FST Polymorphisms Associate with Musculoskeletal Traits and Modulate Exercise Response Differentially by Sex and Modality in Northern Han Chinese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Ethics Statement
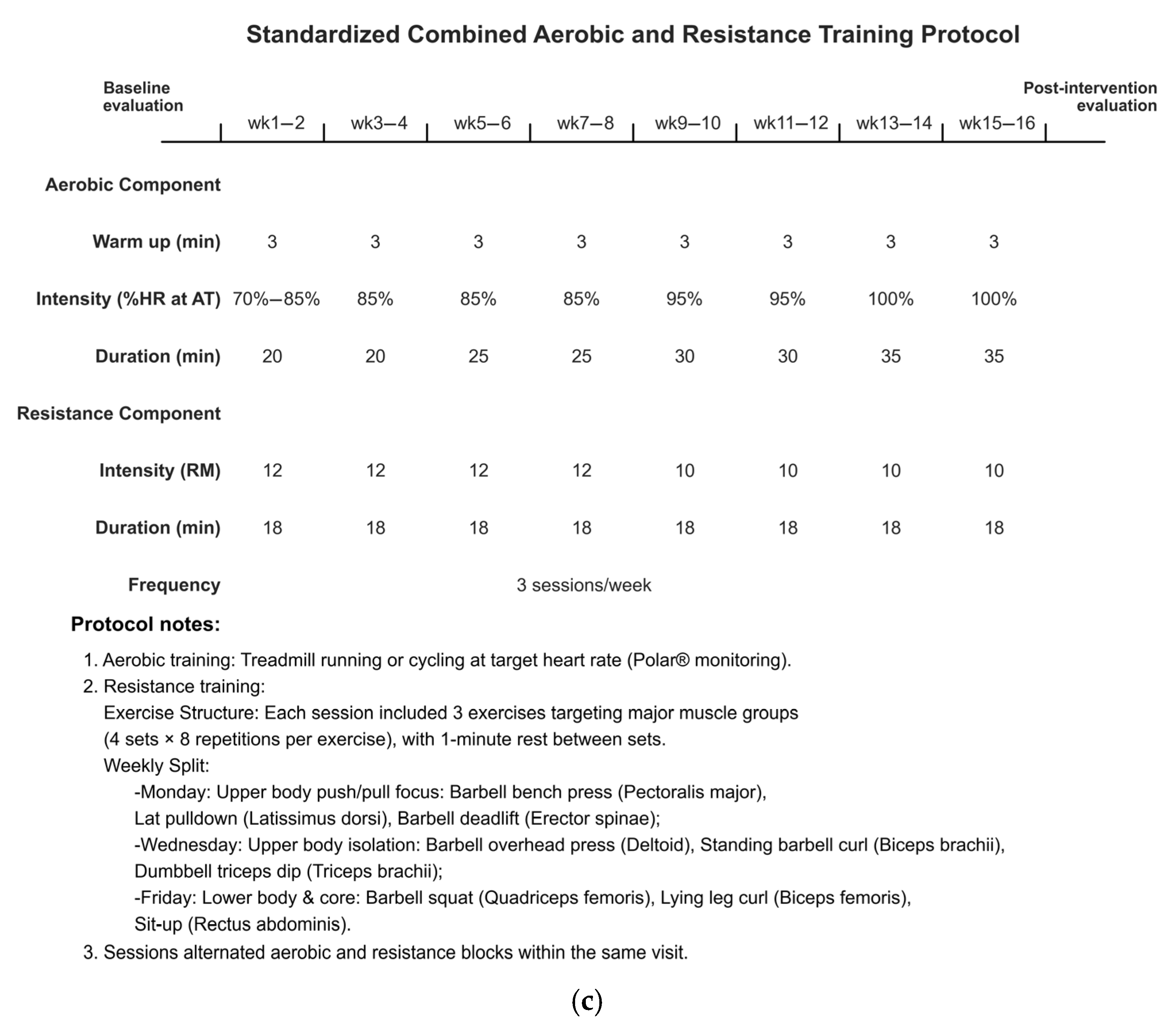
2.3. Exercise Intervention Protocols
2.4. Anaerobic Threshold Testing
2.5. Body Composition Assessment
2.6. DNA Extraction and Genotyping
2.7. Serum Follistatin Measurement
2.8. In Silico Functional Annotation
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
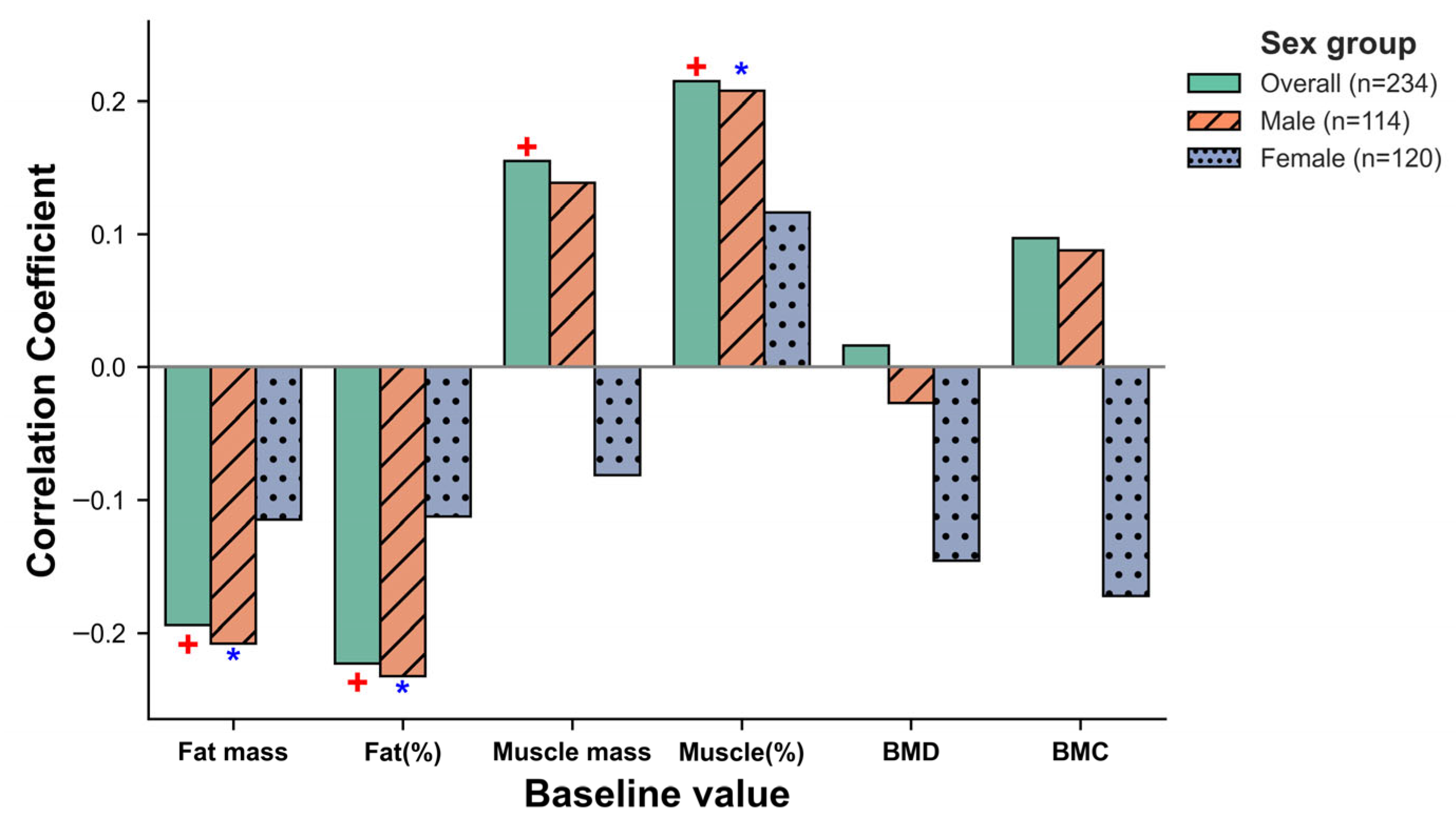
3.2. Associations of FST SNPs with Baseline Body Composition and Bone Mineral Parameters
3.2.1. rs3797297 Association with Baseline Muscle Mass and BMC in Women
3.2.2. Mediation of Muscle Mass in rs3797297 Effect on BMC
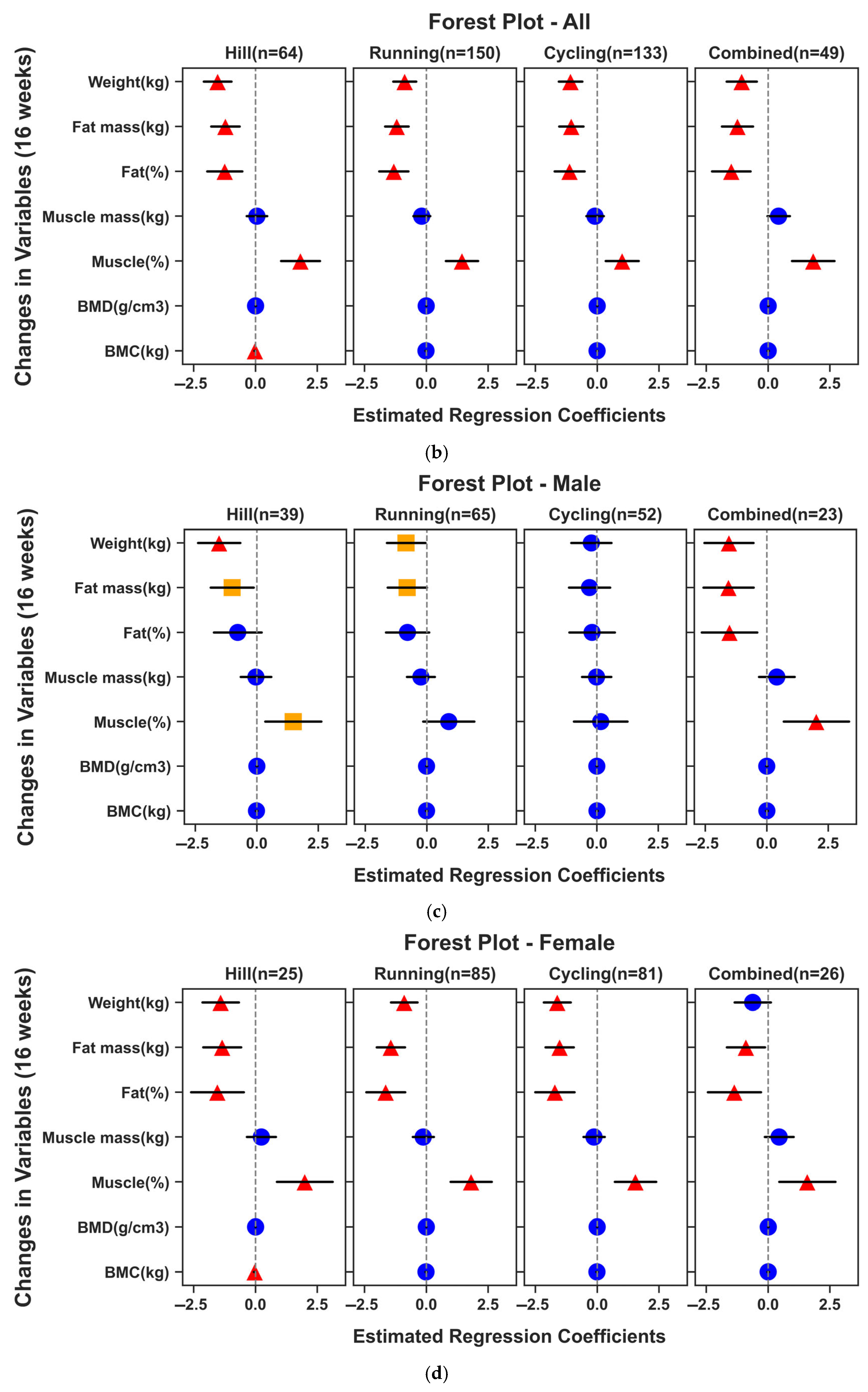
3.3. Effects of the Exercise Intervention on Body Composition and Bone Mineral Parameters
3.4. Interactions of FST SNPs with the 16-Week Exercise Responses
3.5. Follistatin Analysis: Baseline Associations, Exercise-Induced Changes, and Mediation
3.6. In Silico Functional Characterization of rs3797296 and rs3797297
4. Discussion
- (1)
- recruiting broader and more diverse cohorts to enhance generalizability and provide independent external validation of genetic associations;
- (2)
- implementing precisely standardized exercise interventions with objective dose monitoring to enable direct efficacy comparisons and dose–response modeling;
- (3)
- comprehensively assessing and controlling for a wider array of confounding factors like diet, sleep, daily physical activity levels, and stress; and
- (4)
- integrating multi-omics data (e.g., epigenomics, transcriptomics, proteomics) alongside genetic information to gain deeper insights into the complex biological pathways underlying gene–exercise interactions. Ultimately, validating these preliminary findings in larger, independent studies is essential to translate genetic insights into practical, personalized exercise recommendations for optimizing musculoskeletal health. It is crucial to note that while genetic insights can refine individual exercise strategies, regular physical activity remains a cornerstone for musculoskeletal health for everyone, irrespective of their genetic predisposition.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.H.; Keum, N.; Hu, F.B.; Orav, E.J.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Predicted Lean Body Mass, Fat Mass, and All Cause and Cause Specific Mortality in Men: Prospective US Cohort Study. BMJ 2018, 362, k2575. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, X.Q.; Yu, Y.F.; Yu, B.W.; Sun, Y.; Tan, X.; Wang, B.; Lu, Y.L.; Wang, N.J. Revisiting the Obesity Paradox: Lean Body Mass, Fat Mass, and Mortality in Patients with Atrial Fibrillation. Eur. Heart J. 2024, 45, ehae666.2892. [Google Scholar] [CrossRef]
