Exploring Concomitant Ophthalmic Comorbidities in Portuguese Patients with Inherited Retinal Diseases: A Comprehensive Clinical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical and Demographical Data
2.2. Statistical Analysis
3. Results
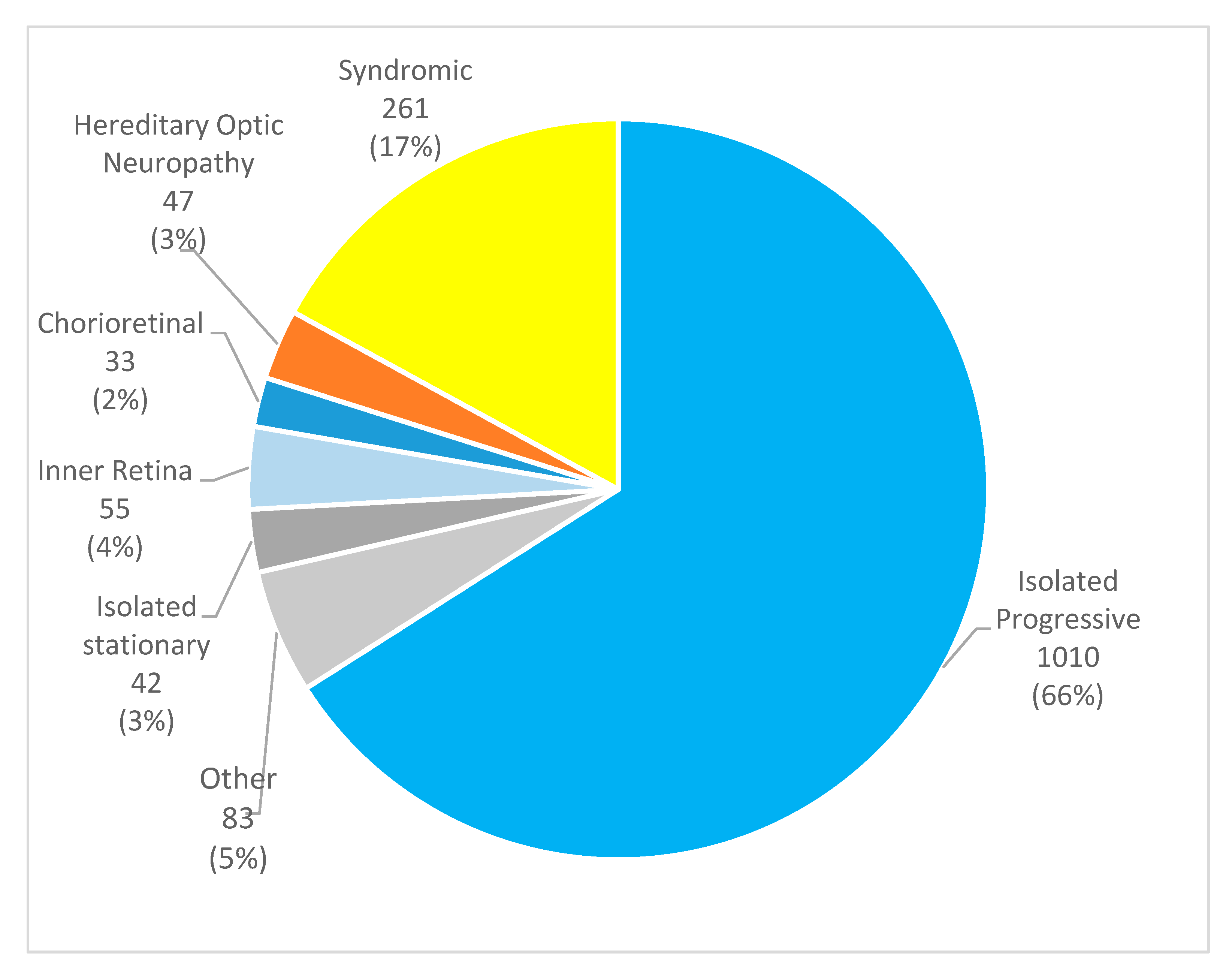
3.1. Clinical and Demographical Characteristics
3.2. Isolated Progressive Retinal Disorders
3.3. Isolated Stationary Retinal Disorders
3.4. Syndromic Retinal Disorders
3.5. Inner Retina and/or Vitreoretinal Dystrophies
3.6. Chorioretinal Disorders
3.7. Hereditary Optic Neuropathy
3.8. Other
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Autosomal Dominant |
| AR | Autosomal Recessive |
| ASD | Anterior Segment Dysgenesis |
| BBS | Bardet–Biedl Syndrome |
| BD | Best Disease |
| CNV | Choroidal Neovascularization |
| CME | Cystoid Macular Edema |
| CSNB | Congenital Stationary Night Blindness |
| ERM | Epiretinal Membrane |
| ICD | International Classification of Diseases |
| IRD | Inherited Retinal Dystrophy |
| PXE | Pseudoxanthoma Elasticum |
| RD | Retinal Detachment |
| RP | Retinitis Pigmentosa |
| STGD | Stargardt Disease |
| ULS | Unidade Local de Saúde |
| XL | X-Linked |
References
- Bowling, B. Kanski’s Clinical Ophthalmology, 8th ed.; Elsevier Ltd.: London, UK, 2016. [Google Scholar]
- Hanany, M.; Shalom, S.; Ben-Yosef, T.; Sharon, D. Comparison of Worldwide Disease Prevalence and Genetic Prevalence of Inherited Retinal Diseases and Variant Interpretation Considerations. Cold Spring Harb. Perspect. Med. 2024, 14, a041277. [Google Scholar] [CrossRef] [PubMed]
