Molecular-Marker-Based Design for Breeding Indica–Japonica Hybrid Rice with Bacterial Blight Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
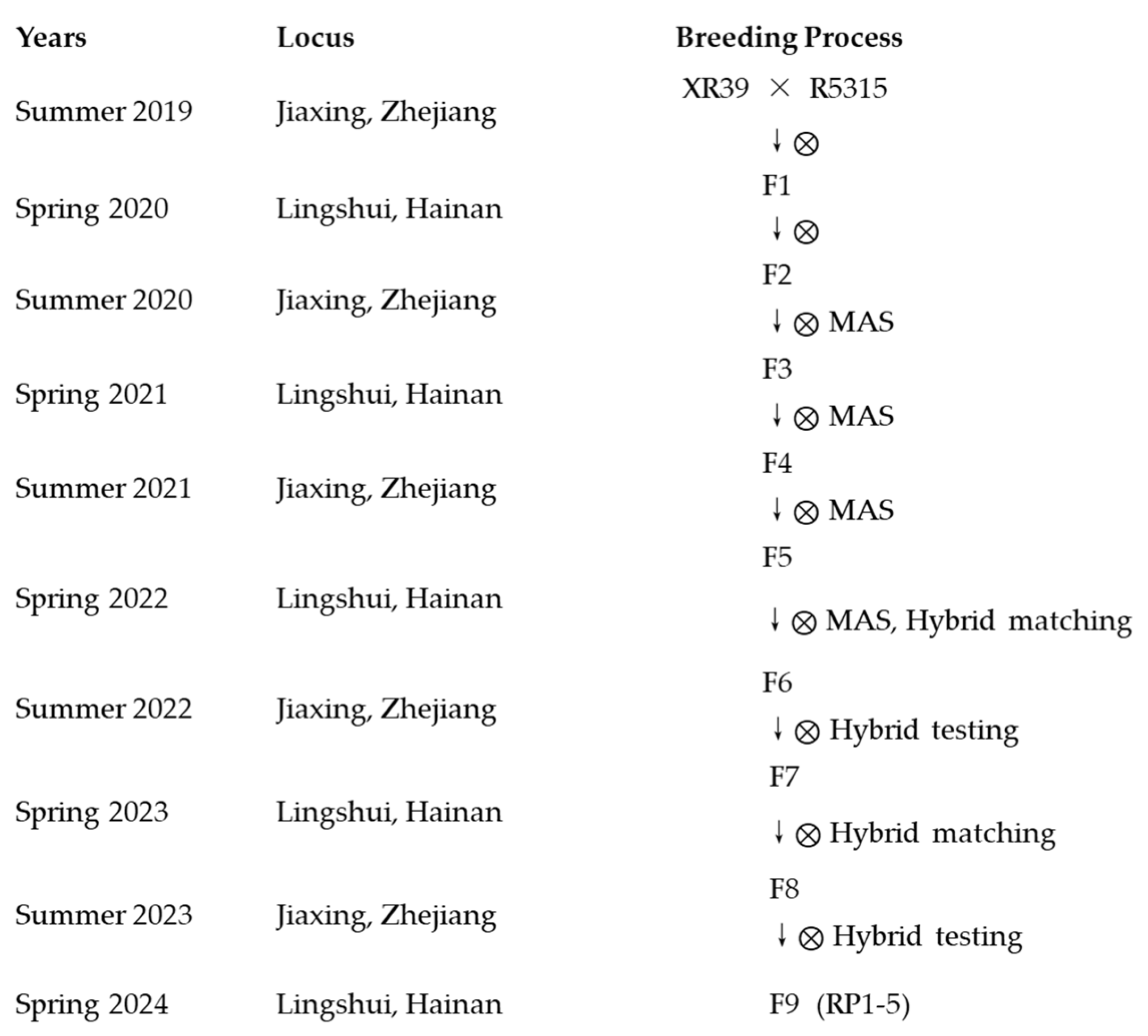
2.2. RP1–5 Breeding Process (Figure 1)
2.2.1. Parental Material Development (2019–2020)
2.2.2. Population Construction and Multi-Generational Selection (2020–2022)
2.2.3. Combining Ability Evaluation (2022–2023)
2.2.4. Line Stabilization and Field Trials (2023–2024)
2.3. Management of Experimental Fields and Evaluation of Agronomic Traits
2.4. Molecular Marker Detection
2.5. Identification of Bacterial Blight Resistance in the Field
2.6. Statistical Analyses
3. Results
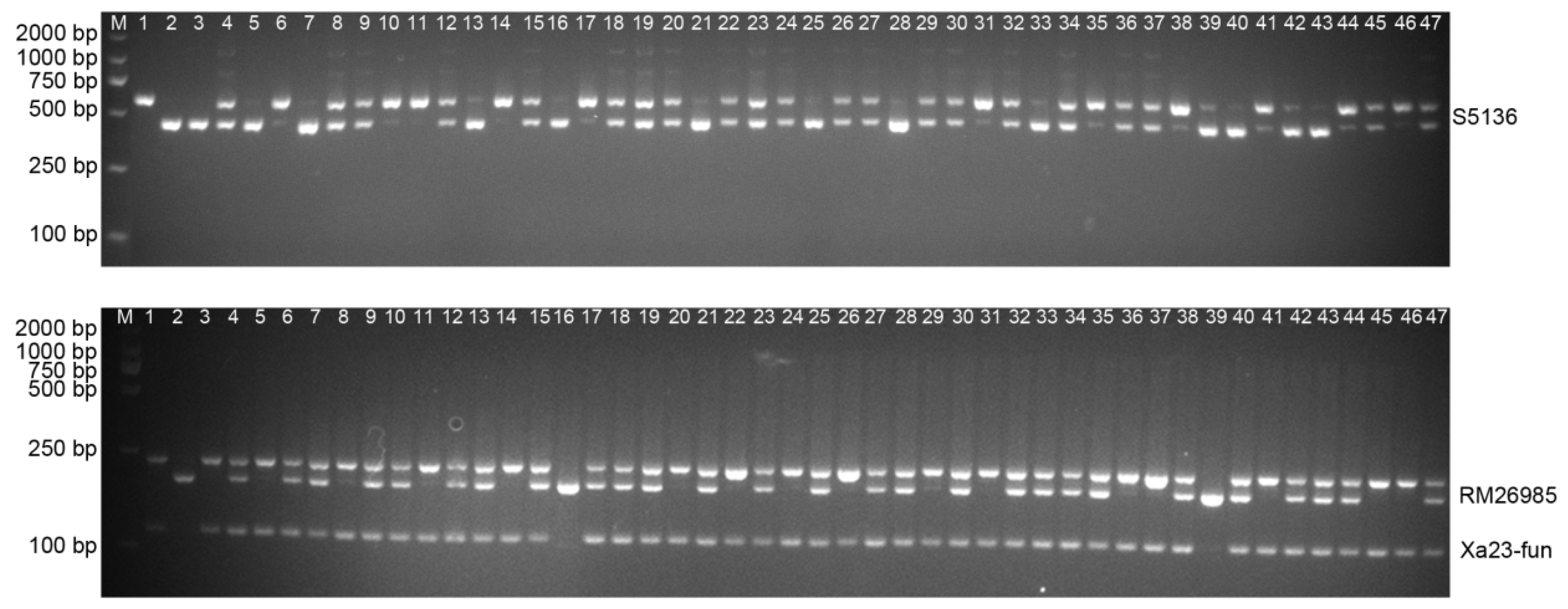
3.1. Molecular Detection of Resistance Gene Xa23 and Wide-Compatibility Gene S5n in F2 Populations
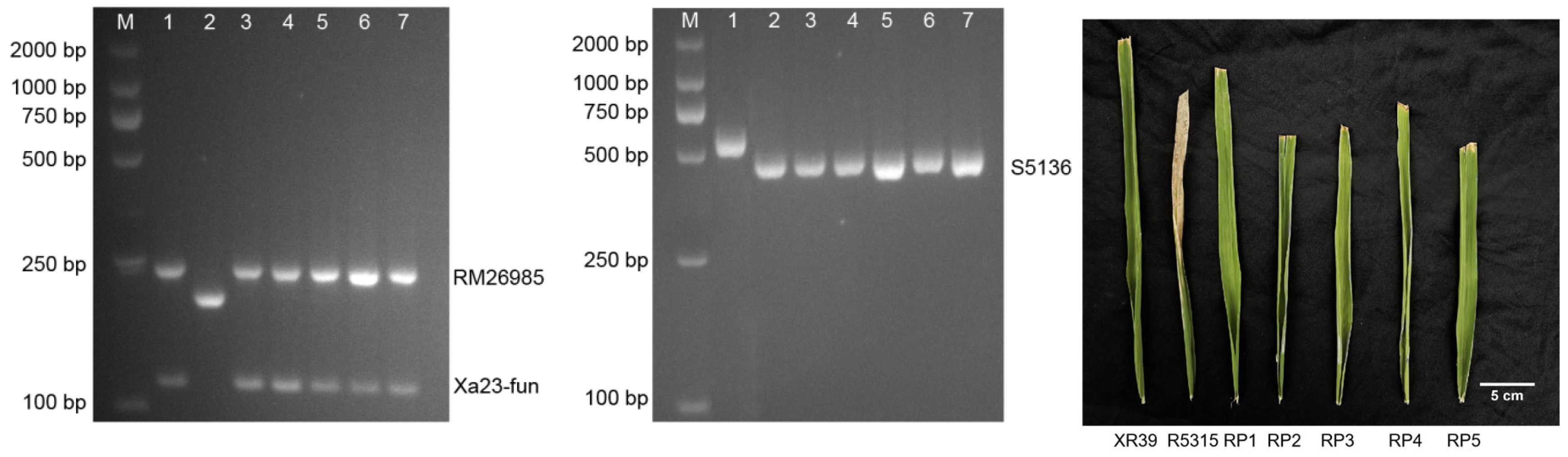
3.2. Breeding of Wide-Compatibility Restorer Lines Resistant to Bacterial Blight
3.3. Bacterial Blight Resistance Performance of Five Wide-Compatibility Restorer Lines and Twenty Hybrid Combinations
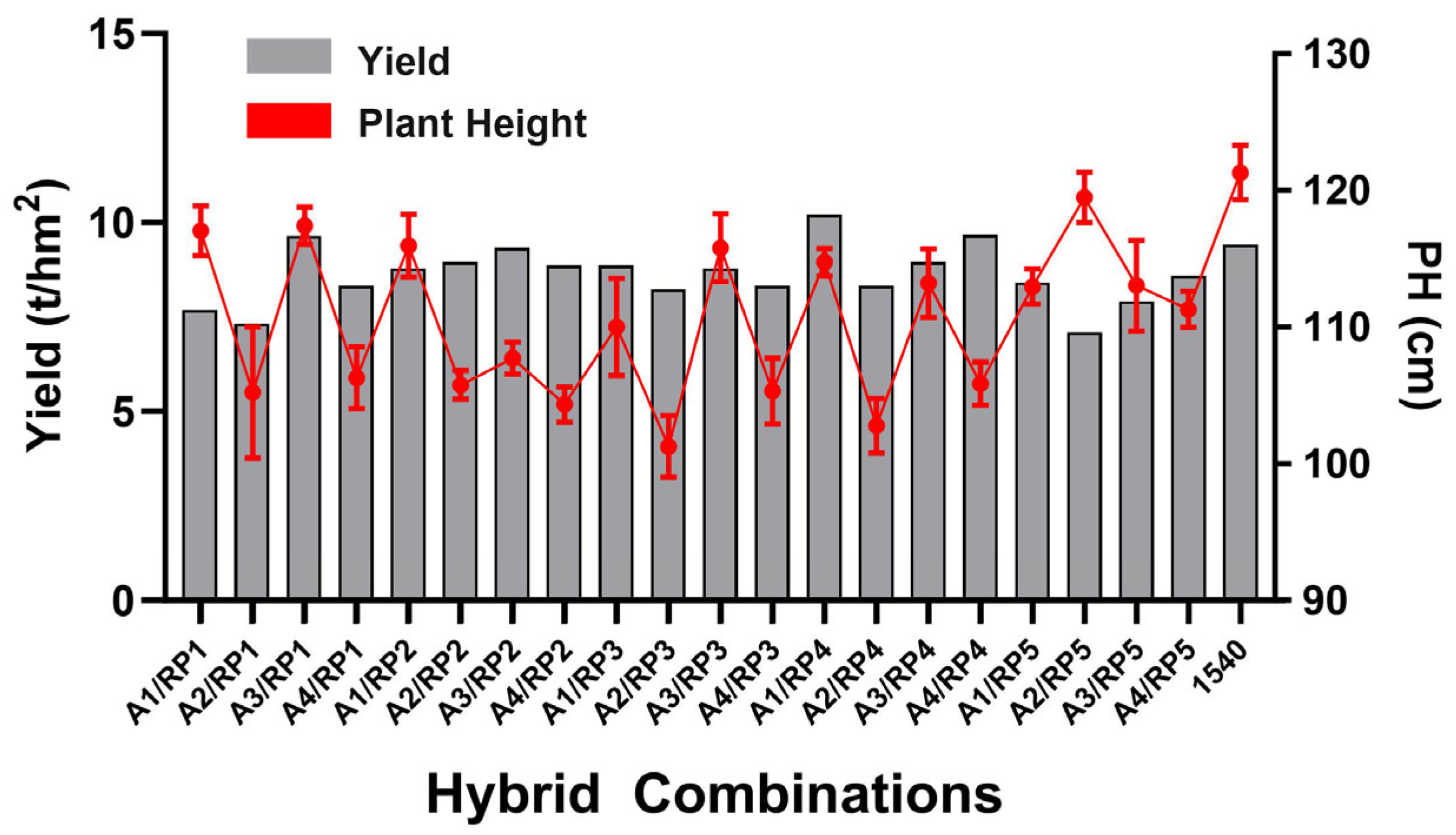
3.4. Main Agronomic Trait Performance of Five Wide-Compatibility Restorer Lines and Twenty Hybrid Combinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhou, Y.; Sun, J.; Liang, W.; Chen, X.; Wang, X.; Zhou, J.; Yu, C.; Wang, J.; Wu, S.; et al. Research Progress on Cloning and Function of Xa Genes Against Rice Bacterial Blight. Front. Plant Sci. 2022, 13, 847199. [Google Scholar] [CrossRef] [PubMed]
- Dossa, G.S.; Quibod, I.; Atienza-Grande, G.; Oliva, R.; Maiss, E.; Vera Cruz, C.; Wydra, K. Rice Pyramided Line IRBB67 (Xa4/Xa7) Homeostasis under Combined Stress of High Temperature and Bacterial Blight. Sci. Rep. 2020, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Zeng, Q.; Liu, X.; Peng, J. Resistance Genes and Their Interactions with Bacterial Blight/Leaf Streak Pathogens (Xanthomonas oryzae) in Rice (Oryza sativa, L.)