- Zhou, H.-H.; Liao, Y.; Peng, Z.; Liu, F.; Wang, Q.; Yang, W. Association of Muscle Wasting with Mortality Risk among Adults: A Systematic Review and Meta-Analysis of Prospective Studies. J. Cachexia Sarcopenia Muscle 2023, 14, 1596–1612. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Park, R.; Oh, C.-M.; Moon, S. Association of Low Muscle Mass and Obesity with Increased All-Cause and Cardiovascular Disease Mortality in US Adults. J. Cachexia Sarcopenia Muscle 2024, 15, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Liu, Q.; Jiang, Z.; Zhou, H.; Wang, Q. Global Burden of Low Bone Mineral Density Related Fractures in Pre- and Post-Menopausal Women from 1990 to 2021, with Projections to 2050. Osteoporos. Int. 2025. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Beaudart, C.; Bruyère, O.; Abrahamsen, B.; Al-Daghri, N.; Burlet, N.; Chandran, M.; Rosa, M.M.; Cortet, B.; Demonceau, C.; et al. Evidence-Based Guideline for the Management of Osteoporosis in Men. Nat. Rev. Rheumatol. 2024, 20, 241–251. [Google Scholar] [CrossRef]
- Brooke-Wavell, K.; Skelton, D.A.; Barker, K.L.; Clark, E.M.; De Biase, S.; Arnold, S.; Paskins, Z.; Robinson, K.R.; Lewis, R.M.; Tobias, J.H.; et al. Strong, Steady and Straight: UK Consensus Statement on Physical Activity and Exercise for Osteoporosis. Br. J. Sports Med. 2022, 56, 837–846. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, R.; Xie, L.; Hu, F. Comparative Efficacy of Exercise Training Modes on Systemic Metabolic Health in Adults with Overweight and Obesity: A Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2023, 14, 1294362. [Google Scholar] [CrossRef]
- Thomaes, T.; Thomis, M.; Onkelinx, S.; Goetschalckx, K.; Fagard, R.; Lambrechts, D.; Vanhees, L. Genetic Predisposition Scores Associate with Muscular Strength, Size, and Trainability. Med. Sci. Sports Exerc. 2013, 45, 1451–1459. [Google Scholar] [CrossRef][Green Version]
- Barber, J.L.; Ruiz-Ramie, J.J.; Robbins, J.M.; Gerszten, R.E.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Bouchard, C.; Sarzynski, M.A. Regular Exercise and Patterns of Response across Multiple Cardiometabolic Traits: The HERITAGE Family Study. Br. J. Sports Med. 2022, 56, 95–100. [Google Scholar] [CrossRef]
- Tynkkynen, N.P.; Törmäkangas, T.; Palviainen, T.; Hyvärinen, M.; Klevjer, M.; Joensuu, L.; Kujala, U.; Kaprio, J.; Bye, A.; Sillanpää, E. Associations of Polygenic Inheritance of Physical Activity with Aerobic Fitness, Cardiometabolic Risk Factors and Diseases: The HUNT Study. Eur. J. Epidemiol. 2023, 38, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.J.; Atkinson, G.; Batterham, A.M. Inter-Individual Responses of Maximal Oxygen Uptake to Exercise Training: A Critical Review. Sports Med. 2017, 47, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.J. Limits to the Evidence That DNA Sequence Differences Contribute to Variability in Fitness and Trainability. Med. Sci. Sports Exerc. 2019, 51, 1786–1789. [Google Scholar] [CrossRef]
