Exploring Cloned Disease Resistance Gene Homologues and Resistance Gene Analogues in Brassica nigra, Sinapis arvensis, and Sinapis alba: Identification, Characterisation, Distribution, and Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference Genomes
2.2. Genome-Wide Mining of Resistance Gene Analogues
2.3. Characterisation of Resistance Gene Analogues
2.4. Mining the Protein Sequences of the Cloned Genes
2.5. Homologue Identification
2.6. Co-Localisation of RLKs and RLPs to Reported Disease Resistance Loci in Brassica Crops
2.7. Phylogenetic Analysis
3. Results
3.1. RGAs Identification and Classification
3.2. RGA Number and Chromosome Size Relationship
3.3. RGAs Distribution and Density
3.4. Physical Clustering
3.5. RGAs Sequence Pairwise Similarities
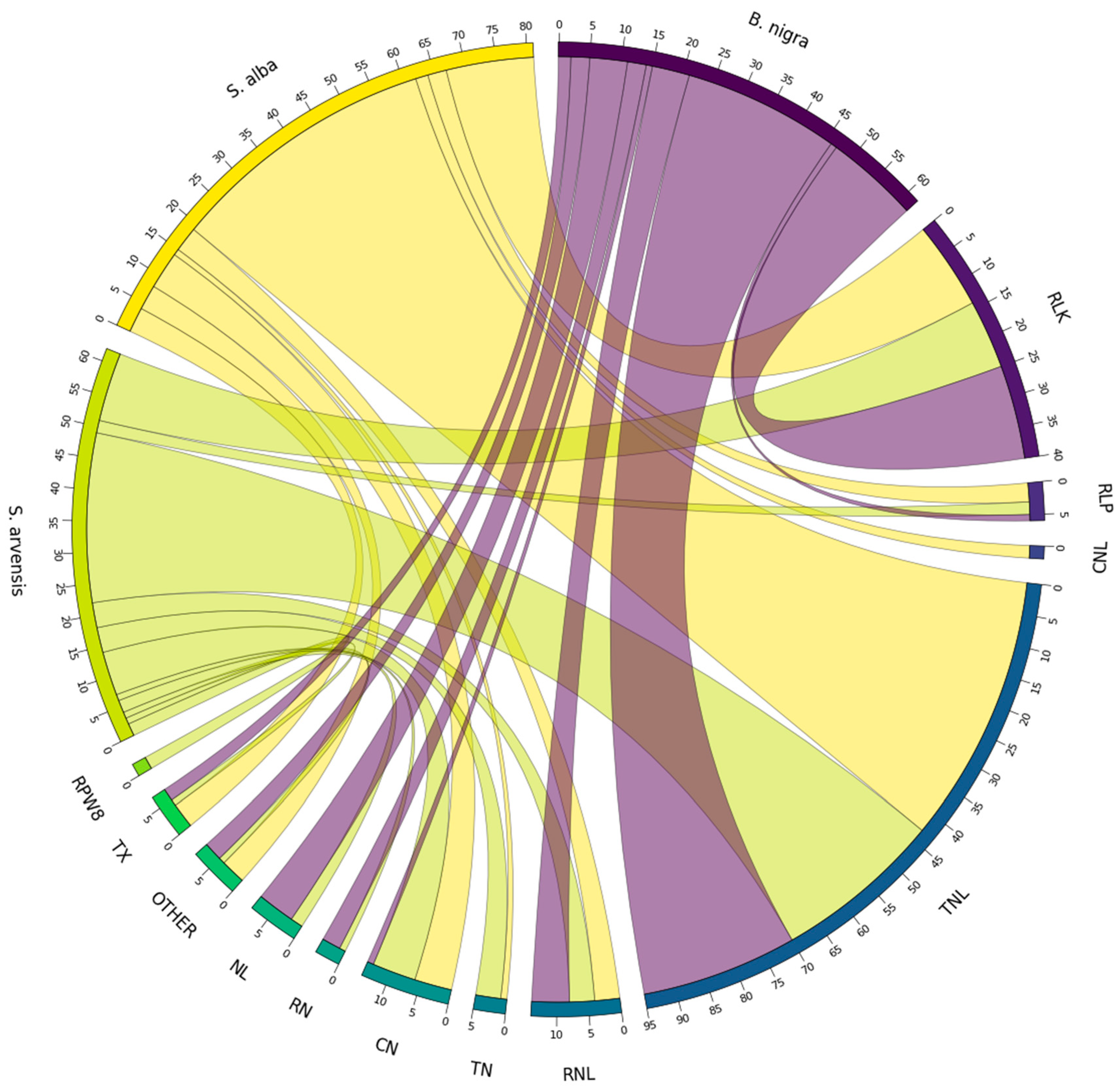
3.6. RGA Homologue Identification
3.7. Non-RGA Homologues Identification
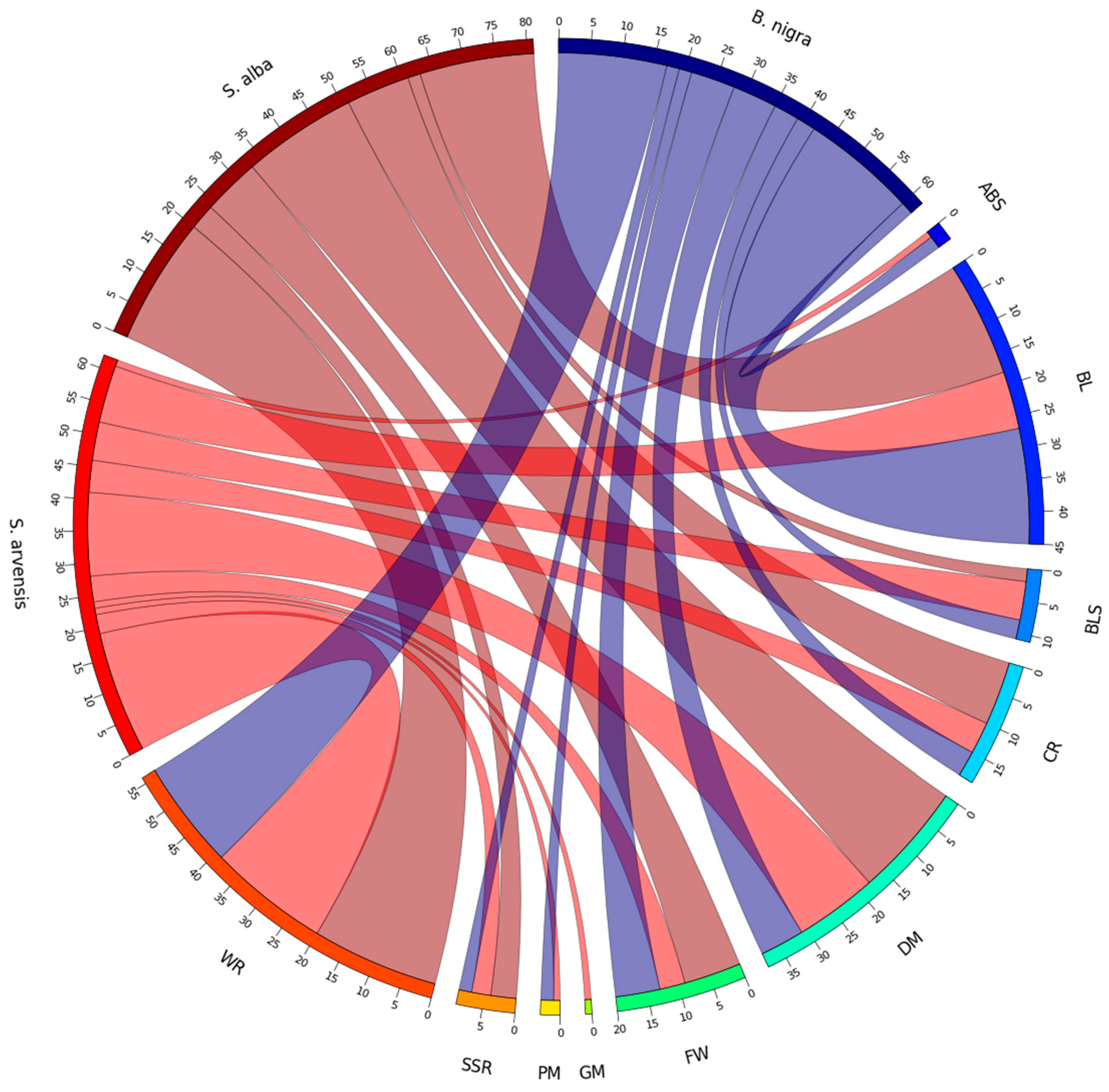
3.8. Co-Localisation of RLKs and RLPs to Reported Disease Resistance Loci
3.9. Phylogenetic Analysis
4. Discussion
4.1. RGA Identification and Classification
4.2. RGAs Distribution, Density and Chromosome Size Relationship
4.3. Physical Clustering
4.4. RGAs Sequence Pairwise Similarities
4.5. RGA and Non-RGA Homologues Identification
4.6. Co-Localisation of RLKs and RLPs to Reported Disease Resistance Loci
4.7. Phylogenetic Analysis
5. Conclusions
Limitations and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohd Saad, N.S.; Severn-Ellis, A.A.; Pradhan, A.; Edwards, D.; Batley, J. Genomics armed with diversity leads the way in Brassica improvement in a changing global environment. Front. Genet. 2021, 12, 600789. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Scheben, A.; Edwards, D.; Spillane, C.; Ortiz, R. Assessing and exploiting functional diversity in germplasm pools to enhance abiotic stress adaptation and yield in cereals and food legumes. Front. Plant Sci. 2017, 8, 1461. [Google Scholar] [CrossRef] [PubMed]
- Greer, S.F.; Surendran, A.; Grant, M.; Lillywhite, R. The current status, challenges, and future perspectives for managing diseases of Brassicas. Front. Microbiol. 2023, 14, 1209258. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Dutta, S.; Mondal, S.; Mondal, B. Clubroot disease on Brassica crops in India. Can. J. Plant Pathol. 2014, 36, 154–160. [Google Scholar] [CrossRef]
- Barbetti, M.J.; Li, C.X.; Banga, S.S.; Banga, S.K.; Singh, D.; Sandhu, P.S.; Singh, R.; Liu, S.Y.; You, M.P. New host resistances in Brassica napus and Brassica juncea from Australia, China and India: Key to managing Sclerotinia stem rot (Sclerotinia sclerotiorum) without fungicides. Crop Prot. 2015, 78, 127–130. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Kolte, S.J.; Waliyar, F. Diseases of Edible Oilseed Crops; CRC Press Inc.: Boca Raton, FL, USA, 2015. [Google Scholar]
- Barbetti, M.J.; Li, C.X.; You, M.P.; Singh, D.; Agnihotri, A.; Banga, S.K.; Sandhu, P.S.; Singh, R.; Banga, S.S. Valuable new leaf or inflorescence resistances ensure improved management of white rust (Albugo candida) in mustard (Brassica juncea) crops. J. Phytopathol. 2016, 164, 404–411. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sekhwal, M.K.; Li, P.; Lam, I.; Wang, X.; Cloutier, S.; You, F.M. Disease resistance gene analogs (RGAs) in plants. Int. J. Mol. Sci. 2015, 16, 19248–19290. [Google Scholar] [CrossRef] [PubMed]
- Tirnaz, S.; Bayer, P.; Inturrisi, F.; Neik, T.; Yang, H.; Dolatabadian, A.; Zhang, F.; Severn-Ellis, A.; Patel, D.; Pradhan, A.; et al. Resistance gene analogs in the Brassicaceae: Identification, characterisation, distribution and evolution. Plant Physiol. 2020, 184, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Wu, S.; Sun, W.; Coaker, G.; Kunkel, B.; He, P.; Shan, L. The Pseudomonas syringae type III effector AvrRpt2 promotes pathogen virulence via stimulating Arabidopsis auxin/indole acetic acid protein turnover. Plant Physiol. 2013, 162, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host—Microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Bao, J.; Li, H.; Hu, W.; Kong, Y.; Zhong, Y.; Fu, Q.; Xu, G.; Liu, F.; Jiao, X.; et al. Structural and biochemical basis of FLS2-mediated signal activation and transduction in rice. Plant Commun. 