—An Updated Review. Rice 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hu, M.; Chen, S.; Hu, A.; Li, S.; Han, H.; Lu, G.; Zeng, L.; Zhou, J. Enterobacter asburiae and Pantoea ananatis Causing Rice Bacterial Blight in China. Plant Dis. 2021, 105, 2078–2088. [Google Scholar] [CrossRef]
- Yu, L.; Yang, C.; Ji, Z.; Zeng, Y.; Liang, Y.; Hou, Y. First Report of New Bacterial Leaf Blight of Rice Caused by Pantoea ananatis in Southeast China. Plant Dis. 2022, 106, 310. [Google Scholar] [CrossRef]
- Huang, F.; He, N.; Yu, M.; Li, D.; Yang, D. Identification and Fine Mapping of a New Bacterial Blight Resistance Gene, Xa43(t), in Zhangpu Wild Rice (Oryza rufipogon). Plant Biol. 2023, 25, 433–439. [Google Scholar] [CrossRef]
- Huang, B.; Xu, J.Y.; Hou, M.S.; Ali, J.; Mou, T.M. Introgression of Bacterial Blight Resistance Genes Xa7, Xa21, Xa22 and Xa23 into Hybrid Rice Restorer Lines by Molecular Marker-Assisted Selection. Euphytica 2012, 187, 449–459. [Google Scholar] [CrossRef]
- Javed, M.A.; Ali, S.W.; Ashfaq, M.; Tabassam, J.; Ali, M.; IhsanUllah, M.; Nayab, S.F.; Kaya, Y.; Khalili, E.; Ali, Q.; et al. Molecular Profiling of Bacterial Blight Resistance in Malaysian Rice Cultivars. Braz. J. Biol. 2022, 82, e256189. [Google Scholar] [CrossRef]
- Wang, C.; Qin, T.; Yu, H.; Zhang, X.; Che, J.; Gao, Y.; Zheng, C.; Yang, B.; Zhao, K. The Broad Bacterial Blight Resistance of Rice Line CBB 23 Is Triggered by a Novel Transcription Activator-like (TAL) Effector of X Anthomonas Oryzae Pv. Oryzae. Mol. Plant Pathol. 2014, 15, 333–341. [Google Scholar] [CrossRef]