- Retnet. Genes and Mapped Loci Causing Retinal Diseases. Available online: https://retnet.org/disease (accessed on 31 August 2024).
- Woon, P.Y.; Chien, J.Y.; Wang, J.H.; Chou, Y.Y.; Lin, M.C.; Huang, S.P. Prevalence and associated relating factors in patients with hereditary retinal dystrophy: A nationwide population-based study in Taiwan. BMJ Open 2022, 12, e054111. [Google Scholar] [CrossRef] [PubMed]
- Hamel, C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef]
- Broadgate, S.; Yu, J.; Downes, S.M.; Halford, S. Unravelling the genetics of inherited retinal dystrophies: Past, present and future. Prog. Retin. Eye Res. 2017, 59, 53–96. [Google Scholar] [CrossRef] [PubMed]
- Garip, G.; Kamal, A. Systematic review and meta-synthesis of coping with retinitis pigmentosa: Implications for improving quality of life. BMC Ophthalmol. 2019, 19, 181. [Google Scholar] [CrossRef]
- Marques, J.P.; Carvalho, A.L.; Henriques, J.; Murta, J.N.; Saraiva, J.; Silva, R. Design, development and deployment of a web-based interoperable registry for inherited retinal dystrophies in Portugal: The IRD-PT. Orphanet J. Rare Dis. 2020, 15, 304. [Google Scholar] [CrossRef]
- Grupo de Estudos da Retina. Retina Study Group—Sponsor GER—Grupo de Estudos da Retina. Available online: https://www.retina.com.pt/index.aspx (accessed on 1 December 2024).