2024, 5, 100785. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.J.; Wood, A.J.; Lightfoot, D.A. Plant receptor—Like serine threonine kinases: Roles in signaling and plant defense. Mol. Plant–Microbe Interact. 2008, 21, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Trotochaud, A.E.; Clark, S.E. The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 1999, 11, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.; Huet, G.; Jauneau, A.; Camborde, L.; Trémousaygue, D.; Kraut, A.; Zhou, B.; Levaillant, M.; Adachi, H.; Yoshioka, H.; et al. A receptor pair with an integrated decoy converts pathogen disabling of transcription factors to immunity. Cell 2015, 161, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Ravensdale, M.; Bernoux, M.; Ve, T.; Kobe, B.; Thrall, P.H.; Ellis, J.G.; Dodds, P.N. Intramolecular interaction influences binding of the flax L5 and L6 resistance proteins to their AvrL567 ligands. PLoS Pathog. 2012, 8, e1003004. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, J.A.; Sack, F.D. Control of stomatal distribution on the Arabidopsis leaf surface. Science 2002, 296, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Martinez, D.; Addo Nyarko, C.P.; Schiessl, S.V.; Mason, A.S. Using wild relatives and related species to build climate resilience in Brassica crops. Theor. Appl. Genet. 2021, 134, 1711–1728. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.J.; Strelkov, S.E.; Howard, R.J.; Rahman, H. Screening of Brassica germplasm for resistance to Plasmodiophora brassicae pathotypes prevalent in Canada for broadening diversity in clubroot resistance. Can. J. Plant Sci. 2012, 92, 501–515. [Google Scholar] [CrossRef]
- Chu, M.; Yu, F.; Falk, K.; Liu, X.; Zhang, X.; Chang, A.; Peng, G. Identification of the clubroot resistance gene Rpb1 and introgression of the resistance gene into canola breeding lines using a marker-assisted selection approach. Acta Hortic. 2013, 1005, 599–605. [Google Scholar] [CrossRef]
- Peng, G.; Falk, K.C.; Gugel, R.K.; Franke, C.; Yu, F.; James, B.; Strelkov, S.E.; Hwang, S.-F.; McGregor, L. Sources of resistance to Plasmodiophora brassicae (clubroot) pathotypes virulent on canola. Can. J. Plant Pathol. 2014, 36, 89–99. [Google Scholar] [CrossRef]
- Chevre, A.M.; Eber, F.; This, P.; Barret, P.; Tanguy, X.; Brun, H.; Delseny, M.; Renard, M. Characterization of Brassica nigra chromosomes and of blackleg resistance in B. napus–B. nigra addition lines. Plant Breed. 1996, 115, 113–118. [Google Scholar] [CrossRef]
- Cantila, A.Y.; Thomas, W.J.W.; Bayer, P.E.; Edwards, D.; Batley, J. In silico prediction and analysis of transmembrane-coiled-coil resistance gene analogues in 27 Brassicaceae species. Plant Pathol. 2024, 73, 115–130. [Google Scholar] [CrossRef]
- Yang, T.; Cai, B.; Jia, Z.; Wang, Y.; Wang, J.; King, G.J.; Ge, X.; Li, Z. Sinapis genomes provide insights into whole-genome triplication and divergence patterns within tribe Brassiceae. Plant J. 2023, 113, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Quan, X.; Jia, G.; Xiao, J.; Cloutier, S.; You, F.M. RGAugury: A pipeline for genome-wide prediction of resistance gene analogs (RGAs) in plants. BMC Genom. 2016, 17, 852. [Google Scholar] [CrossRef] [PubMed]
- Anand, L.; Rodriguez Lopez, C.M. ChromoMap: An R package for interactive visualization of multi-omics data and annotation of chromosomes. BMC Bioinform. 2022, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Cantila, A.Y.; Neik, T.X.; Tirnaz, S.; Thomas, W.J.W.; Bayer, P.E.; Edwards, D.; Batley, J. Mining of cloned disease resistance gene homologs (CDRHs) in Brassica species and Arabidopsis thaliana. Biology 2022, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Al-Mamun, H.A.; Edwards, D.; Batley, J.; Dolatabadian, A. Genome-wide identification and prediction of disease resistance genes in Hirschfeldia incana. Agric. Commun. 2024, 2, 100049. [Google Scholar] [CrossRef]