- Wang, C.; Fan, Y.; Zheng, C.; Qin, T.; Zhang, X.; Zhao, K. High-Resolution Genetic Mapping of Rice Bacterial Blight Resistance Gene Xa23. Mol. Genet. Genom. 2014, 289, 745–753. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Fan, Y.; Gao, Y.; Zhu, Q.; Zheng, C.; Qin, T.; Li, Y.; Che, J.; Zhang, M.; et al. XA23 Is an Executor R Protein and Confers Broad-Spectrum Disease Resistance in Rice. Mol. Plant 2015, 8, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.Y.; Fang, Y.L.; Shen, Q.; Wang, H.J.; Chen, X.F.; Guo, W.; Zhao, K.J.; Wang, C.L.; Ji, Z.Y. Genotypic Diversity and Pathogenisity of Xanthomonas Oryzae Pv. Oryzae Isolated from Southern China in 2019-2021. Chin. Bull. Bot. 2023, 5, 743–749. [Google Scholar]
- Yuan, L.P. Breeding Strategy of Hybrid Rice. Hybrid Rice 1987, 1, 1–3. [Google Scholar]
- Ouyang, Y.D. Progress of Indica-Japonica Hybrid Sterility and Wide-Compatibility in Rice. Chin. Sci. Bull. 2016, 35, 3833–3841. [Google Scholar] [CrossRef]
- Ikehashi, H.; Araki, H. Genetics of F1 sterility in remote crosses of rice. In Rice Genetics; World Scientific: Singapore, 1986; pp. 119–130. [Google Scholar]
- Ji, Q.; Lu, J.; Chao, Q.; Gu, M.; Xu, M. Delimiting a Rice Wide-Compatibility Gene S 5 n to a 50 Kb Region. Theor. Appl. Genet. 2005, 111, 1495–1503. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Liu, K.; Jiang, J.X.; Song, X.; Xu, C.G.; Li, X.H.; Zhang, Q. Delimitation of the Rice Wide Compatibility Gene S5 n to a 40-Kb DNA Fragment. Theor. Appl. Genet. 2005, 111, 1080–1086. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Ouyang, Y.; Du, H.; Yang, J.; Cheng, K.; Zhao, J.; Qiu, S.; Zhang, X.; Yao, J.; et al. A Triallelic System of S5 Is a Major Regulator of the Reproductive Barrier and Compatibility of Indica–Japonica Hybrids in Rice. Proc. Natl. Acad. Sci. USA 2008, 105, 11436–11441. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Cao, Q.; Chen, Z.D.; Zhong, W.G. Development and Application of a Functional Marker for Wide Compatibility Gene S5-n of Rice. Acta Agron. Sin. 2009, 35, 2000–2007. [Google Scholar] [CrossRef]