- Batrakoulis, A.; Jamurtas, A.Z.; Metsios, G.S.; Perivoliotis, K.; Liguori, G.; Feito, Y.; Riebe, D.; Thompson, W.R.; Angelopoulos, T.J.; Krustrup, P.; et al. Comparative Efficacy of 5 Exercise Types on Cardiometabolic Health in Overweight and Obese Adults: A Systematic Review and Network Meta-Analysis of 81 Randomized Controlled Trials. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e008243. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, J.; Liu, Y.; Zhou, Y. Effects of Different Exercise Modalities and Intensities on Body Composition in Overweight and Obese Children and Adolescents: A Systematic Review and Network Meta-Analysis. Front. Physiol. 2023, 14, 1193223. [Google Scholar] [CrossRef]
- Mallinson, J.E.; Taylor, T.; Constantin-Teodosiu, D.; Billeter-Clark, R.; Constantin, D.; Franchi, M.V.; Narici, M.V.; Auer, D.; Greenhaff, P.L. Longitudinal Hypertrophic and Transcriptional Responses to High-Load Eccentric-Concentric vs Concentric Training in Males. Scand. J. Med. Sci. Sports 2020, 30, 2101–2115. [Google Scholar] [CrossRef]
- Rector, R.S.; Rogers, R.; Ruebel, M.; Widzer, M.O.; Hinton, P.S. Lean Body Mass and Weight-Bearing Activity in the Prediction of Bone Mineral Density in Physically Active Men. J. Strength Cond. Res. 2009, 23, 427–435. [Google Scholar] [CrossRef]
- Rector, R.S.; Rogers, R.; Ruebel, M.; Hinton, P.S. Participation in Road Cycling vs Running Is Associated with Lower Bone Mineral Density in Men. Metabolism 2008, 57, 226–232. [Google Scholar] [CrossRef]
- Bouamra, M.; Zouhal, H.; Ratel, S.; Makhlouf, I.; Bezrati, I.; Chtara, M.; Behm, D.G.; Granacher, U.; Chaouachi, A. Concurrent Training Promotes Greater Gains on Body Composition and Components of Physical Fitness Than Single-Mode Training (Endurance or Resistance) in Youth with Obesity. Front. Physiol. 2022, 13, 869063. [Google Scholar] [CrossRef]
- Tao, R.; Stöhr, O.; Wang, C.; Qiu, W.; Copps, K.D.; White, M.F. Hepatic Follistatin Increases Basal Metabolic Rate and Attenuates Diet-Induced Obesity during Hepatic Insulin Resistance. Mol. Metab. 2023, 71, 101703. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Rodopaios, N.E.; Makras, P.; Kumar, A.; Kalra, B.; Mantzoros, C.S. Activins, Follistatins and Inhibins in Postmenopausal Osteoporosis: A Proof of Concept, Case-Control Study. Metabolism 2023, 141, 155397. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Kim, N.-K.; Lee, S.-H.; Eom, J.-H.; Lee, Y.; Park, J.-C.; Woo, K.M.; Baek, J.-H.; Kim, J.-E.; Ryoo, H.-M.; et al. GDF11 Promotes Osteogenesis as Opposed to MSTN, and Follistatin, a MSTN/GDF11 Inhibitor, Increases Muscle Mass but Weakens Bone. Proc. Natl. Acad. Sci. USA 2020, 117, 4910–4920. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Schirwis, E.; Blaauw, B.; Bortolanza, S.; Zhao, J.; Enzo, E.; Stantzou, A.; Mouisel, E.; Toniolo, L.; Ferry, A.; et al. BMP Signaling Controls Muscle Mass. Nat. Genet. 2013, 45, 1309–1318. [Google Scholar] [CrossRef]