- Yang, J.; Liu, D.; Wang, X.; Ji, C.; Cheng, F.; Liu, B.; Hu, Z.; Chen, S.; Pental, D.; Ju, Y.; et al. The genome sequence of allopolyploid Brassica juncea and analysis of differential homeolog gene expression influencing selection. Nat. Genet. 2016, 48, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Parkin, I.A.P.; Koh, C.; Tang, H.; Robinson, S.J.; Kagale, S.; E Clarke, W.; Town, C.D.; Nixon, J.; Krishnakumar, V.; Bidwell, S.L.; et al. Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 2014, 15, R77. [Google Scholar] [CrossRef] [PubMed]
- Stotz, H.U.; Harvey, P.J.; Haddadi, P.; Mashanova, A.; Kukol, A.; Larkan, N.J.; Borhan, M.H.; Fitt, B.D.L.; Raman, H. Genomic evidence for genes encoding leucine-rich repeat receptors linked to resistance against the eukaryotic extra- and intracellular Brassica napus pathogens Leptosphaeria maculans and Plasmodiophora brassicae. PLoS ONE 2018, 13, e0198201. [Google Scholar] [CrossRef] [PubMed]
- Inturrisi, F.; Bayer, P.E.; Cantila, A.Y.; Tirnaz, S.; Edwards, D.; Batley, J. In silico integration of disease resistance QTL, genes and markers with the Brassica juncea physical map. Mol. Breed. 2022, 42, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Izzah, N.K.; Jayakodi, M.; Perumal, S.; Joh, H.J.; Lee, H.J.; Lee, S.-C.; Park, J.Y.; Yang, K.-W.; Nou, I.-S.; et al. Genome-wide SNP identification and QTL mapping for black rot resistance in cabbage. BMC Plant Biol. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, M.J.; Hossain, M.R.; Park, J.-I.; Kim, H.-T.; Robin, A.H.K.; Natarajan, S.; Biswas, M.K.; Jung, H.-J.; Nou, I.-S. In silico characterization and expression of disease-resistance-related genes within the collinear region of Brassica napus blackleg resistant locus LepR1 in B. oleracea. J. Gen. Plant Pathol. 2020, 86, 442–456. [Google Scholar] [CrossRef]
- Hossain, M.R.; Ferdous, M.J.; Park, J.I.; Robin, A.H.K.; Natarajan, S.; Jung, H.-J.; Kim, H.-T.; Nou, I.-S. In-silico identification and differential expression of putative disease resistance-related genes within the collinear region of Brassica napus blackleg resistance locus LepR2′ in Brassica oleracea. Hortic. Environ. Biotechnol. 2020, 61, 879–890. [Google Scholar] [CrossRef]
- Ferdous, M.J.; Hossain, M.R.; Park, J.-I.; Robin, A.H.K.; Natarajan, S.; Jesse, D.M.I.; Jung, H.-J.; Kim, H.-T.; Nou, I.-S. In-silico identification and differential expressions of LepR4-syntenic disease resistance-related domain containing genes against blackleg causal fungus Leptosphaeria maculans in Brassica oleracea. Gene Rep. 2020, 19, 100598. [Google Scholar] [CrossRef]
- Ferdous, M.J.; Hossain, M.R.; Park, J.-I.; Robin, A.H.K.; Jesse, D.M.I.; Jung, H.-J.; Kim, H.-T.; Nou, I.-S. Inheritance pattern and molecular markers for resistance to blackleg disease in cabbage. Plants 2019, 8, 583. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Lamara, M.; Wei, Y.; Hu, H.; Parkin, I.A.P.; Gossen, B.D.; Peng, G.; Yu, F. Clubroot resistance gene Rcr6 in Brassica nigra resides in a genomic region homologous to chromosome A08 in B. rapa. BMC Plant Biol. 2019, 19, 224. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Saad, N.S.M.; Ibrahim, M.I.; Bayer, P.E.; Neik, T.X.; Severn-Ellis, A.A.; Pradhan, A.; Tirnaz, S.; Edwards, D.; Batley, J. Candidate Rlm6 resistance genes against Leptosphaeria maculans identified through a genome-wide association study in Brassica juncea (L.) Czern. Theor. Appl. Genet. 2021, 134, 2035–2050. [Google Scholar] [CrossRef] [PubMed]
- Raman, H.; Raman, R.; Qiu, Y.; Zhang, Y.; Batley, J.; Liu, S. The Rlm13 gene, a new player of Brassica napus–Leptosphaeria maculans interaction maps on chromosome C03 in canola. Front. Plant Sci. 2021, 12, 654604. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Bayer, P.E.; Golicz, A.A.; Tirnaz, S.; Chan, C.K.K.; Edwards, D.; Batley, J. Variation in abundance of predicted resistance genes in the Brassica oleracea pangenome. Plant Biotechnol. J. 2019, 17, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.H.; Bleecker, A.B. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003, 132, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003, 15, 809–834. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.H.; Karlowski, W.M.; Pan, R.; Tzeng, Y.H.; Mayer, K.F.; Li, W.H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004, 16, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, Y.; Chen, J.Q.; Araki, H.; Jing, Z.; Jiang, K.; Shen, J.; Tian, D. Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol. Genet. Genom. 2004, 271, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Fritz-Laylin, L.K.; Krishnamurthy, N.; Tör, M.; Sjölander, K.V.; Jones, J.D. Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis. Plant Physiol. 2005, 138, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, W.; Feng, J.; Luo, H.; Pi, M.; Liu, Z.; Kang, C. Genome re-annotation of the wild strawberry Fragaria vesca using extensive Illumina- and SMRT-based RNA-seq datasets. DNA Res. 2018, 25, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Bayer, P.E.; Tirnaz, S.; Edwards, D.; Batley, J. Genome-wide identification and evolution of receptor-like kinases (RLKs) and receptor-like proteins (RLPs) in Brassica juncea. Biology 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Larkan, N.J.; Ma, L.; Haddadi, P.; Buchwaldt, M.; Parkin, I.A.P.; Djavaheri, M.; Borhan, M.H. The Brassica napus wall-associated kinase-like (WAKL) gene Rlm9 provides race-specific blackleg resistance. Plant J. 2020, 104, 892–900. [Google Scholar] [CrossRef] [PubMed]
- McHale, L.; Tan, X.; Koehl, P.; Michelmore, R.W. Plant NBS-LRR proteins: Adaptable guards. Genome Biol. 2006, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Salman, A.; Guo, C.; Yu, J.; Cao, S.; Gao, X.; Li, W.; Li, H.; Guo, Y. Identification and characterization of LRR-RLK family genes in potato reveal their involvement in peptide signaling of cell fate decisions and biotic/abiotic stress responses. Cells 2018, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Rai, K.M.; Balasubramanian, V.K.; Upadhyay, S.K.; Luo, H.; Mendu, V. Genome-wide identification and characterization of LRR-RLKs reveal functional conservation of the SIF subfamily in cotton (Gossypium hirsutum). BMC Plant Biol. 2018, 18, 185. [Google Scholar] [CrossRef] [PubMed]
- Shumayla Sharma, S.; Kumar, R.; Mendu, V.; Singh, K.; Upadhyay, S.K. Genomic dissection and expression profiling revealed functional divergence in Triticum aestivum leucine rich repeat receptor like kinases (TaLRRKs). Front. Plant Sci. 2016, 7, 1374. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, G.L. Genome-wide identification, characterization and phylogenetic analysis of the rice LRR-kinases. PLoS ONE 2011, 6, e16079. [Google Scholar] [CrossRef] [PubMed]
- Tomé, F.; Nägele, T.; Adamo, M.; Garg, A.; Marco-Llorca, C.; Nukarinen, E.; Pedrotti, L.; Peviani, A.; Simeunovic, A.; Tatkiewicz, A.; et al. The low energy signaling network. Front. Plant Sci. 2014, 5, 353. [Google Scholar] [CrossRef] [PubMed]
- Narusaka, M.; Shirasu, K.; Noutoshi, Y.; Kubo, Y.; Shiraishi, T.; Iwabuchi, M.; Narusaka, Y. RRS1 and RPS4 provide a dual resistance-gene system against fungal and bacterial pathogens. Plant J. 2009, 60, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, M.; Wang, Y.; Yuan, W.; Zhang, H. Plant NLRs: Evolving with pathogen effectors and engineerable to improve resistance. Front. Microbiol. 2022, 13, 1018504. [Google Scholar] [CrossRef] [PubMed]
- Blommaert, J. Genome size evolution: Towards new model systems for old questions. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201441. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Giordano, F.; Ning, Z. Oxford Nanopore MinION sequencing and genome assembly. Genom. Proteom. Bioinform. 2016, 14, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.L.; Jockusch, E.L. Jumping genomic gigantism. Nat. Ecol. Evol. 2018, 2, 1687–1688. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-H. (Ed.) Chapter 5—The Genome. In Diagnostic Molecular Biology; Academic Press: Cambridge, MA, USA, 2019; pp. 117–141. [Google Scholar]
- Gebhardt, C.; Valkonen, J.P. Organization of genes controlling disease resistance in the potato genome. Annu. Rev. Phytopathol. 2001, 39, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Ponce, O.; Ramirez, M.; Mostajo, N.; Orjeda, G. Genome-wide identification and mapping of NBS-encoding resistance genes in Solanum tuberosum group Phureja. PLoS ONE 2012, 7, e34775. [Google Scholar] [CrossRef] [PubMed]