- Jeffery, R.C.H.; Chen, F.K. Macular neovascularization in inherited retinal diseases: A review. Surv. Ophthalmol. 2024, 69, 1–23. [Google Scholar] [CrossRef]
- Boycott, K.M.; Pearce, W.G.; Bech-Hansen, N.T. Clinical variability among patients with incomplete X-linked congenital stationary night blindness and a founder mutation in CACNA1F. Can. J. Ophthalmol. 2000, 35, 204–213. [Google Scholar] [CrossRef]
- Lee, H.; Purohit, R.; Sheth, V.; McLean, R.J.; Kohl, S.; Leroy, B.P.; Sundaram, V.; Michaelides, M.; Proudlock, F.A.; Gottlob, I. Retinal Development in Infants and Young Children with Achromatopsia. Ophthalmology 2015, 122, 2145–2147. [Google Scholar] [CrossRef]
- Tatour, Y.; Ben-Yosef, T. Syndromic Inherited Retinal Diseases: Genetic, Clinical and Diagnostic Aspects. Diagnostics 2020, 10, 779. [Google Scholar] [CrossRef]
- Edwards, A.O. Clinical features of the congenital vitreoretinopathies. Eye 2008, 22, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Newman, N.J.; Biousse, V. Hereditary optic neuropathies. Eye 2004, 18, 1144–1160. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, L.; Fogliato, G.; Corbelli, E.; Bandello, F.; Codenotti, M. Blind patients in end-stage inherited retinal degeneration: Multimodal imaging of candidates for artificial retinal prosthesis. Eye 2021, 35, 289–298. [Google Scholar] [CrossRef]
- Bakthavatchalam, M.; Lai, F.H.P.; Rong, S.S.; Ng, D.S.; Brelen, M.E. Treatment of cystoid macular edema secondary to retinitis pigmentosa: A systematic review. Surv. Ophthalmol. 2018, 63, 329–339. [Google Scholar] [CrossRef]
- Khojasteh, H.; Riazi-Esfahani, H.; Mirghorbani, M.; Pour, E.K.; Mahmoudi, A.; Mahdizad, Z.; Akhavanrezayat, A.; Ghoraba, H.M.; Do, D.V.; Nguyen, Q.D.M. Cataract surgery in patients with retinitis pigmentosa: Systematic review. J. Cataract Refract. Surg. 2023, 49, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Sergouniotis, P.I.; Maxime, E.; Leroux, D.; Olry, A.; Thompson, R.; Rath, A.; Robinson, P.N.; Dollfus, H. An ontological foundation for ocular phenotypes and rare eye diseases. Orphanet J. Rare Dis. 2019, 14, 8. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Robman, L.; Taylor, H. External factors in the development of cataract. Eye 2005, 19, 1074–1082. [Google Scholar] [CrossRef]
- Knowledge Action Change. Tobacco Smoking in Portugal. Global State of Tobacco Harm Reduction. Available online: https://gsthr.org/countries/profile/PRT/1/ (accessed on 10 June 2025).
- Knowledge Action Change. Tobacco Smoking in Taiwan. Global State of Tobacco Harm Reduction. Available online: https://gsthr.org/countries/profile/twn/1/ (accessed on 10 June 2025).
- Tan, L.; Long, Y.; Li, Z.; Ying, X.; Ren, J.; Sun, C.; Meng, X.; Li, S. Ocular abnormalities in a large patient cohort with retinitis pigmentosa in Western China. BMC Ophthalmol 2021, 21, 43. [Google Scholar] [CrossRef]
- Testa, F.; Rossi, S.; Colucci, R.; Gallo, B.; Di Iorio, V.; della Corte, M.; Azzolini, C.; Melillo, P.; Simonelli, F. Macular abnormalities in Italian patients with retinitis pigmentosa. Br. J. Ophthalmol. 2014, 98, 98946–98950. [Google Scholar] [CrossRef]
- Hong, Y.; Li, H.; Sun, Y.; Ji, Y. A Review of Complicated Cataract in Retinitis Pigmentosa: Pathogenesis and Cataract Surgery. J. Ophthalmol. 2020, 2020, 6699103. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.D.; Georgiou, M.; Alswaiti, Y.; Kabbani, J.; Fujinami, K.; Fujinami-Yokokawa, Y.; Khoda, S.; Mahroo, O.A.; Robson, A.G.; Webster, A.R.; et al. CRB1-Associated Retinal Dystrophies: Genetics, Clinical Characteristics, and Natural History. Am. J. Ophthalmol. 2023, 246, 107–121. [Google Scholar] [CrossRef]