- Qian, S.; Tang, Y.; Tang, Q.-Q. Adipose Tissue Plasticity and the Pleiotropic Roles of BMP Signaling. J. Biol. Chem. 2021, 296, 100678. [Google Scholar] [CrossRef]
- Pervin, S.; Reddy, S.T.; Singh, R. Novel Roles of Follistatin/Myostatin in Transforming Growth Factor-β Signaling and Adipose Browning: Potential for Therapeutic Intervention in Obesity Related Metabolic Disorders. Front. Endocrinol. 2021, 12, 653179. [Google Scholar] [CrossRef]
- Liu, Y.; Lehar, A.; Rydzik, R.; Chandok, H.; Lee, Y.-S.; Youngstrom, D.W.; George, J.; Matzuk, M.M.; Germain-Lee, E.L.; Lee, S.-J. Local versus Systemic Control of Bone and Skeletal Muscle Mass by Components of the Transforming Growth Factor-β Signaling Pathway. Proc. Natl. Acad. Sci. USA 2021, 118, e2111401118. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.-F.; Liu, Y.-Z.; Yang, X.-L.; Zhang, H.; Feng, G.-J.; Wei, X.-T.; Zhang, L. The Genetic Architecture of Appendicular Lean Mass Characterized by Association Analysis in the UK Biobank Study. Commun. Biol. 2020, 3, 608. [Google Scholar] [CrossRef] [PubMed]
- He, L. Genetic and DNA Methylation Markers of Ageing Muscle. Ph.D. Thesis, Manchester Metropolitan University in Collaboration with Katholieke Universiteit Leuven, Manchester, UK, 2020. [Google Scholar]
- Tian, Y.; He, Z.; Xu, C.; Huang, C.; Lee, J.-H.; Li, R.; Zhou, J.; Zhao, J.; Wang, M.; Hong, P.; et al. Energy Expenditure and Fitness Response Following Once Weekly Hill Climbing at Low Altitude. Int. J. Sports Med. 2015, 36, 357–364. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A New Method for Detecting Anaerobic Threshold by Gas Exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; Abecasis, G.R. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and Visualization of LD and Haplotype Maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Farrell, C.M.; Feldgarden, M.; Fine, A.M.; Funk, K.; et al. Database Resources of the National Center for Biotechnology Information in 2023. Nucleic Acids Res. 2023, 51, D29–D38. [Google Scholar] [CrossRef]
- Machiela, M.J.; Chanock, S.J. LDlink: A Web-Based Application for Exploring Population-Specific Haplotype Structure and Linking Correlated Alleles of Possible Functional Variants. Bioinformatics 2015, 31, 3555–3557. [Google Scholar] [CrossRef] [PubMed]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of Published Genome-Wide Association Studies, Targeted Arrays and Summary Statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving Access to Variant Interpretations and Supporting Evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Ghoussaini, M.; Mountjoy, E.; Carmona, M.; Peat, G.; Schmidt, E.M.; Hercules, A.; Fumis, L.; Miranda, A.; Carvalho-Silva, D.; Buniello, A.; et al. Open Targets Genetics: Systematic Identification of Trait-Associated Genes Using Large-Scale Genetics and Functional Genomics. Nucleic Acids Res. 2021, 49, D1311–D1320. [Google Scholar] [CrossRef]