- Ameline-Torregrosa, C.; Wang, B.B.; O’Bleness, M.S.; Deshpande, S.; Zhu, H.; Roe, B.; Young, N.D.; Cannon, S.B. Identification and characterization of nucleotide-binding site-leucine-rich repeat genes in the model plant Medicago truncatula. Plant Physiol. 2008, 146, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tehrim, S.; Zhang, F.; Tong, C.; Huang, J.; Cheng, X.; Dong, C.; Zhou, Y.; Qin, R.; Hua, W.; et al. Genome-wide comparative analysis of NBS-encoding genes between Brassica species and Arabidopsis thaliana. BMC Genom. 2014, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Golicz, A.A.; Bayer, P.E.; Barker, G.C.; Edger, P.P.; Kim, H.; Martinez, P.A.; Chan, C.K.K.; Severn-Ellis, A.; McCombie, W.R.; Parkin, I.A.; et al. The pangenome of an agronomically important crop plant Brassica oleracea. Nat. Commun. 2016, 7, 13390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Shao, Z.Q.; Wang, Q.; Hang, Y.Y.; Xue, J.Y.; Wang, B.; Chen, J.Q. Uncovering the dynamic evolution of nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes in Brassicaceae. J. Integr. Plant Biol. 2016, 58, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadian, A.; Bayer, P.E.; Tirnaz, S.; Hurgobin, B.; Edwards, D.; Batley, J. Characterisation of disease resistance genes in the Brassica napus pangenome reveals significant structural variation. Plant Biotechnol. J. 2020, 18, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Alamery, S.; Tirnaz, S.; Bayer, P.; Tollenaere, R.; Chaloub, B.; Edwards, D.; Batley, J. Genome-wide identification and comparative analysis of NBS-LRR resistance genes in Brassica napus. Crop Pasture Sci. 2018, 69, 72–93. [Google Scholar] [CrossRef]
- Ma, Y.; Chhapekar, S.S.; Lu, L.; Oh, S.; Singh, S.; Kim, C.S.; Kim, S.; Choi, G.J.; Lim, Y.P.; Choi, S.R. Genome-wide identification and characterization of NBS-encoding genes in Raphanus sativus L. and their roles related to Fusarium oxysporum resistance. BMC Plant Biol. 2021, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Katagiri, S.; Kanamori, H.; Mukai, Y.; Sasaki, T.; Matsumoto, T.; Wu, J. Evolutionary dynamics and impacts of chromosome regions carrying R-gene clusters in rice. Sci. Rep. 2020, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Benbow, H.R.; Ślęczka-Brady, K.; Malla, K.B.; Doohan, F.M. Analysis of the chromosomal clustering of Fusarium-responsive wheat genes uncovers new players in the defense against head blight disease. Sci. Rep. 2021, 11, 7446. [Google Scholar] [CrossRef] [PubMed]
- van Wersch, S.; Li, X. Stronger when together: Clustering of plant NLR disease resistance genes. Trends Plant Sci. 2019, 24, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.H.; Webb, C.A.; Smith, S.M.; Sun, Q. Resistance gene complexes: Evolution and utilization. Annu. Rev. Phytopathol. 2001, 39, 285–312. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, A.C.; Fonseca, F.C.D.A.; Cotta, M.G.; Alves, G.S.C.; Miller, R.N.G. Plant NLR receptor proteins and their potential in the development of durable genetic resistance to biotic stresses. Biotechnol. Res. Innov. 2019, 3, 80–94. [Google Scholar] [CrossRef]
- Sanseverino, W.; Hermoso, A.; D’aLessandro, R.; Vlasova, A.; Andolfo, G.; Frusciante, L.; Lowy, E.; Roma, G.; Ercolano, M.R. PRGdb 2.0: Towards a community-based database model for the analysis of R-genes in plants. Nucleic Acids Res. 2013, 41, D1167–D1171. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.S.; Michelmore, R.W. Arabidopsis thaliana genes encoding defense signaling and recognition proteins exhibit contrasting evolutionary dynamics. Genetics 2009, 181, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wei, Y.; Xiao, D.; Gong, K.; Sun, P.; Ren, Y.; Yuan, J.; Wu, T.; Yang, Q.; Li, X.; et al. Brassica carinata genome characterization clarifies U’s triangle model of evolution and polyploidy in Brassica. Plant Physiol. 2021, 186, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.S.; Takahashi, H.; Miyazaki, A.; Hamamoto, H.; Yamaguchi, I.; Kusano, T.; Shah, J. Up-regulation of Arabidopsis thaliana NHL10 in the hypersensitive response to cucumber mosaic virus infection and in senescing leaves is controlled by signalling pathways that differ in salicylate involvement. Planta 2004, 218, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, G.; Zhou, J.M. Receptor kinases in plant-pathogen interactions: More than pattern recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Chen, J.; Outram, M.A. Pathogen perception and signaling in plant immunity. Plant Cell 2024, 36, 1465–1481. [Google Scholar] [CrossRef] [PubMed]