- Bujakowska, K.; Audo, I.; Mohand-Saïd, S.; Lancelot, M.-E.; Antonio, A.; Germain, A.; Léveillard, T.; Letexier, M.; Saraiva, J.-P.; Lonjou, C.; et al. CRB1 mutations in inherited retinal dystrophies. Hum. Mutat. 2012, 33, 306–315. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, A.C.; Marmoy, O.R.; Prise, K.L.; Henderson, R.H.; Thompson, D.A.; Moosajee, M. Expanding the Clinical Spectrum of CRB1-Retinopathies: A Novel Genotype–Phenotype Correlation with Macular Dystrophy and Elevated Intraocular Pressure. Int. J. Mol. Sci. 2025, 26, 2836. [Google Scholar] [CrossRef]
- Martins, E.A.d.S.; Rodrigues, G.D.; Ivama, K.J.; Pereira, M.C.; Texeira, C.H.M.; Amaral, R.A.S.; Sallum, J.M.F. Multimodal imaging in retinitis pigmentosa related to the EYS gene. Arq. Bras. Oftalmol. 2025, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Oishi, A.; Yang, L.; Arno, G.; Pontikos, N.; Yoshitake, K.; Fujinami-Yokokawa, Y.; Liu, X.; Hayashi, T.; Katagiri, S.; et al. Clinical and genetic characteristics of 10 Japanese patients with PROM1-associated retinal disorder: A report of the phenotype spectrum and a literature review in the Japanese population. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 656–674. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Yin, J.; Huo, W.; Chaum, E. Prominin-1 interacts with vascular endothelial growth factor and regulates its secretion in human retinal pigment epithelial cells. Invest Ophthalmol. Vis. Sci. 2018, 59, 3999. [Google Scholar]
- Sieving, P.A.; Fishman, G.A. Refractive errors of retinitis pigmentosa patients. Br. J. Ophthalmol. 1978, 62, 163–167. [Google Scholar] [CrossRef]
- Lee, S.H.; Yu, H.G.; Seo, J.M.; Moon, S.W.; Moon, J.W.; Kim, S.J.; Chung, H. Hereditary and clinical features of retinitis pigmentosa in Koreans. J. Korean Med. Sci. 2010, 25, 918–923. [Google Scholar] [CrossRef]
- Testa, F.; Melillo, P.; Rossi, S.; Marcelli, V.; de Benedictis, A.; Colucci, R.; Gallo, B.; Brunetti-Pierri, R.; Donati, S.; Azzolini, C.; et al. Prevalence of macular abnormalities assessed by optical coherence tomography in patients with Usher syndrome. Ophthalmic Genet. 2018, 39, 17–21. [Google Scholar] [CrossRef]
- Castro-Sánchez, S.; Álvarez-Satta, M.; Cortón, M.; Guillén, E.; Ayuso, C.; Valverde, D. Exploring genotype-phenotype relationships in Bardet-Biedl syndrome families. J. Med. Genet. 2015, 52, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Testa, F.; Carreño, E.; Born, L.I.v.D.; Melillo, P.; Perea-Romero, I.; Di Iorio, V.; Risca, G.; Iodice, C.M.; Pennings, R.J.E.; Karali, M.; et al. Multicentric Longitudinal Prospective Study in a European Cohort of MYO7A Patients: Disease Course and Implications for Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 25. [Google Scholar] [CrossRef] [PubMed]
- Romo-Aguas, J.C.; de Guimarães, T.A.C.; Kalitzeos, A.; Aychoua, N.; Tsika, C.; Robson, A.G.; Fujinami-Yokokawa, Y.; Fujinami, K.; Mahroo, O.A.; Webster, A.R.; et al. Detailed Clinical, Ophthalmic, and Genetic Characterization of MYO7A-Associated Usher Syndrome. Investig. Ophthalmol. Vis. Sci. 2025, 66, 60. [Google Scholar] [CrossRef]
- Bartstra, J.W.; Risseeuw, S.; de Jong, P.A.; van Os, B.; Kalsbeek, L.; Mol, C.; Baas, A.F.; Verschuere, S.; Vanakker, O.; Florijn, R.J.; et al. Genotype-phenotype correlation in pseudoxanthoma elasticum. Atherosclerosis 2021, 324, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.D.; Meyer, D.; Xu, S.; Elfervig, J.L. Long-term follow-up of Stargardt’s disease and fundus flavimaculatus. Ophthalmology 1998, 105, 448–457. [Google Scholar] [CrossRef]
- National Eye Institute. 2010 U.S. Age-Specific Prevalence Rates for Cataract by Age, Gender, and Race/Ethnicity. 2020. Available online: https://www.statista.com/statistics/521211/ethnic-groups-with-age-related-eye-diseases-in-us/ (accessed on 3 January 2024).