- The GTEx Consortium. The GTEx Consortium Atlas of Genetic Regulatory Effects across Human Tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Ward, L.D.; Kellis, M. HaploReg v4: Systematic Mining of Putative Causal Variants, Cell Types, Regulators and Target Genes for Human Complex Traits and Disease. Nucleic Acids Res. 2016, 44, D877–D881. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of Functional Variation in Personal Genomes Using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef]
- Jiang, B.-C.; Ling, Y.-J.; Xu, M.-L.; Gu, J.; Wu, X.-B.; Sha, W.-L.; Tian, T.; Bai, X.-H.; Li, N.; Jiang, C.-Y.; et al. Follistatin Drives Neuropathic Pain in Mice through IGF1R Signaling in Nociceptive Neurons. Sci. Transl. Med. 2024, 16, eadi1564. [Google Scholar] [CrossRef]
- Kostek, M.A.; Angelopoulos, T.J.; Clarkson, P.M.; Gordon, P.M.; Moyna, N.M.; Visich, P.S.; Zoeller, R.F.; Price, T.B.; Seip, R.L.; Thompson, P.D.; et al. Myostatin and Follistatin Polymorphisms Interact with Muscle Phenotypes and Ethnicity. Med. Sci. Sports Exerc. 2009, 41, 1063–1071. [Google Scholar] [CrossRef]
- Amthor, H.; Nicholas, G.; McKinnell, I.; Kemp, C.F.; Sharma, M.; Kambadur, R.; Patel, K. Follistatin Complexes Myostatin and Antagonises Myostatin-Mediated Inhibition of Myogenesis. Dev. Biol. 2004, 270, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Skouvaklidou, E.C.; Kynigopoulos, G.; Saridakis, Z.G.; Apostolou, A.; Triantafyllou, G.A.; Karagiozoglou-Lampoudi, T.; Mantzoros, C.S. Circulating Follistatin Displays a Day-Night Rhythm and Is Associated with Muscle Mass and Circulating Leptin Levels in Healthy, Young Humans. Metabolism 2016, 65, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Sun, J.; Li, Y.; Zhao, J.; Shen, J. Association between Adiposity and Bone Mineral Density in Adults: Insights from a National Survey Analysis. Nutrients 2023, 15, 3492. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, M.; Liu, J.; Tong, L.; Ding, W. Impact of High Fat and Low Lean Mass Phenotype on Bone Mineral Content: A Cross-Sectional Study of Chinese Adolescent Population. Bone 2024, 186, 117170. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, K.; Guan, Q.; Guo, Q.; Zhao, C. Mechanism and Physical Activities in Bone-Skeletal Muscle Crosstalk. Front. Endocrinol. 2024, 14, 1287972. [Google Scholar] [CrossRef]
- Maïmoun, L.; Mura, T.; Attalin, V.; Dupuy, A.M.; Cristol, J.-P.; Avignon, A.; Mariano-Goulart, D.; Sultan, A. Modification of Muscle-Related Hormones in Women with Obesity: Potential Impact on Bone Metabolism. J. Clin. Med. 2020, 9, 1150. [Google Scholar] [CrossRef]