- Kibby, E.M.; Conte, A.N.; Burroughs, A.M.; Nagy, T.A.; Vargas, J.A.; Whalen, L.A.; Aravind, L.; Whiteley, A.T. Bacterial NLR-related proteins protect against phage. Cell 2023, 186, 2410–2424. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, Z.; Wu, C.-H.; Ding, P. A comparative overview of the intracellular guardians of plants and animals: NLRs in innate immunity and beyond. Annu. Rev. Plant Biol. 2021, 72, 155–184. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, J.; Sakai, T.; Adachi, H.; Kamoun, S. RefPlantNLR is a comprehensive collection of experimentally validated plant disease resistance proteins from the NLR family. PLoS Biol. 2021, 19, e3001124. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.; Dickerman, A.; Michelmore, R.; Sivaramakrishnan, S.; Sobral, B.; Young, N. Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant J. 1999, 20, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Larkan, N.J.; Lydiate, D.J.; Parkin, I.A.P.; Nelson, M.N.; Epp, D.J.; Cowling, W.A.; Rimmer, S.R.; Borhan, M.H. The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytol. 2013, 197, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Larkan, N.J.; Ma, L.; Borhan, M.H. The Brassica napus receptor-like protein RLM2 is encoded by a second allele of the LepR3/Rlm2 blackleg resistance locus. Plant Biotechnol. J. 2015, 13, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Borhan, M.H. The receptor-like kinase SOBIR1 interacts with Brassica napus LepR3 and is required for Leptosphaeria maculans AvrLm1-triggered immunity. Front. Plant Sci. 2015, 6, 933. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Zipfel, C. Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; VandenLangenberg, K.; Wehner, T.C.; Weng, Y. QTLs for downy mildew resistance and their association with LRR-RLK/RLP resistance gene homologs in cucumber. In Cucurbitaceae; Michigan State University: East Lansing, MI, USA, 2014. [Google Scholar]

| QTL | Species | Disease | Chromosome (QTL Coordinates) | Reference |
|---|---|---|---|---|
| A7 | Bna | BL | A08 (9,926,520–14,644,781) | [33] |
| A8.dy09 | Bna | BL | A08 (9,514,104–15,735,553) | |
| A9.dy05 | Bna | BL | A09 (5,371,869–20,078,473) | |
| C6.dy13 | Bna | BL | C06 (13,138,327–20,245,793) | |
| C8 | Bna | BL | C08 (23,938,650–35,120,753) | |
| CRQTL-GN_2 | Bna | CR | C03 (1,185,066–2,835,468) | |
| Dw12 | Bna | SSR | C02 (3,710,868–6,707,092) | |
| Dw16 | Bna | SSR | C06 (28,554,990–35,465,622) | |
| Dw3 | Bna | SSR | A03 (15,547,362–16,064,878) | |
| LepR1 | Bna | BL | A02 (11,756,829–18,651,329) | |
| LRA9-1 | Bna | SSR | A09 (22,050,676–23,688,607) | |
| LRA9-2 | Bna | SSR | A09 (22,050,676–22,586,868) | |
| LRC5 | Bna | SSR | C04 (3,065,391–7,930,616) | |
| qFR10-1 | Bna | SSR | C02 (1,027,454–3,174,971) | |
| qFR10-2 | Bna | SSR | C02 (1,099,275–6,816,020) | |
| qFR11-1 | Bna | SSR | A09 (29,166,099–29,361,292) | |
| qFR11-3 | Bna | SSR | C02 (1,027,454–3,174,971) | |
| qSR10-1 | Bna | SSR | A02 (1,610,851–7,705,833) | |
| qSR10-2 | Bna | SSR | A03 (3,131,828–6,786,833) | |
| qSR10-3 | Bna | SSR | C02 (1,027,454–3,953,336) | |
| qSR11-1 | Bna | SSR | A09 (27,128,147–28,071,597) | |
| qSR11-2 | Bna | SSR | C02 (1,027,454–3,953,336) | |
| Rlm3 | Bna | BL | A07 (15,120,000–16,290,000) | |
| SCR-C6 | Bna | CR | C06 (25,090,000–26,220,000) | |
| Sll14a | Bna | SSR | C04 (396,764–9,418,160) | |
| Sll14b | Bna | SSR | C04 (11,691,778–28,720,453) | |
| Sll2 | Bna | SSR | A02 (32,458–3,454,175) | |
| SRA1 | Bna | SSR | A01 (12,444,829–19,857,639) | |