- Mi, J.; Li, G.; Huang, J.; Yu, H.; Zhou, F.; Zhang, Q.; Ouyang, Y.; Mou, T. Stacking S5-n and F5-n to Overcome Sterility in Indica-Japonica Hybrid Rice. TAG. Theor. Appl. Genet. 2016, 129, 563–575. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Arremsetty, H.P.S.; Singh, A.K.; Khandekar, D.; Ulaganathan, K.; Shenoy, V.; Sinha, P.; Singh, V.K. Marker-Assisted Improvement of the Elite Maintainer Line of Rice, IR 58025B for Wide Compatibility (S5n) Gene. Front. Plant Sci. 2018, 9, 1051. [Google Scholar] [CrossRef]
- Zeng, Y.; Ji, Z.; Wen, Z.; Liang, Y.; Yang, C. Combination of Eight Alleles at Four Quantitative Trait Loci Determines Grain Length in Rice. PLoS ONE 2016, 11, e0150832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Fan, Y.L.; Ji, X.Y.; Xia, Z.H. Variation and Functional Markers of Xa23 Promoter of Rice Bacterial Blight Resistance Gene. Mol. Plant Breed. 2020, 18, 7088–7094. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, D.; Ali, J.; Mou, T. Molecular Marker-Assisted Pyramiding of Broad-Spectrum Disease Resistance Genes, Pi2 and Xa23, into GZ63-4S, an Elite Thermo-Sensitive Genic Male-Sterile Line in Rice. Mol. Breed. 2015, 35, 83. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, D.; Yin, F.; Zhong, Q.; Wang, B.; Xiao, S.; Ke, X.; Wang, L.; Zhang, Y.; Zhao, C.; et al. Identification and Fine-Mapping of a New Bacterial Blight Resistance Gene, Xa47(t), in G252, an Introgression Line of Yuanjiang Common Wild Rice (Oryza rufipogon). Plant Dis. 2021, 105, 4106–4112. [Google Scholar] [CrossRef]
- Huang, Y.L.; Yan, Z.; Shen, G.L.; Wang, H.; Zhang, C.H.; Yang, W.; Chen, L.; Zhang, Y.H.; Pang, Z.S.; Qiao, M.; et al. Bacterial Blight Resistance of Rice Sterile Line ‘Quan 2l1S’ Improved by Molecular Marker-Assisted Selection. Chin. Agric. Sci. Bull. 2022, 38, 133–139. [Google Scholar]