- Jürimäe, J.; Remmel, L.; Tamm, A.-L.; Purge, P.; Maasalu, K.; Tillmann, V. Follistatin Is Associated with Bone Mineral Density in Lean Adolescent Girls with Increased Physical Activity. Children 2023, 10, 1226. [Google Scholar] [CrossRef]
- Tang, R.; Harasymowicz, N.S.; Wu, C.-L.; Collins, K.H.; Choi, Y.-R.; Oswald, S.J.; Guilak, F. Gene Therapy for Follistatin Mitigates Systemic Metabolic Inflammation and Post-Traumatic Arthritis in High-Fat Diet-Induced Obesity. Sci. Adv. 2020, 6, eaaz7492. [Google Scholar] [CrossRef]
- Bellicha, A.; van Baak, M.A.; Battista, F.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Dicker, D.; Encantado, J.; Ermolao, A.; et al. Effect of Exercise Training on Weight Loss, Body Composition Changes, and Weight Maintenance in Adults with Overweight or Obesity: An Overview of 12 Systematic Reviews and 149 Studies. Obes. Rev. 2021, 22 (Suppl. S4), e13256. [Google Scholar] [CrossRef] [PubMed]
- Oppert, J.-M.; Ciangura, C.; Bellicha, A. Physical Activity and Exercise for Weight Loss and Maintenance in People Living with Obesity. Rev. Endocr. Metab. Disord. 2023, 24, 937–949. [Google Scholar] [CrossRef]
- Ji, H.; Gulati, M.; Huang, T.Y.; Kwan, A.C.; Ouyang, D.; Ebinger, J.E.; Casaletto, K.; Moreau, K.L.; Skali, H.; Cheng, S. Sex Differences in Association of Physical Activity with All-Cause and Cardiovascular Mortality. J. Am. Coll. Cardiol. 2024, 83, 783–793. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine/Adipokine Response to “Aerobic” Exercise: Is It Just a Matter of Exercise Load? Front. Physiol. 2019, 10, 691. [Google Scholar] [CrossRef] [PubMed]
- Moqaddam, M.A.; Nemati, M.; Dara, M.M.; Hoteit, M.; Sadek, Z.; Ramezani, A.; Rand, M.K.; Abbassi-Daloii, A.; Pashaei, Z.; Almaqhawi, A.; et al. Exploring the Impact of Astaxanthin Supplementation in Conjunction with a 12-Week CrossFit Training Regimen on Selected Adipo-Myokines Levels in Obese Males. Nutrients 2024, 16, 2857. [Google Scholar] [CrossRef]
- Perakakis, N.; Mougios, V.; Fatouros, I.; Siopi, A.; Draganidis, D.; Peradze, N.; Ghaly, W.; Mantzoros, C.S. Physiology of Activins/Follistatins: Associations with Metabolic and Anthropometric Variables and Response to Exercise. J. Clin. Endocrinol. Metab. 2018, 103, 3890–3899. [Google Scholar] [CrossRef]
- Yin, Z.; Agip, A.-N.A.; Bridges, H.R.; Hirst, J. Structural Insights into Respiratory Complex I Deficiency and Assembly from the Mitochondrial Disease-Related Ndufs4-/- Mouse. EMBO J. 2024, 43, 225–249. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, M.; Zhou, B.; Yoshii, A.; Bugg, D.; Villet, O.; Sahu, A.; Olson, G.S.; Davis, J.; Tian, R. Mitochondrial Dysfunction in Macrophages Promotes Inflammation and Suppresses Repair after Myocardial Infarction. J. Clin. Investig. 2023, 133, e159498. [Google Scholar] [CrossRef]