| SRA2-1 | Bna | SSR | A02 (16,670,964–20,474,897) | |
| SRA2-2 | Bna | SSR | A02 (21,084,362–24,719,312) | |
| SRA6 | Bna | SSR | A06 (20,965,425–23,324,273) | |
| SRA8 | Bna | SSR | A08 (7,467,851–8,338,138) | |
| SRA9-1 | Bna | SSR | A09 (22,586,748–26,573,318) | |
| SRC6-1 | Bna | SSR | C06 (30,278,840–34,585,422) | |
| SRC6-2 | Bna | SSR | C06 (30,278,840–34,585,422) | |
| SRC7 | Bna | SSR | C07 (29,634,609–31,761,057) | |
| TS A09 | Bna | BL | A09 (24,341,296–25,991,630) | |
| AcB1-A4.1 | Bju | WR | A04 (9,446,467–11,808,704) | [34] |
| AcB1-A5.1 | Bju | WR | A05 (3,795,221–6,894,070) | |
| AcB1-A5.1 | Bju | WR | B06 (4,226,533–7,156,115) | |
| BjCHI1 | Bju | HR | A03 (9,353,574–21,355,565) | |
| LMJR1 | Bju | BL | B03 (498,805–10,675,185) | |
| LMJR2 | Bju | BL | B08 (1–21,282,056) | |
| PhR2 | Bju | BL | A08 (21,485,767–24,843,799) | |
| PhR2 | Bju | BL | B03 (1,554,162–4,778,538) | |
| BRQTL-C1_1 | Bol | BR | C01 (14,884,502–16,579,946) | [35] |
| BRQTL-C1_2 | Bol | BR | C01 (18,227,386–37,119,290) | |
| BRQTL-C3 | Bol | BR | C03 (19,714,632–22,846,644) | |
| BRQTL-C6 | Bol | BR | C06 (7,423,787–10,466,894) | |
| LepR1 | Bol | BL | C02 (23,420,917–39,667,823) | [36] |
| LepR2 | Bol | BL | C09 (36,661,274–41,215,564) | [37] |
| LepR4 | Bol | BL | C03 (35,912,191–49,368,477) | [38] |
| Rlm1 | Bol | BL | C06 (20,455,085–36,165,661) | [39] |
| Rcr6 | Bni | CR | B03 (6,100,000–6,600,000) | [40] |
| Rlm6 | Bju | BL | A07 (28,140,000–28,631,000) | [41] |
| Rlm6 | Bju | BL | B04 (19,804,000–22,303,000) | |
| Rlm13 | Bna | BL | C03 (2,573,230–5,711,418) | [42] |
| Species | Position (Mbp) | RLK | LRR-RLK | LysM-RLK | Other Receptor | RLP | LRR-RLP | LysM-RLP | Other Receptor | TM-CC | TNL | CNL | RNL | TX | TN | NL | RN | CN | OTHER | RPW8 | Total | RGA/Mbp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. nigra | Chr01 (54.73) | 74 | 28 | 4 | 42 | 10 | 10 | 0 | 0 | 24 | 9 | 0 | 0 | 4 | 3 | 3 | 0 | 0 | 2 | 2 | 131 | 2.39 |
| Chr02 (73.74) | 147 | 57 | 0 | 90 | 23 | 23 | 0 | 0 | 53 | 20 | 6 | 3 | 16 | 5 | 8 | 2 | 2 | 4 | 4 | 293 | 3.97 | |
| Chr03 (59.02) | 117 | 44 | 0 | 73 | 17 | 17 | 0 | 0 | 40 | 22 | 8 | 0 | 3 | 9 | 4 | 2 | 2 | 6 | 0 | 230 | 3.89 | |
| Chr04 (51.41) | 87 | 38 | 1 | 48 | 28 | 27 | 1 | 0 | 30 | 35 | 13 | 0 | 23 | 5 | 5 | 2 | 4 | 4 | 1 | 237 | 4.60 | |
| Chr05 (67.89) | 132 | 47 | 0 | 85 | 29 | 28 | 1 | 0 | 29 | 7 | 3 | 1 | 12 | 6 | 11 | 2 | 1 | 3 | 1 | 237 | 3.49 | |
| Chr06 (61.87) | 83 | 37 | 0 | 46 | 22 | 22 | 0 | 0 | 30 | 3 | 4 | 2 | 4 | 0 | 2 | 1 | 1 | 2 | 0 | 154 | 2.48 | |
| Chr07 (59.87) | 61 | 33 | 1 | 27 | 17 | 17 | 0 | 0 | 32 | 4 | 1 | 3 | 3 | 1 | 1 | 0 | 3 | 2 | 0 | 128 | 2.13 | |
| Chr08 (71.98) | 118 | 39 | 0 | 79 | 15 | 15 | 0 | 0 | 30 | 19 | 2 | 0 | 3 | 1 | 3 | 0 | 2 | 7 | 3 | 203 | 2.82 | |
| Contig 005 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 011 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 013 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 032 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 041 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 048 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 067 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 158 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 193 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 296 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 323 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 353 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Total | 821 | 325 | 6 | 490 | 164 | 162 | 2 | 0 | 272 | 119 | 37 | 9 | 71 | 30 | 37 | 9 | 15 | 30 | 11 | 1625 | ||
| S. arvensis | Chr01 (46.40) | 75 | 23 | 2 | 50 | 13 | 13 | 0 | 0 | 20 | 10 | 1 | 0 | 7 | 1 | 0 | 0 | 0 | 1 | 1 | 129 | 2.78 |
| Chr02 (43.41) | 124 | 45 | 1 | 78 | 15 | 15 | 0 | 0 | 38 | 14 | 4 | 1 | 13 | 4 | 3 | 2 | 1 | 3 | 0 | 222 | 5.11 | |
| Chr03 (37.28) | 65 | 28 | 1 | 36 | 23 | 23 | 0 | 0 | 26 | 11 | 5 | 0 | 5 | 8 | 0 | 0 | 0 | 4 | 0 | 147 | 3.51 | |
| Chr04 (47.78) | 91 | 34 | 0 | 57 | 13 | 13 | 0 | 0 | 28 | 26 | 0 | 0 | 5 | 6 | 3 | 1 | 2 | 3 | 4 | 182 | 3.80 | |
| Chr05 (52.17) | 68 | 35 | 0 | 33 | 16 | 16 | 0 | 0 | 31 | 39 | 9 | 5 | 28 | 7 | 15 | 1 | 13 | 3 | 1 | 236 | 4.52 | |