- Yang, D.Y.; Xiong, L.Z.; Mou, T.M.; Mi, J.M. Improving the Resistance of the Rice PTGMS Line Feng39S by Pyramiding Blast, Bacterial Blight, and Brown Planthopper Resistance Genes. Crop J. 2022, 10, 1187–1197. [Google Scholar] [CrossRef]
- Huang, X.; Jin, L.C.; Ye, C.H.; Jiang, J.F.; Shi, X.B. Molecular Detection and Breeding Application of Some Disease and Inseet Resistance Genes Ofjaponica Rice Varieties/Lines Recently Developed in Zhejiang Province. Acta Agric. Zhejiangensis 2021, 33, 1159–1169. [Google Scholar]
- Dong, J.J.; Zhang, X.Y.; Fu, H.W.; Li, Y.F. Analysis of Genetic Parameters of Main Agronomic Characters of Hybrid Rice Between the Japonica CMS Line and Wide-Compatibility Indica Restorer Line. Hybrid Rice 2023, 38, 14–21. [Google Scholar]
- Song, J.; Cui, Y.; Fan, H.; Tang, L.; Wang, J. Molecular Breeding of Zheyou810, an Indica–Japonica Hybrid Rice Variety with Superior Quality and High Yield. Agriculture 2023, 13, 1807. [Google Scholar] [CrossRef]

| Lesion Rating | Resistance Level | Lesion Length |
|---|---|---|
| 0 | HR | <1 cm |
| 1 | R | 1–3 cm |
| 3 | MR | <1/4 of the inoculated leaf length |
| 5 | MS | 1/4–1/2 of the inoculated leaf length |
| 7 | S | 1/2–3/4 of the inoculated leaf length |
| 9 | HS | >3/4 of the inoculated leaf length |
| Gene | Primer | Primer Sequence (5′-3′) | Fragment Length (bp) |
|---|---|---|---|
| Xa23 | RM26985-F | CACAAGACAACCTTCAATGG | 183/166 |
| RM26985-R | GGCTTAGGAGCGTTTATAGG | ||
| Xa23-fun-F | AAAGTCCCTTCCGAAACATC | 105/- | |
| Xa23-fun-R | ATGAGGAAGTGCTGCCAGA | ||
| S5n | S5136-F | ATCAACCCATTTCCTTTCCT | 577/441 |
| S5136-R | ATACGCTCGATCGGATTAAC |
| Material | Lesion Rating | Resistance Level | Material | Lesion Rating | Resistance Level |
|---|---|---|---|---|---|
