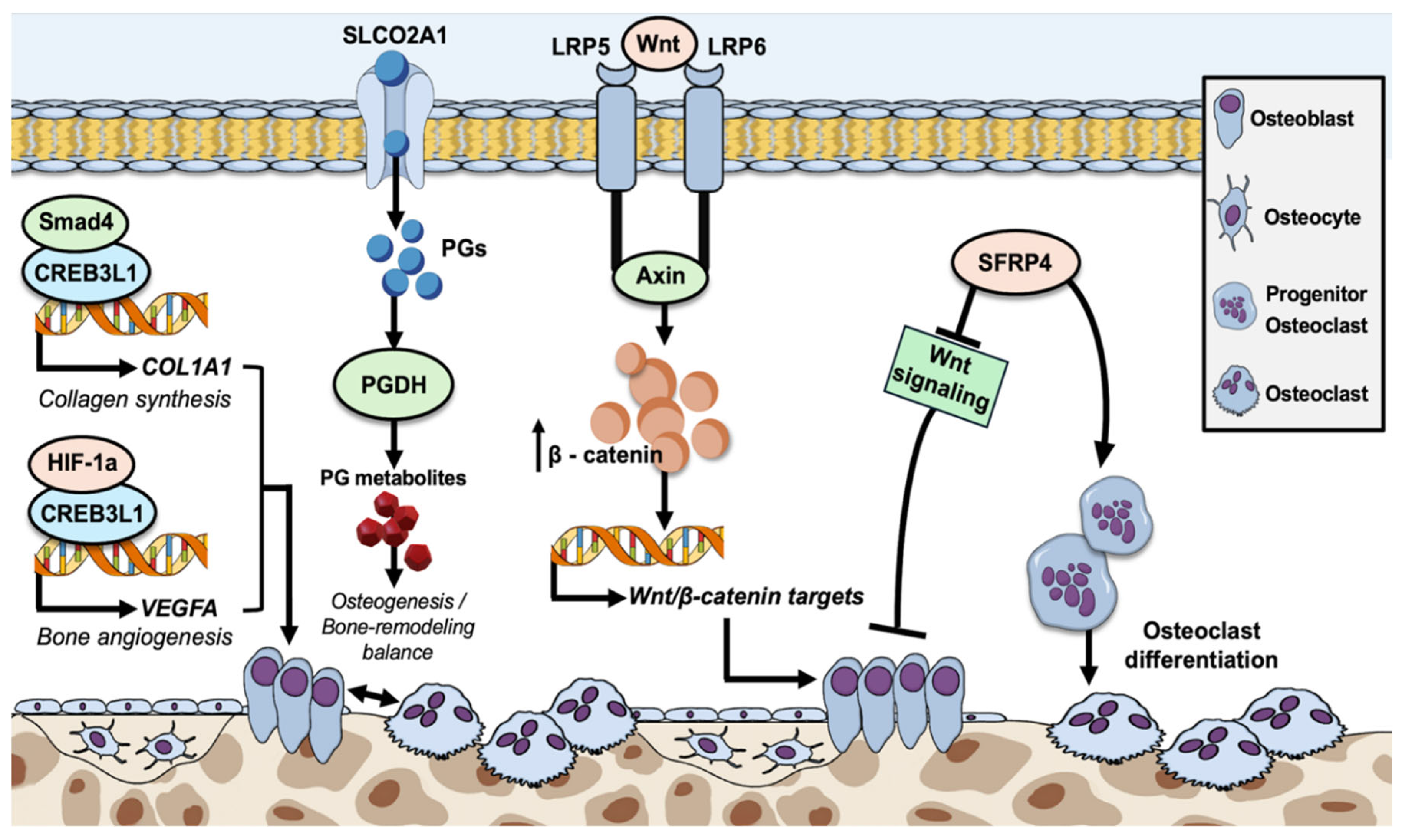
4.1. CREB3L1 in Osteogenesis Imperfecta Type XVI
4.2. SLCO2A1 in Primary Hypertrophic Osteoarthropathy
4.3. SFRP4 in Metaphyseal Dysplasia/Pyle’s Disease
4.4. LRP5 in Endosteal Hyperostosis/Osteosclerosis
4.5. LRP6 Associated with Osteoporosis and an Increased Risk of Fractures
4.6. Wnt Signaling Pathway Variants
4.7. Limitations of the Study
4.8. Potential Future Prospects of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unger, S.; Ferreira, C.R.; Mortier, G.R.; Ali, H.; Bertola, D.R.; Calder, A.; Cohn, D.H.; Cormier-Daire, V.; Girisha, K.M.; Hall, C.; et al. Nosology of genetic skeletal disorders: 2023 revision. Am. J. Med. Genet. 2023, 191, 1164–1209. [Google Scholar] [CrossRef] [PubMed]
- Colares Neto, G.P.; Alves, C.A.D. Demystifying Skeletal Dysplasias: A Practical Approach for the Pediatric Endocrinologist. Horm. Res. Paediatr. 2025, 98, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Warman, M.L.; Cormier-Daire, V.; Hall, C.; Krakow, D.; Lachman, R.; LeMerrer, M.; Mortier, G.; Mundlos, S.; Nishimura, G.; Rimoin, D.L.; et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am. J. Med. Genet. 2011, 155, 943–968. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, D.; Shchelochkov, O.; Kelley, B.; Lee, B. Signaling pathways in human skeletal dysplasias. Annu. Rev. Genom. Hum. Genet. 2010, 11, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Kronenberg, H.M.; Settembre, C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 629–648. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Geister, K.A.; Camper, S.A. Advances in Skeletal Dysplasia Genetics. Annu. Rev. Genom. Hum. Genet. 2015, 16, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.C.; Blissett, A.R. New genes in bone development: What’s new in osteogenesis imperfecta. J. Clin. Endocrinol. Metab. 2013, 98, 3095–3103. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.; Lima, J.; Vieira, J.D.; Costa, J.N. An unusual presentation of osteogenesis imperfecta type I. Int. Med. Case Rep. J. 2011, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Bamshad, M.J.; Ng, S.B.; Bigham, A.W.; Tabor, H.K.; Emond, M.J.; Nickerson, D.A.; Shendure, J. Exome sequencing as a tool for Mendelian disease gene discovery. Nat. Rev. Genet. 2011, 12, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Yapijakis, C.; Pachis, N.; Sotiriadou, T.; Vaila, C.; Michopoulou, V.; Vassiliou, S. Molecular Mechanisms Involved in Craniosynostosis. In Vivo 2023, 37, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Yapijakis, C.; Davaria, S.; Gintoni, I.; Chrousos, G.P. The Impact of Genetic Variability of TGF-Beta Signaling Biomarkers in Major Craniofacial Syndromes. Adv. Exp. Med. Biol. 2023, 1423, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Paltoglou, G.; Ziakas, N.; Chrousos, G.P.; Yapijakis, C. Cephalometric Evaluation of Children with Short Stature of Genetic Etiology: A Review. Children 2024, 11, 792. [Google Scholar] [CrossRef] [PubMed]
- Prado, H.V.; Soares, E.C.B.; Carneiro, N.C.R.; Oliveira Vilar, I.C.; Abreu, L.G.; Borges-Oliveira, A.C. Dental anomalies in individuals with osteogenesis imperfecta: A systematic review and meta-analysis of prevalence and comparative studies. J. Appl. Oral Sci. Rev. FOB 2023, 31, e20230040. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, K.; Åström, E.; Dragomir, A.; Symoens, S.; Coucke, P.; Larsson, S.; Paschalis, E.; Roschger, P.; Gamsjaeger, S.; Klaushofer, K.; et al. Homozygosity for CREB3L1 premature stop codon in first case of recessive osteogenesis imperfecta associated with OASIS-deficiency to survive infancy. Bone 2018, 114, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Kanemoto, S.; Cui, X.; Kaneko, M.; Asada, R.; Matsuhisa, K.; Tanimoto, K.; Yoshimoto, Y.; Shukunami, C.; Imaizumi, K. OASIS