| Chr06 (49.85) | 125 | 39 | 0 | 86 | 27 | 26 | 1 | 0 | 33 | 14 | 3 | 0 | 4 | 5 | 3 | 1 | 0 | 3 | 2 | 220 | 4.41 | |
| Chr07 (49.15) | 86 | 38 | 0 | 48 | 16 | 16 | 0 | 0 | 30 | 3 | 9 | 2 | 3 | 3 | 6 | 1 | 1 | 2 | 0 | 162 | 3.29 | |
| Chr08 (42.09) | 104 | 33 | 1 | 70 | 11 | 11 | 0 | 0 | 33 | 8 | 5 | 0 | 1 | 1 | 3 | 2 | 3 | 2 | 1 | 174 | 4.13 | |
| Chr09 (36.73) | 80 | 25 | 0 | 55 | 20 | 19 | 1 | 0 | 25 | 7 | 5 | 0 | 3 | 4 | 0 | 0 | 2 | 1 | 3 | 150 | 4.08 | |
| Contig 357 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 358 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 452 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Total | 818 | 300 | 5 | 513 | 155 | 153 | 2 | 0 | 266 | 132 | 41 | 8 | 69 | 39 | 33 | 8 | 22 | 22 | 12 | 1625 | ||
| S. alba | Chr01 (57.15) | 55 | 19 | 2 | 34 | 7 | 7 | 0 | 0 | 17 | 3 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 86 | 1.50 |
| Chr02 (31.68) | 55 | 28 | 1 | 26 | 11 | 11 | 0 | 0 | 35 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 108 | 3.40 | |
| Chr03 (31.00) | 78 | 21 | 0 | 57 | 0 | 0 | 0 | 0 | 15 | 3 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 101 | 3.25 | |
| Chr04 (32.85) | 62 | 31 | 0 | 31 | 2 | 2 | 0 | 0 | 33 | 6 | 1 | 0 | 11 | 1 | 4 | 2 | 1 | 2 | 3 | 128 | 3.89 | |
| Chr05 (28.50) | 60 | 20 | 1 | 39 | 10 | 9 | 1 | 0 | 12 | 6 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 94 | 3.29 | |
| Chr06 (38.87) | 60 | 22 | 1 | 37 | 7 | 7 | 0 | 0 | 20 | 7 | 1 | 0 | 6 | 4 | 3 | 1 | 1 | 0 | 1 | 111 | 2.85 | |
| Chr07 (29.24) | 65 | 27 | 0 | 38 | 9 | 9 | 0 | 0 | 23 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 107 | 3.65 | |
| Chr08 (32.51) | 48 | 19 | 1 | 28 | 14 | 13 | 1 | 0 | 16 | 2 | 4 | 0 | 4 | 1 | 1 | 0 | 0 | 0 | 0 | 90 | 2.76 | |
| Chr09 (44.42) | 79 | 31 | 0 | 48 | 13 | 12 | 1 | 0 | 18 | 4 | 2 | 0 | 2 | 0 | 1 | 1 | 2 | 1 | 1 | 124 | 2.79 | |
| Chr10 (28.29) | 60 | 24 | 0 | 36 | 8 | 8 | 0 | 0 | 18 | 7 | 0 | 1 | 6 | 2 | 3 | 0 | 2 | 1 | 0 | 108 | 3.81 | |
| Chr11 (30.85) | 48 | 23 | 0 | 25 | 7 | 7 | 0 | 0 | 23 | 3 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 89 | 2.88 | |
| Chr12 (30.69) | 62 | 22 | 0 | 40 | 15 | 15 | 0 | 0 | 17 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 100 | 3.25 | |
| Contig 191 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 373 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Contig 456 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | - | |
| Total | 734 | 287 | 6 | 441 | 104 | 101 | 3 | 0 | 247 | 47 | 15 | 4 | 37 | 13 | 14 | 7 | 11 | 8 | 8 | 1249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolatabadian, A.; Amas, J.C.; Thomas, W.J.W.; Sayari, M.; Al-Mamun, H.A.; Edwards, D.; Batley, J. Exploring Cloned Disease Resistance Gene Homologues and Resistance Gene Analogues in Brassica nigra, Sinapis arvensis, and Sinapis alba: Identification, Characterisation, Distribution, and Evolution. Genes 2025, 16, 849. https://doi.org/10.3390/genes16080849
Dolatabadian A, Amas JC, Thomas WJW, Sayari M, Al-Mamun HA, Edwards D, Batley J. Exploring Cloned Disease Resistance Gene Homologues and Resistance Gene Analogues in Brassica nigra, Sinapis arvensis, and Sinapis alba: Identification, Characterisation, Distribution, and Evolution. Genes. 2025; 16(8):849. https://doi.org/10.3390/genes16080849
Chicago/Turabian StyleDolatabadian, Aria, Junrey C. Amas, William J. W. Thomas, Mohammad Sayari, Hawlader Abdullah Al-Mamun, David Edwards, and Jacqueline Batley. 2025. "Exploring Cloned Disease Resistance Gene Homologues and Resistance Gene Analogues in Brassica nigra, Sinapis arvensis, and Sinapis alba: Identification, Characterisation, Distribution, and Evolution" Genes 16, no. 8: 849. https://doi.org/10.3390/genes16080849
APA StyleDolatabadian, A., Amas, J. C., Thomas, W. J. W., Sayari, M., Al-Mamun, H. A., Edwards, D., & Batley, J. (2025). Exploring Cloned Disease Resistance Gene Homologues and Resistance Gene Analogues in Brassica nigra, Sinapis arvensis, and Sinapis alba: Identification, Characterisation, Distribution, and Evolution. Genes, 16(8), 849. https://doi.org/10.3390/genes16080849