| XR39 | 0 | HR | A4/RP2 | 1 | R |
| R5315 | 9 | S | A1/RP3 | 0 | R |
| RP1 | 0 | HR | A2/RP3 | 1 | R |
| RP2 | 0 | HR | A3/RP3 | 1 | R |
| RP3 | 0 | HR | A4/RP3 | 1 | R |
| RP4 | 0 | HR | A1/RP4 | 0 | R |
| RP5 | 0 | HR | A2/RP4 | 1 | R |
| A1/RP1 | 0 | HR | A3/RP4 | 1 | R |
| A2/RP1 | 1 | R | A4/RP4 | 1 | R |
| A3/RP1 | 1 | R | A1/RP5 | 0 | R |
| A4/RP1 | 1 | R | A2/RP5 | 1 | R |
| A1/RP2 | 0 | R | A3/RP5 | 1 | R |
| A2/RP2 | 1 | R | A4/RP5 | 1 | R |
| A3/RP2 | 1 | R | 1540 | 9 | S |
| Traits | RP1 | RP2 | RP3 | RP4 | RP5 | R5315 |
|---|---|---|---|---|---|---|
| DSH (d) | 77.00 | 81.00 | 81.00 | 80.00 | 77.00 | 81.00 |
| PH (cm) | 103.4 ± 1.4 a | 95.0 ± 1.8 c | 96.5 ± 1.2 bc | 96.5 ± 1.3 bc | 97.6 ± 1.2 b | 104.8 ± 1.3 a |
| EPNPP | 7.4 ± 0.5 a | 7.8 ± 0.8 a | 8.0 ± 1.0 a | 8.0 ± 0.7 a | 8.2 ± 0.8 a | 7.2 ± 0.8 a |
| TGW (g) | 19.3 ± 0.2 e | 22.3 ± 0.3 a | 20.9 ± 0.4 c | 21.4 ± 0.2 b | 20.3 ± 0.2 d | 20.1 ± 0.3 d |
| TGNPP | 378.1 ± 3.0 a | 217.9 ± 1.5 e | 251.7 ± 3.8 d | 344.7 ± 5.6 b | 303.4 ± 5.5 c | 311.5 ± 6.4 c |
| SSR (%) | 85.2 ± 0.6 a | 80.6 ± 1.8 b | 76.9 ± 1.9 c | 79.1 ± 0.9 bc | 77.3 ± 1.2 c | 81.3 ± 0.3 b |
| SPW (g) | 6.2 ± 0.1 a | 3.9 ± 0.1 e | 4.0 ± 0.1 e | 5.8 ± 0.1 b | 4.8 ± 0.1 d | 5.1 ± 0.2 c |
| GL (mm) | 7.66 ± 0.14 d | 8.75 ± 0.07 a | 8.18 ± 0.09 b | 8.17 ± 0.04 b | 7.93 ± 0.04 c | 8.28 ± 0.07 b |
| GW (mm) | 2.48 ± 0.04 b | 2.79 ± 0.03 a | 2.42 ± 0.03 c | 2.29 ± 0.03 d | 2.41 ± 0.02 c | 2.42 ± 0.01 c |
| GL/GW | 3.09 ± 0.01 f | 3.13 ± 0.01 e | 3.39 ± 0.01 c | 3.56 ± 0.02 a | 3.29 ± 0.01 d | 3.42 ± 0.01 b |
| Hybrid Combinations | DSH (d) | PH (cm) | EPNPP | TGW (g) | TGNPP | SSR (%) | SPW (g) | Yield (t/hm2) | GL (mm) | GW (mm) | GL/GW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1/RP1 | 97.0 | 117.0 ± 1.8 ** | 9.2 ± 0.8 | 18.9 ± 0.1 ** | 300.2 ± 5.0 ** | 65.2 ± 0.9 ** | 3.7 ± 0.1 ** | 7.68 | 7.68 | 2.68 | 2.89 |