modulates hypoxia pathway activity to regulate bone angiogenesis. Sci. Rep. 2015, 5, 16455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Yu, Z.; Song, Y.; Fan, L.; Lei, T.; He, Y.; Hu, S. The Regulatory Network of CREB3L1 and Its Roles in Physiological and Pathological Conditions. Int. J. Med. Sci. 2024, 21, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gong, Y.; Wang, Z.; Meng, X.; Luo, Z.; Papasian, C.J.; Greenbaum, J.; Li, Y.; Liang, Q.; Chen, Y.; et al. Regulon active landscape reveals cell development and functional state changes of human primary osteoblasts in vivo. Hum. Genom. 2023, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Guterman-Ram, G.; Marini, J.C. Osteogenesis Imperfecta: Mechanisms and Signaling Pathways Connecting Classical and Rare OI Types. Endocr. Rev. 2022, 43, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.; Malmgren, B.; Åström, E.; Nordgren, A.; Taylan, F.; Dahllöf, G. Mutations in COL1A1/A2 and CREB3L1 are associated with oligodontia in osteogenesis imperfecta. Orphanet J. Rare Dis. 2020, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Yanai, S.; Yamaguchi, S.; Nakamura, S.; Kawasaki, K.; Toya, Y.; Yamada, N.; Eizuka, M.; Uesugi, N.; Umeno, J.; Esaki, M.; et al. Distinction between chronic enteropathy associated with the slco2a1 gene and crohn’s disease. Gut Liver 2019, 13, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Xu, Y.; Zhang, Z.; Li, S.; Zhang, Z. Primary hypertrophic osteoarthropathy: Genetics, clinical features and management. Front. Endocrinol. 2023, 14, 1235040. [Google Scholar] [CrossRef] [PubMed]