- Peng, Y.; Jia, L.; Hu, X.; Shi, X.; Fang, X.; Qiu, Y.; Gan, Z.; Wang, Y. Cellular Feimin Enhances Exercise Performance by Suppressing Muscle Thermogenesis. Nat. Metab. 2025, 7, 84–101. [Google Scholar] [CrossRef]


| Total (n = 470) | Male (n = 208) | Female (n = 262) | |
|---|---|---|---|
| Age (years) a | 37 (23–50) | 33.5 (22–49) | 40 (24–50) |
| Height (cm) b | 165.68 ± 7.95 | 172.14 ± 5.63 | 160.55 ± 5.36 *** |
| Weight (kg) b | 63.82 ± 11.36 | 71.31 ± 10.60 | 57.88 ± 7.92 *** |
| BMI (kg/m2) b | 23.21 ± 3.25 | 24.06 ± 3.35 | 22.53 ± 3.01 *** |
| Waist circumference b | 81.32 ± 9.94 | 84.64 ± 9.95 | 78.67 ± 9.12 *** |
| Hip circumference b | 93.42 ± 5.85 | 94.18 ± 6.10 | 92.81 ± 5.58 * |
| Waist-Hip ratio b | 0.87 ± 0.07 | 0.90 ± 0.07 | 0.85 ± 0.07 *** |
| Fat mass (kg) | 17.22 (12.43–22.00) | 15.66 (9.15–20.99) | 17.92 (14.43–22.93) ### |
| Fat (%) | 27.74 (21.32–33.65) | 22.41 (14.05–27.60) | 31.79 (27.00–36.61) ### |
| Muscle mass (kg) | 40.75 (36.17–51.44) | 52.48 (48.75–56.54) | 36.54 (34.30–38.77) ### |
| Muscle (%) | 67.75 (62.11–74.24) | 72.83 (68.47–81.40) | 63.35 (58.93–68.53) ### |
| BMD (g/cm3) | 1.15 ± 0.10 | 1.20 ± 0.09 | 1.11 ± 0.08 *** |
| BMC (kg) | 2.56 (2.24–2.90) | 2.91 (2.67–3.15) | 2.33 (2.11–2.55) ### |
| Chromosome (Forward Strand) | ID | Sex | Allele | MAF | Genotype: Frequency (Count) | χ2 | P_HWE | |
|---|---|---|---|---|---|---|---|---|
| 5: 52780353 | rs12152850 | Total | C/T | T: 0.020 | CC: 0.960 (451) | CT: 0.040 (19) | 0.002 | 0.964 |
| Male | C/T | T: 0.019 | CC: 0.962 (200) | CT: 0.038 (8) | 0.001 | 0.978 | ||
| Female | C/T | T: 0.021 | CC: 0.958 (251) | CT: 0.042 (11) | 0.001 | 0.971 | ||
| 5: 52777721 | rs3797296 | Total | A/G | G: 0.188 | AA: 0.655 (308) | AG/GG: 0.345 (162) | 1.922 | 0.166 |
| Male | A/G | G: 0.151 | AA: 0.721 (150) | AG/GG: 0.279 (58) | 0.409 | 0.522 | ||
| Female | A/G | G: 0.218 | AA: 0.603 (158) | AG/GG: 0.397 (104) | 1.770 | 0.183 | ||
| 5: 52777656 | rs3797297 | Total | G/T | T: 0.124 | GG: 0.770 (362) | GT/TT: 0.230 (108) | 0.483 | 0.487 |
| Male | G/T | T: 0.161 | GG: 0.726 (151) | GT/TT: 0.274 (57) | 0.386 | 0.535 | ||
| Female | G/T | T: 0.103 | GG: 0.805 (211) | GT/TT: 0.195 (51) | 0.156 | 0.693 | ||
| Males (n = 208) | Females (n = 262) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs3797297 | rs3797297 | |||||||||
| GG (n = 151) | GT/TT (n = 57) | Beta (95% CI) | P | P_adj | GG (n = 211) | GT/TT (n = 51) | Beta (95% CI) | P | P_adj | |
| Fat mass (kg) | 15.28 (9.14, 20.78) | 16.34 (9.52, 21.34) | −0.714 (−1.719, 0.290) | 0.163 | 0.259 | 17.58 (14.01, 21.91) | 21.06 (15.66, 24.28) | 0.606 (−0.173, 1.384) | 0.127 | 0.206 |
| Fat (%) | 22.42 (14.00, 27.60) | 22.40 (14.66, 27.30) | −0.662 (−2.049, 0.725) | 0.349 | 0.463 | 31.40 (26.57, 36.49) | 33.66 (30.09, 38.00) | 0.080 (−1.058, 1.218) | 0.890 | 0.963 |