- Han, I.C.; Jaffe, G.J.; Schuerch, K.; Zanzottera, E.C.; Freund, K.B.; Yannuzzi, L.A. Choroidal neovascularization is common in Best vitelliform macular dystrophy and plays a role in vitelliform lesion evolution. Ophthalmol. Retina 2023, 7, 441–449. [Google Scholar] [CrossRef]
- Chowers, I.; Boon, C.J.F. The Pattern Dystrophies. In Macular Dystrophies; Springer International Publishing: Cham, Switzerland, 2016; pp. 11–23. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Sun, L.; Zhou, X.; Ke, S.; Ding, X. Choroidal neovascularisation secondary to X-linked retinoschisis. Br. J. Ophthalmol. 2024, 108, 1564–1570. [Google Scholar] [CrossRef]
- Naravane, A.V.; Belin, P.J.; Pierce, B.; Quiram, P.A. Risk and prevention of retinal detachments in patients with Stickler syndrome. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 7–11. [Google Scholar] [CrossRef]
- Zhou, Q.; Weis, E.; Ye, M.; Benjaminy, S.; MacDonald, I.M. An internet-based health survey on the co-morbidities of choroideremia patients. Ophthalmic Physiol. Opt. 2013, 33, 157–163. [Google Scholar] [CrossRef]
- Pal, B. A new phenotype of recessively inherited foveal hypoplasia and anterior segment dysgenesis maps to a locus on chromosome 16q23.2-24.2. J. Med. Genet. 2004, 41, 772–777. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.; Yi, Z.; Zheng, Y.; Ouyang, J.; Guo, D.; Li, S.; Xiao, X.; Wang, P.; Sun, W.; Zhang, Q. The Systemic Genotype-Phenotype Characterization of PAX6-Related Eye Disease in 164 Chinese Families. Investig. Ophthalmol. Vis. Sci. 2024, 65, 46. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.; Ferreira, N.; Moreno, N.; Marta, A.; Vaz-Pereira, S.; Estrela-Silva, S.; Costa, J.; Cardoso, A.R.; Neves, P.; Duarte, L.; et al. Current management of inherited retinal degenerations in Portugal (IRD-PT survey). Sci. Rep. 2024, 14, 21473. [Google Scholar] [CrossRef] [PubMed]

| Disease Group | Associated Comorbidities | References |
|---|---|---|
| Isolated progressive IRDs | Cataract, choroidal neovascularization, and refractive error | [5,10] |
| Isolated stationary IRDs | Foveal hypoplasia, strabismus, and myopia | [11,12] |
| Syndromic IRDs | Cataract, strabismus, and ophthalmoplegia | [13] |
| Inner retina and/or vitreoretinal dystrophies | Cataract and retinal detachment | [14] |
| Chorioretinal dystrophies | Choroidal neovascularization | [10] |
| Hereditary optic neuropathies | Cataract, ptosis, and ophthalmoplegia | [15] |
| Characteristics | Results |
|---|---|
| Female, n (%) | 751 (49.1%) |
| Male, n (%) | 780 (50.9%) |
| Age, mean ± SD * (years) | 45.8 ± 19.3 |
| Age at diagnosis, mean ± SD * (years) | 39.4 ± 19.5 |
| Family history, n (%) | 787 (52.9%) |
| Families, n | 1254 |
| Consanguinity, n (%) | 285 (19.4%) |
| Number of follow-ups, mean ± SD * | 4.8 ± 5.6 |
| Visual acuity, mean ± SD * (ETDRS letters) | 46.1 ± 30.1 |
| Ocular Comorbidities | Frequency, n (%) |
|---|---|
| None | 887 (57.9%) |
| Cataract | 324 (21.2%) |
| Amblyopia | 96 (6.3%) |
| High myopia | 90 (5.9%) |
| Glaucoma | 89 (5.8%) |
| Pseudohole | 77 (5.0%) |
| Cystoid macular edema | 63 (4.1%) |
| Vitreomacular traction | 58 (3.8%) |
| High hyperopia | 55 (3.6%) |
| Keratoconus | 50 (3.3%) |
| Anterior segment dysgenesis | 48 (3.1%) |
| Strabismus | 32 (2.1%) |