| A2/RP1 | 101.0 | 105.2 ± 4.8 ** | 8.8 ± 0.8 | 20.6 ± 0.2 | 369.7 ± 4.5 ** | 74.6 ± 1.1 ** | 5.7 ± 0.1 * | 7.32 | 7.75 | 2.67 | 2.92 |
| A3/RP1 | 98.0 | 117.4 ± 1.4 ** | 9.2 ± 0.8 | 20.6 ± 0.2 | 359.2 ± 5.1 ** | 85.7 ± 0.5 ** | 6.3 ± 0.1 | 9.64 | 7.77 | 2.68 | 2.92 |
| A4/RP1 | 83.0 | 106.3 ± 2.2 ** | 9.2 ± 0.8 | 19.5 ± 0.2 ** | 349.6 ± 4.0 ** | 77.7 ± 1.9 ** | 5.3 ± 0.1 ** | 8.32 | 7.35 | 2.47 | 3 |
| A1/RP2 | 100.0 | 115.9 ± 2.3 ** | 8.8 ± 0.8 | 22.8 ± 0.2 ** | 298.4 ± 7.6 ** | 74.5 ± 0.9 ** | 5.1 ± 0.2 ** | 8.78 | 7.71 | 2.76 | 2.82 |
| A2/RP2 | 104.0 | 105.8 ± 1.1 ** | 6.8 ± 0.8 * | 23.9 ± 0.2 ** | 326.8 ± 9.3 | 76.9 ± 1.1 ** | 6.0 ± 0.2 | 8.96 | 8.26 | 2.75 | 3.02 |
| A3/RP2 | 100.0 | 107.7 ± 1.2 ** | 9.0 ± 1.0 | 23.9 ± 0.1 ** | 267.4 ± 4.4 ** | 84.9 ± 0.9 ** | 5.4 ± 0.1 ** | 9.33 | 8.06 | 2.65 | 3.07 |
| A4/RP2 | 84.0 | 104.3 ± 1.3 ** | 8.2 ± 0.8 | 22.3 ± 0.2 ** | 343.0 ± 3.6 ** | 75.0 ± 1.1.0 ** | 5.7 ± 0.1 * | 8.87 | 7.7 | 2.52 | 3.07 |
| A1/RP3 | 98.0 | 110.0 ± 3.5 ** | 10.2 ± 0.8 ** | 22.9 ± 0.1 ** | 298.4 ± 7.4 ** | 82.4 ± 0.6 ** | 5.6 ± 0.1 ** | 8.87 | 7.83 | 2.65 | 2.97 |
| A2/RP3 | 103.0 | 101.3 ± 2.3 ** | 8.2 ± 0.8 | 22.6 ± 0.2 ** | 272.8 ± 12 ** | 82.8 ± 1.1 ** | 5.1 ± 0.1 ** | 8.23 | 8.23 | 2.73 | 3.03 |
| A3/RP3 | 100.0 | 115.8 ± 2.5 ** | 9.6 ± 1.1 | 23.6 ± 0.1 ** | 227.4 ± 13.9 ** | 87.8 ± 1.5 * | 4.7 ± 0.2 ** | 8.78 | 7.78 | 2.77 | 2.84 |
| A4/RP3 | 85.0 | 105.3 ± 2.4 ** | 9.2 ± 0.8 | 21.1 ± 0.2 | 326.7 ± 15 | 81.2 ± 0.2 ** | 5.6 ± 0.2 * | 8.32 | 7.67 | 2.52 | 3.06 |
| A1/RP4 | 98.0 | 114.7 ± 1.0 ** | 8.4 ± 0.9 | 23.3 ± 0.2 ** | 356.1 ± 5.5 ** | 79.5 ± 0.9 ** | 6.6 ± 0.1 ** | 10.21 | 7.74 | 2.63 | 2.96 |
| A2/RP4 | 103.0 | 102.8 ± 2.0 ** | 7.4 ± 0.9 | 21.4 ± 0.2 ** | 270.4 ± 3.1 ** | 72.4 ± 1.2 ** | 4.2 ± 0.1 ** | 8.32 | 8.18 | 2.63 | 3.13 |