| Muscle mass (kg) | 52.54 ± 5.21 | 52.61 ± 5.74 | 0.155 (−1.257, 1.567) | 0.830 | 0.905 | 36.28 ± 3.33 | 37.62 ± 3.62 | 1.159 (0.202, 2.116) | 0.018 | 0.034 † |
| Muscle (%) | 72.55 (68.58, 81.56) | 73.07 (68.36, 81.30) | 0.559 (−0.858, 1.977) | 0.439 | 0.550 | 63.67 (59.25, 68.68) | 62.13 (57.64, 65.72) | −0.046 (−1.192, 1.100) | 0.937 | 0.963 |
| BMD (g/cm3) | 1.20 ± 0.09 | 1.19 ± 0.07 | −0.011 (−0.035, 0.014) | 0.390 | 0.501 | 1.10 ± 0.08 | 1.13 ± 0.09 | 0.024 (0.001, 0.047) | 0.040 | 0.074 |
| BMC (kg) | 2.94 ± 0.41 | 2.86 ± 0.31 | −0.071 (−0.173 0.032) | 0.176 | 0.270 | 2.30 ± 0.30 | 2.43 ± 0.34 | 0.127 (0.039, 0.215) | 0.005 | 0.009 † |
| Mediation Effect | Beta (95% CI) | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| Total effect | 0.106 | 0.016 | 0.198 | 0.021 |
| Indirect effect | 0.056 | 0.005 | 0.113 | 0.043 |
| Direct effect | 0.050 | −0.023 | 0.123 | 0.181 |
| Males | Females | |||||
|---|---|---|---|---|---|---|
| AA (n = 29) | AG/GG (n = 10) | AA (n = 17) | AG/GG (n = 8) | |||
| Beta (95% CI) | P | P_adj | Beta (95% CI) | P | P_adj | |
| Fat mass (kg) | 0.173 (−0.807, 1.153) | 0.729 | 0.921 | −0.284 (−1.469, 0.900) | 0.638 | 0.868 |
| Fat (%) | 0.038 (−1.013, 1.089) | 0.944 | 0.980 | 0.301 (−1.083, 1.685) | 0.670 | 0.886 |
| Muscle mass (kg) | 0.161 (−0.729, 1.052) | 0.723 | 0.921 | −1.126 (−1.767, −0.485) | 0.001 | 0.034 † |
| Muscle (%) | −0.213 (−1.658, 1.233) | 0.773 | 0.938 | −0.451 (−2.025, 1.123) | 0.575 | 0.844 |
| BMD (g/cm3) | −0.008 (−0.027, 0.012) | 0.442 | 0.785 | −0.010 (−0.025, 0.005) | 0.180 | 0.658 |
| BMC (kg) | 0.015 (−0.030, 0.060) | 0.523 | 0.833 | 0.014 (−0.038, 0.066) | 0.594 | 0.854 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Gu, Z.; Fu, R.; Chen, Y.; He, Y.; Yang, R.; Yang, X.; He, Z. FST Polymorphisms Associate with Musculoskeletal Traits and Modulate Exercise Response Differentially by Sex and Modality in Northern Han Chinese Adults. Genes 2025, 16, 810. https://doi.org/10.3390/genes16070810
Cao W, Gu Z, Fu R, Chen Y, He Y, Yang R, Yang X, He Z. FST Polymorphisms Associate with Musculoskeletal Traits and Modulate Exercise Response Differentially by Sex and Modality in Northern Han Chinese Adults. Genes. 2025; 16(7):810. https://doi.org/10.3390/genes16070810
Chicago/Turabian StyleCao, Wei, Zhuangzhuang Gu, Ronghua Fu, Yiru Chen, Yong He, Rui Yang, Xiaolin Yang, and Zihong He. 2025. "FST Polymorphisms Associate with Musculoskeletal Traits and Modulate Exercise Response Differentially by Sex and Modality in Northern Han Chinese Adults" Genes 16, no. 7: 810. https://doi.org/10.3390/genes16070810
APA StyleCao, W., Gu, Z., Fu, R., Chen, Y., He, Y., Yang, R., Yang, X., & He, Z. (2025). FST Polymorphisms Associate with Musculoskeletal Traits and Modulate Exercise Response Differentially by Sex and Modality in Northern Han Chinese Adults. Genes, 16(7), 810. https://doi.org/10.3390/genes16070810