- Mangupli, R.; Daly, A.F.; Cuauro, E.; Camperos, P.; Krivoy, J.; Beckers, A. Primary hypertrophic osteoarthropathy due to a novel SLCO2A1 mutation masquerading as acromegaly. Endocrinol. Diabetes Metab. Case Rep. 2017, 2017, 0013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martínez-Lavín, M. Hypertrophic osteoarthropathy. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101507. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Z.; Yue, H.; Li, S.; Zhang, Z. Monoallelic mutations in SLCO2A1 cause autosomal dominant primary hypertrophic osteoarthropathy. J. Bone Min. Res. 2021, 36, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Bloch, A.; Couture, G.; Isidor, B.; Ricquebourg, M.; Bourrat, E.; Lipsker, D.; Taillan, B.; Combier, A.; Chiaverini, C.; Moufle, F.; et al. Novel pathogenic variants in SLCO2A1 causing autosomal dominant primary hypertrophic osteoarthropathy. Eur. J. Med. Genet. 2023, 66, 104689. [Google Scholar] [CrossRef] [PubMed]
- Narayananan, V.S.; Ashok, L.; Mamatha, G.P.; Rajeshwari, A.; Prasad, S.S. Pyle’s disease: An incidental finding in a routine dental patient. Dentomaxillofacial Radiol. 2006, 35, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Wonkam, A.; Makubalo, N.; Roberts, T.; Chetty, M. Pyle metaphyseal dysplasia in an African child: Case report and review of the literature. S. Afr. Med. J. 2020, 106 (Suppl. 1), S110–S113. [Google Scholar] [CrossRef] [PubMed]
- Kiper, P.O.S.; Saito, H.; Gori, F.; Unger, S.; Hesse, E.; Yamana, K.; Kiviranta, R.; Solban, N.; Liu, J.; Brommage, R.; et al. Cortical-Bone Fragility--Insights from sFRP4 Deficiency in Pyle’s Disease. N. Engl. J. Med. 2016, 374, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Baron, R.; Gori, F. Sfrp4 and the Biology of Cortical Bone. Curr. Osteoporos. Rep. 2022, 20, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, L.; Queiro, R.; Alperi, M.; Lorenzo, J.A.; Ballina, J. Pyle’s Disease: A human model of differentiated cortical and trabecular homeostasis. Reum. Clin. (Engl. Ed.) 2020, 16, 56–58, English, Spanish. [Google Scholar] [CrossRef]