| A3/RP4 | 101.0 | 113.2 ± 2.5 ** | 9.2 ± 0.8 | 23.1 ± 0.1 ** | 244.3 ± 7.1 ** | 85.4 ± 0.2 ** | 4.8 ± 0.2 ** | 8.96 | 8.11 | 2.62 | 3.12 |
| A4/RP4 | 84.0 | 105.9 ± 1.6 ** | 8.2 ± 0.8 | 20.5 ± 0.1 | 371.1 ± 11.8 ** | 85.4 ± 0.8 ** | 6.5 ± 0.1 * | 9.67 | 7.62 | 2.49 | 3.09 |
| A1/RP5 | 100.0 | 113.0 ± 1.3 ** | 7.8 ± 0.8 | 21.8 ± 0.1 ** | 336.4 ± 14.5 | 80.7 ± 0.7 ** | 5.9 ± 0.2 | 8.41 | 7.58 | 2.68 | 2.85 |
| A2/RP5 | 106.0 | 119.5 ± 1.8 | 8.4 ± 0.9 | 21.7 ± 0.3 ** | 325.8 ± 9.6 | 74.7 ± 1.0 ** | 5.3 ± 0.1 ** | 7.10 | 7.67 | 2.71 | 2.85 |
| A3/RP5 | 99.0 | 113.0 ± 3.3 ** | 8.0 ± 0.7 | 21.9 ± 0.3 ** | 222.8 ± 2.7 ** | 78.3 ± 1.6 ** | 3.8 ± 0.1 ** | 7.90 | 7.71 | 2.68 | 2.9 |
| A4/RP5 | 86.0 | 111.3 ± 1.3 ** | 7.8 ± 0.8 | 23.4 ± 0.3 ** | 330.7 ± 6.2 | 72.8 ± 1.3 ** | 5.6 ± 0.2 * | 8.59 | 8.2 | 2.63 | 3.14 |
| Yongyou 1540 | 105.0 | 121.3 ± 2.0 | 8.2 ± 0.8 | 20.8 ± 0.2 | 321.2 ± 5.4 | 91.2 ± 1.2 | 6.1 ± 0.1 | 9.40 | 7.44 | 2.63 | 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Zhang, X.; Li, Y.; Fu, H. Molecular-Marker-Based Design for Breeding Indica–Japonica Hybrid Rice with Bacterial Blight Resistance. Genes 2025, 16, 719. https://doi.org/10.3390/genes16060719
Dong J, Zhang X, Li Y, Fu H. Molecular-Marker-Based Design for Breeding Indica–Japonica Hybrid Rice with Bacterial Blight Resistance. Genes. 2025; 16(6):719. https://doi.org/10.3390/genes16060719
Chicago/Turabian StyleDong, Junjie, Xinyue Zhang, Youfa Li, and Haowei Fu. 2025. "Molecular-Marker-Based Design for Breeding Indica–Japonica Hybrid Rice with Bacterial Blight Resistance" Genes 16, no. 6: 719. https://doi.org/10.3390/genes16060719
APA StyleDong, J., Zhang, X., Li, Y., & Fu, H. (2025). Molecular-Marker-Based Design for Breeding Indica–Japonica Hybrid Rice with Bacterial Blight Resistance. Genes, 16(6), 719. https://doi.org/10.3390/genes16060719