| Epiretinal membrane | 31 (2.0%) |
| Choroidal neovascularization | 26 (1.7%) |
| Macular hole | 23 (1.5%) |
| Lamellar hole | 20 (1.3%) |
| Retinal detachment | 12 (0.8%) |
| Disease | Sample Size | Associated Findings | Frequency |
|---|---|---|---|
| Stargardt disease | 124 (12.3%) | None | 109 (87.9%) |
| Cataract | 7 (5.6%) | ||
| Amblyopia | 4 (3.2%) | ||
| Strabismus | 2 (1.6%) | ||
| Best disease | 28 (2.7%) | None | 17 (60.7%) |
| Cataract | 5 (17.9%) | ||
| CNV * | 5 (17.9%) | ||
| Unspecified macular dystrophy | 33 (3.3%) | None | 20 (60.6%) |
| Cataract | 6 (18.2%) | ||
| High myopia | 2 (6.1%) | ||
| CNV * | 2 (6.1%) | ||
| Glaucoma | 2 (6.1%) |
| Disease | Sample Size | Associated Findings | Frequency |
|---|---|---|---|
| Usher syndrome | 100 (38.3%) | Cataract | 43 (43.0%) |
| None | 40 (40.0%) | ||
| CME * | 14 (14.0%) | ||
| ERM * | 13 (13.0%) | ||
| Strabismus | 3 (3.0%) | ||
| Glaucoma | 2 (2.0%) | ||
| Other | 2 (2.0%) | ||
| High myopia | 2 (2.0%) | ||
| BBS * | 37 (14.2%) | Cataract | 16 (43.2%) |
| None | 15 (40.5%) | ||
| Strabismus | 4 (10.8%) | ||
| ERM * | 2 (5.4%) | ||
| RD * | 2 (2.7%) | ||
| PXE * | 31 (11.9%) | CNV* | 15 (48.4%) |
| None | 11 (35.5%) | ||
| Cataract | 10 (32.3%) | ||
| Glaucoma | 2 (6.5%) | ||
| Other—RP | 14 (5.4%) | None | 7 (50.0%) |
| Cataract | 3 (21.4%) | ||
| CME * | (14.3%) | ||
| Senior–Løken syndrome | 10 (3.8%) | None | 4 (40.0%) |
| CME * | 3 (30.0%) | ||
| Cataract | 3 (30.0%) | ||
| Syndromic cone-rod/cone | 7 (2.7%) | None | 6 (85.7%) |
| C3 glomerulopathy | 7 (2.7%) | None | 7 (100.0%) |
| NBIA * | 6 (2.3%) | None | 4 (66.7%) |
| Cataract | 2 (33.3%) | ||
| Knobloch syndrome | 6 (2.3%) | High myopia | 4 (66.7%) |
| RD * | 2 (33.3%) | ||
| Strabismus | 2 (33.3%) | ||
| PHARC | 5 (1.9%) | None | 3 (60.0%) |
| Alport syndrome | 5 (1.9%) | None | 3 (60.0%) |
| Other | 2 (40.0%) | ||
| MELAS * | 5 (1.9%) | None | 4 (80.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesquita, R.; Marta, A.; Marques-Couto, P.; Costa, J.; Estrela-Silva, S.; Cabral, D.; Marques, J.P.; Vaz-Pereira, S. Exploring Concomitant Ophthalmic Comorbidities in Portuguese Patients with Inherited Retinal Diseases: A Comprehensive Clinical Study. Genes 2025, 16, 743. https://doi.org/10.3390/genes16070743
Mesquita R, Marta A, Marques-Couto P, Costa J, Estrela-Silva S, Cabral D, Marques JP, Vaz-Pereira S. Exploring Concomitant Ophthalmic Comorbidities in Portuguese Patients with Inherited Retinal Diseases: A Comprehensive Clinical Study. Genes. 2025; 16(7):743. https://doi.org/10.3390/genes16070743
Chicago/Turabian StyleMesquita, Rita, Ana Marta, Pedro Marques-Couto, José Costa, Sérgio Estrela-Silva, Diogo Cabral, João Pedro Marques, and Sara Vaz-Pereira. 2025. "Exploring Concomitant Ophthalmic Comorbidities in Portuguese Patients with Inherited Retinal Diseases: A Comprehensive Clinical Study" Genes 16, no. 7: 743. https://doi.org/10.3390/genes16070743
APA StyleMesquita, R., Marta, A., Marques-Couto, P., Costa, J., Estrela-Silva, S., Cabral, D., Marques, J. P., & Vaz-Pereira, S. (2025). Exploring Concomitant Ophthalmic Comorbidities in Portuguese Patients with Inherited Retinal Diseases: A Comprehensive Clinical Study. Genes, 16(7), 743. https://doi.org/10.3390/genes16070743