- Chen, K.; Ng, P.Y.; Chen, R.; Hu, D.; Berry, S.; Baron, R.; Gori, F. Sfrp4 repression of the Ror2/Jnk cascade in osteoclasts protects cortical bone from excessive endosteal resorption. Proc. Natl. Acad. Sci. USA 2019, 116, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Beals, R.K.; McLoughlin, S.W.; Teed, R.L.; McDonald, C. Dominant endosteal hyperostosis. Skeletal characteristics and review of literature. J. Bone Jt. Surg. Am. 2001, 83, 1643–1649. [Google Scholar] [CrossRef]
- Frey, J.L.; Li, Z.; Ellis, J.M.; Zhang, Q.; Farber, C.R.; Aja, S.; Wolfgang, M.J.; Clemens, T.L.; Riddle, R.C. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol. Cell Biol. 2015, 35, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.O. LRP5: From bedside to bench to bone. Bone 2017, 102, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Huh, Y.H.; Kim, K.; Kim, S.; Park, K.H.; Koh, J.T.; Chun, J.S.; Ryu, J.H. Low-density lipoprotein receptor-related protein 5 governs Wnt-mediated osteoarthritic cartilage destruction. Arthritis Res. Ther. 2014, 16, R37. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Yui, H.; Kikugawa, S.; Tokida, R.; Sakai, N.; Kondo, N.; Endo, N.; Haro, H.; Shimodaira, H.; Suzuki, T.; et al. Associations of LRP5 and MTHFR Gene Variants with Osteoarthritis Prevalence in Elderly Women: A Japanese Cohort Survey Randomly Sampled from a Basic Resident Registry. Ther. Clin. Risk Manag. 2021, 17, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- van Meurs, J.B.J.; Rivadeneira, F.; Jhamai, M.; Hugens, W.; Hofman, A.; van Leeuwen, J.P.T.M.; Pols, H.A.P.; Uitterlinden, A.G. Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J. Bone Min. Res. 2006, 21, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Souza, G.; de Oliveira, L.B.; Wambier, L.M.; Scariot, R.; Feltrin-Souza, J. Tooth abnormalities associated with non-syndromic cleft lip and palate: Systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 5089–5103. [Google Scholar] [CrossRef] [PubMed]
- Dinckan, N.; Du, R.; Petty, L.E.; Coban-Akdemir, Z.; Jhangiani, S.N.; Paine, I.; Baugh, E.H.; Erdem, A.P.; Kayserili, H.; Doddapaneni, H.; et al. Whole-Exome Sequencing Identifies Novel Variants for Tooth Agenesis. J. Dent. Res. 2018, 97, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Kimura, M.; Machida, J.; Ota, A.; Nakashima, M.; Tsuchida, N.; Adachi, J.; Aoki, Y.; Tatematsu, T.; Takahashi, K.; et al. A novel LRP6 variant in a Japanese family with oligodontia. Hum. Genome Var. 2021, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Gao, C.Y.; Zheng, C.Y.; Chen, X.; Yan, Y.S.; Sun, Y.Q.; Dong, X.Y.; Yang, K.; Zhang, D.L. Investigation of a Novel LRP6 Variant Causing Autosomal-Dominant Tooth Agenesis. Front. Genet. 2021, 12, 688241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, M.; Sun, K.; Fan, Z.; Liu, H.; Feng, H.; Liu, Y.; Han, D. Rare phenotype: Hand preaxial polydactyly associated with LRP6-related tooth agenesis in humans. NPJ Genom. Med. 2021, 6, 93. [Google Scholar] [CrossRef] [PubMed]

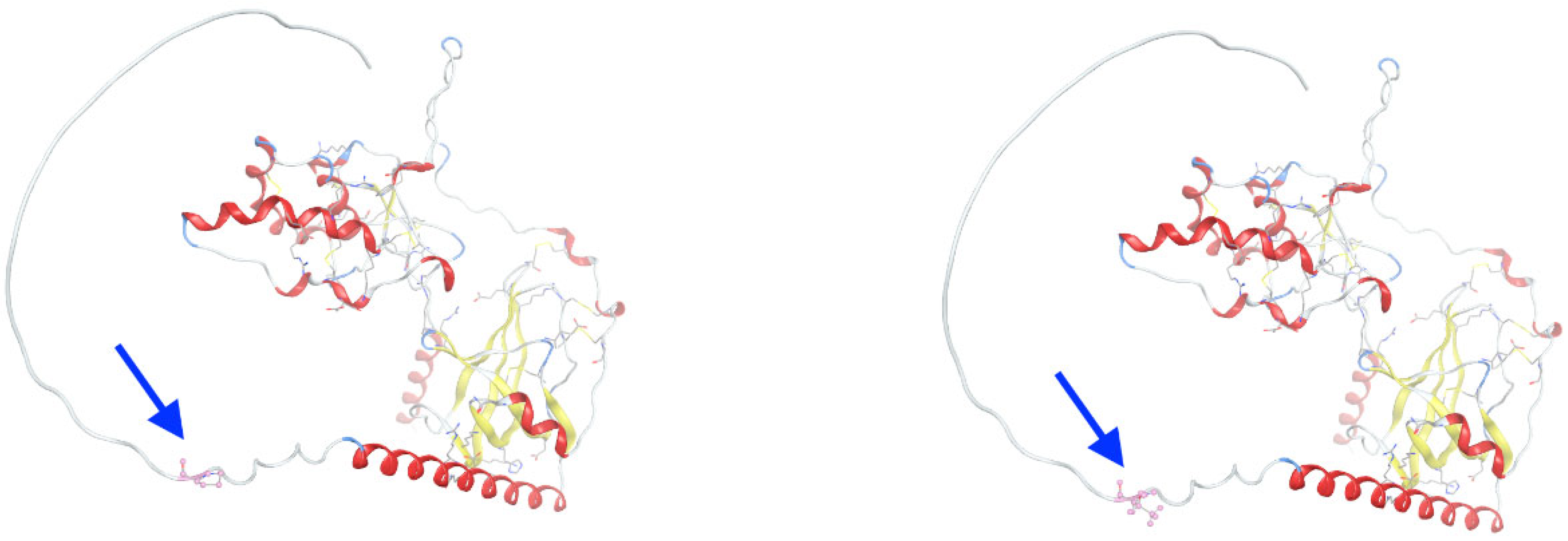
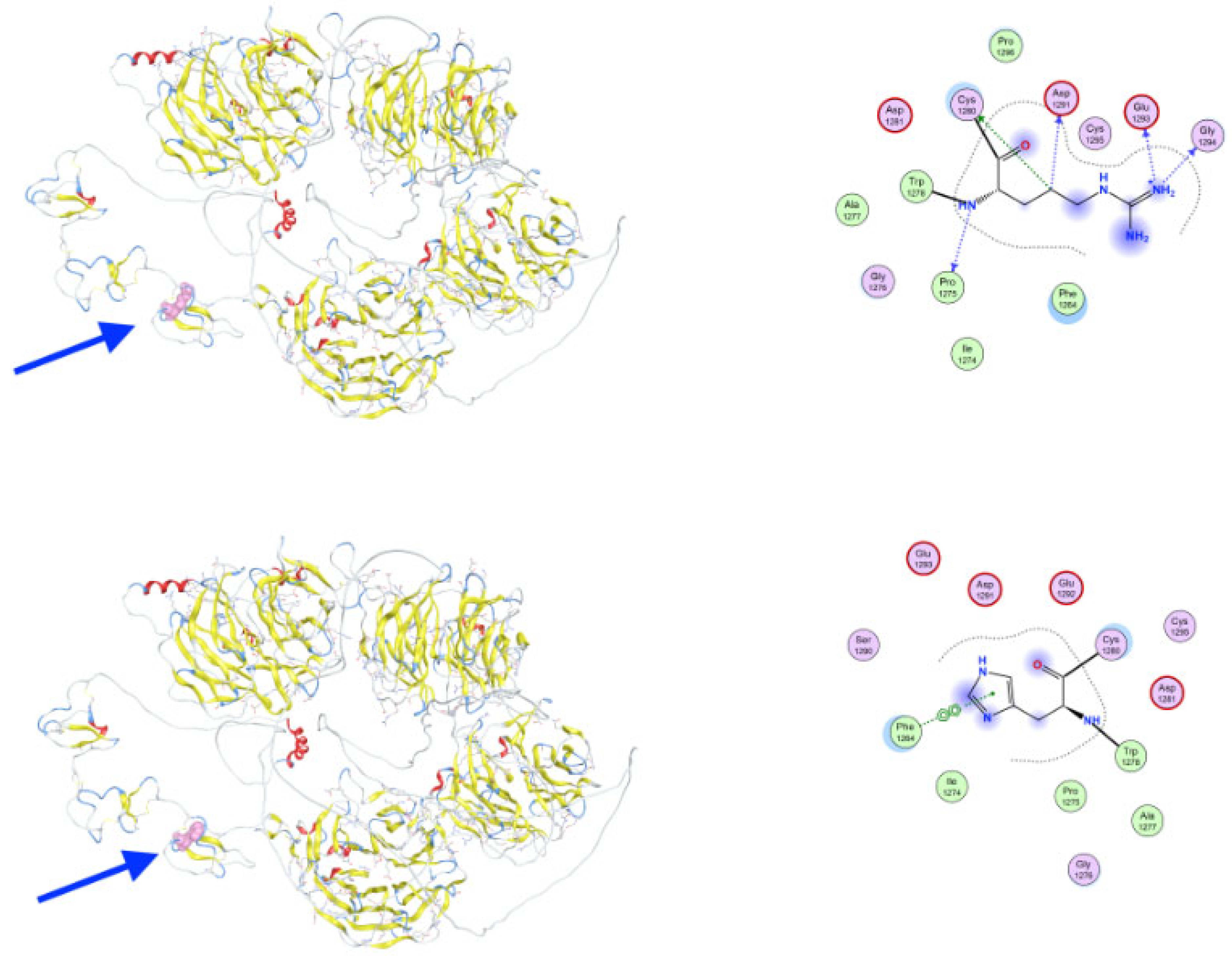
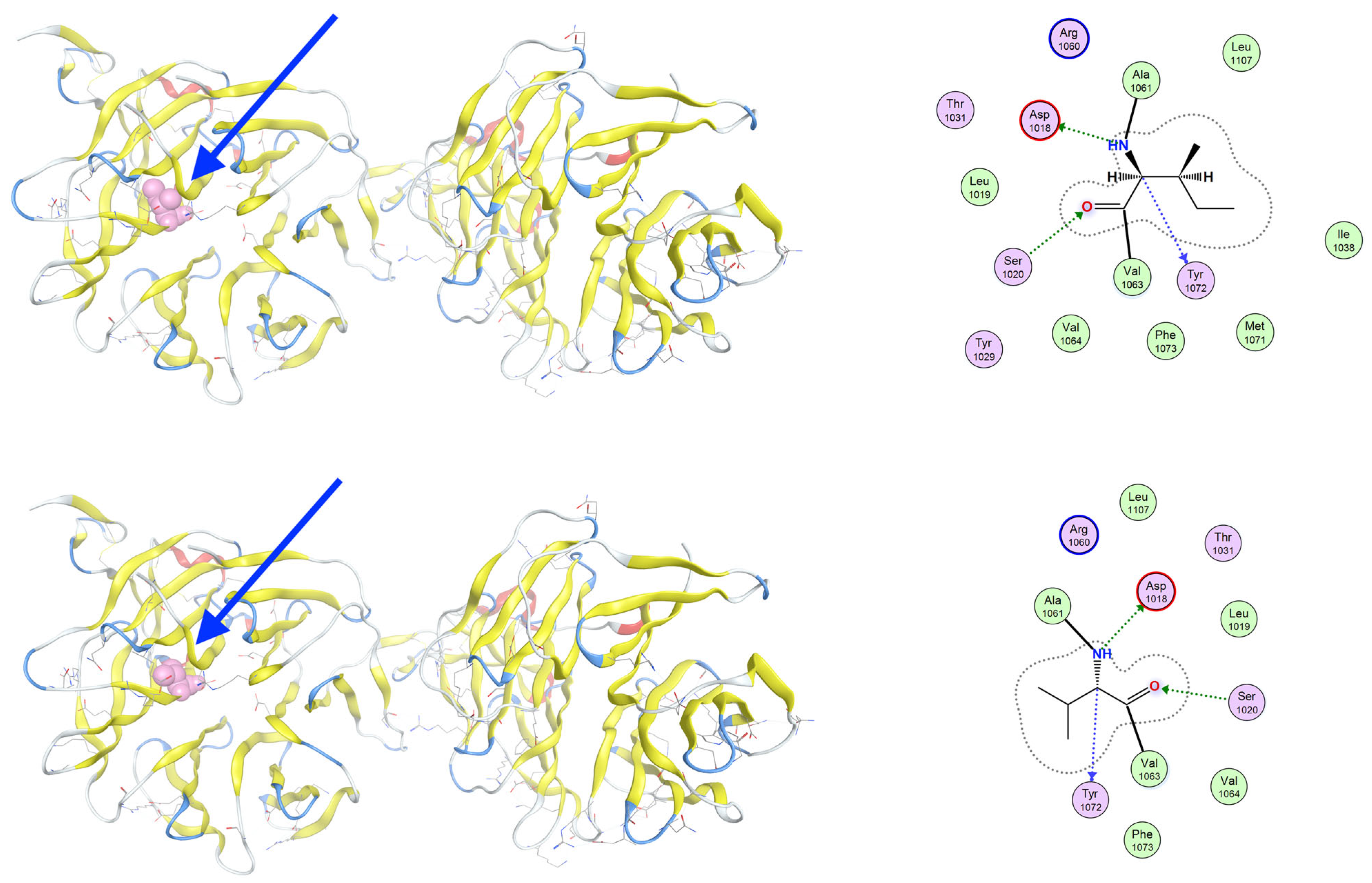
| Gene | Variant | Genotype | Protein Role | Potential Effect | Inheritance | Disease Links |
|---|---|---|---|---|---|---|
| CREB3L1 | c.1523+3G>- | Homozygote | Transcription factor | Abnormal splicing | AR | Osteogenesis imperfecta type XVI |
| SLCO2A1 | c.397+10T>C | Homozygote | Membrane anion transporter | Abnormal splicing | AR | Primary hypertrophic osteoarthropathy |
| SFRP4 | c.958C>A P320T | Homozygote | Wnt antagonist | May impair Wnt inhibition | AR | Pyle’s Metaphyseal Dysplasia |
| LRP5 | c.3184A>G R638H | Heterozygote | Wnt co-receptor | May affect ligand binding or folding | AD | Endosteal Hyperostosis |
| LRP6 | c.3184A>G I1062V | Heterozygote | Wnt co-receptor | Conservative change; may subtly impair signaling | AD | Osteoporosis |
| Variant | Gene | Protein Role | Potential Effect | Disease Links |
|---|---|---|---|---|
| P320T | SFRP4 | Wnt antagonist | May impair Wnt inhibition | Pyle’s Metaphyseal Dysplasia (bone dysplasia) |
| R638H | LRP5 | Wnt co-receptor | May affect ligand binding or folding | Endosteal Hyperostosis (increased bone density) |
| I1062V | LRP6 | Wnt co-receptor | Conservative change; may subtly impair signaling | Osteoporosis (decreased bone density) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yapijakis, C.; Gintoni, I.; Chamakioti, M.; Koniari, E.; Papanikolaou, E.; Kassi, E.; Vlachakis, D.; Chrousos, G.P. Genotype–Phenotype Correlation Insights in a Rare Case Presenting with Multiple Osteodysplastic Syndromes. Genes 2025, 16, 871. https://doi.org/10.3390/genes16080871
Yapijakis C, Gintoni I, Chamakioti M, Koniari E, Papanikolaou E, Kassi E, Vlachakis D, Chrousos GP. Genotype–Phenotype Correlation Insights in a Rare Case Presenting with Multiple Osteodysplastic Syndromes. Genes. 2025; 16(8):871. https://doi.org/10.3390/genes16080871
Chicago/Turabian StyleYapijakis, Christos, Iphigenia Gintoni, Myrsini Chamakioti, Eleni Koniari, Eleni Papanikolaou, Eva Kassi, Dimitrios Vlachakis, and George P. Chrousos. 2025. "Genotype–Phenotype Correlation Insights in a Rare Case Presenting with Multiple Osteodysplastic Syndromes" Genes 16, no. 8: 871. https://doi.org/10.3390/genes16080871
APA StyleYapijakis, C., Gintoni, I., Chamakioti, M., Koniari, E., Papanikolaou, E., Kassi, E., Vlachakis, D., & Chrousos, G. P. (2025). Genotype–Phenotype Correlation Insights in a Rare Case Presenting with Multiple Osteodysplastic Syndromes. Genes, 16(8), 871. https://doi.org/10.3390/genes16080871







