Identification of Novel Genetic Loci Involved in Testis Traits of the Jiangxi Local Breed Based on GWAS Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Birds and Phenotypic Data for GWAS
2.3. RNA Extraction, Synthesis of cDNA and qPCR
2.4. Bioinformatic and Statistical Analysis
3. Results
3.1. Genome-Wide Association Studies of the Testicle Traits
3.2. Identification of Candidate Genes
3.3. Go and KEGG Analysis
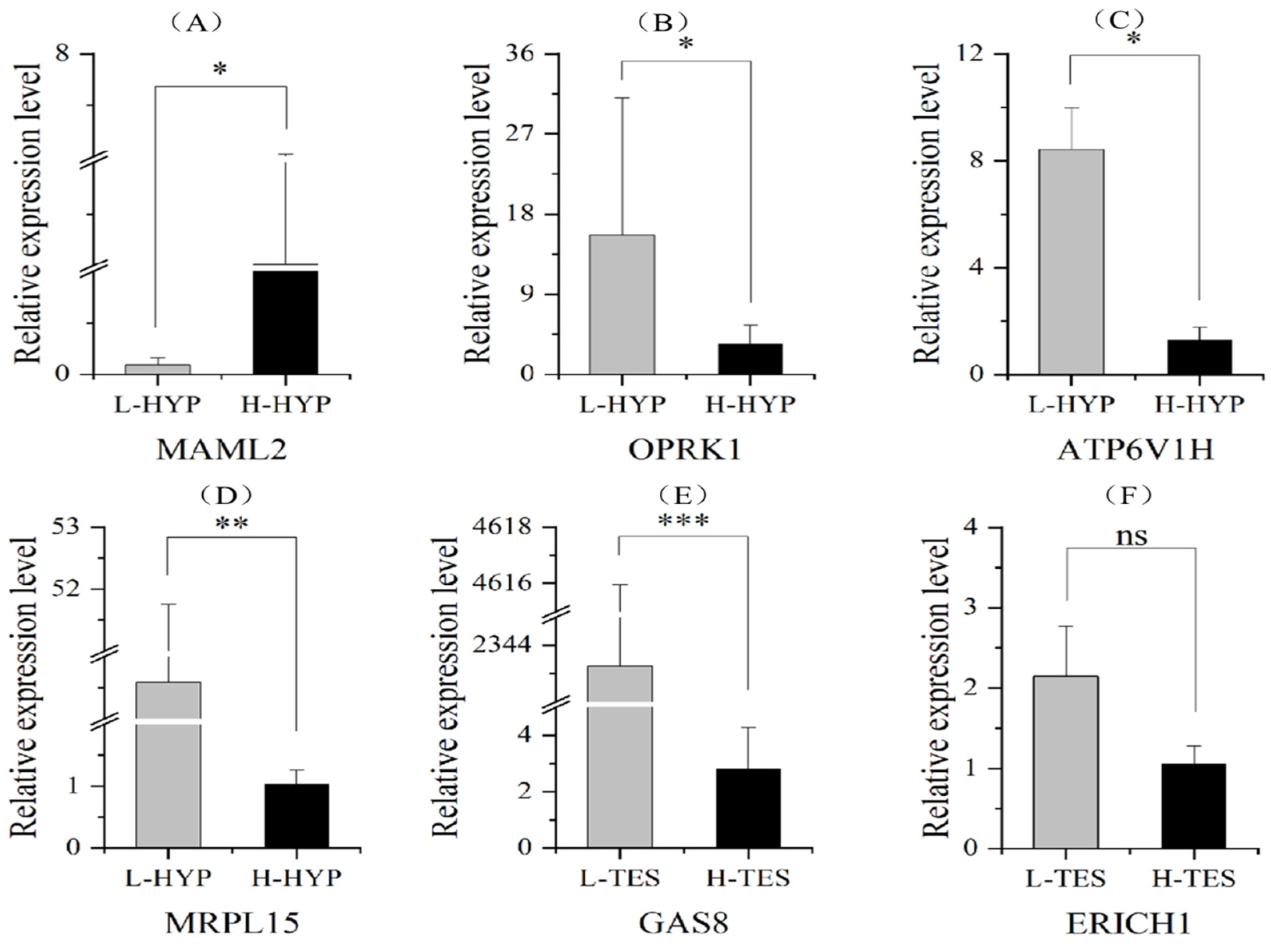
3.4. Gene Function Prediction
3.5. Protein–Protein Interaction Network
4. Discussion
4.1. Genomic Region Analysis for Testicle Traits
4.2. The Gene Associated with Testicle Traits Related to GWAS
4.3. Enriched Gene Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Estermann, M.A.; Major, A.T.; Smith, C.A. Genetic Regulation of Avian Testis Development. Genes 2021, 12, 1459. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, R.; Zhao, G.; Zheng, M.; Li, P.; Liu, L.; Wen, J. Genome-Wide Linkage Analysis Identifies Loci for Testicle and Ovary Traits in Chickens. Anim. Biotechnol. 2018, 29, 309–315. [Google Scholar] [CrossRef]
- Kastelic, J.P. Understanding and evaluating bovine testes. Theriogenology 2014, 81, 18–23. [Google Scholar] [CrossRef]
- Huang, Y.T.; Johnson, R.K. Effect of selection for size of testes in boars on semen and testis traits. Anim. Sci. J. 1996, 74, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Bai, X.; Wang, Y.; Leng, L.; Zhang, H.; Li, Y.; Cao, Z.; Luan, P.; Xiao, F.; et al. Genetic parameters estimation and genome-wide association studies for internal organ traits in an F(2) chicken population. J. Anim. Breed. Genet. 2022, 139, 434–446. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, J.Q.; Yang, L.L.; Kramer, L.M.; Zhang, X.Y.; Na, W.; Reecy, J.M.; Li, H. Identification of genome-wide SNP-SNP interactions associated with important traits in chicken. BMC Genom. 2017, 18, 892. [Google Scholar] [CrossRef]
- Zhang, H.; Na, W.; Zhang, H.L.; Du, Z.Q.; Wang, S.Z.; Wang, Z.P.; Zhang, Z.; Li, H. TCF21 is related to testis growth and development in broiler chickens. Genet. Sel. Evol. 2017, 49, 25. [Google Scholar] [CrossRef]
- Ma, J.E.; Zhang, H.Y.; Wang, S.Q.; Xu, J.G.; Cai, X.W.; Xiong, X.W.; Wang, Z.F.; Rao, Y.S. Correlation analysis between reproductive traits and early body weight of Kangle Yellow roosters. Heilongjiang Anim. Sci. Vet. Med. 2024, 16, 38–41. (In Chinese) [Google Scholar]
- Liu, R.; Xing, S.; Wang, J.; Zheng, M.; Cui, H.; Crooijmans, R.; Li, Q.; Zhao, G.; Wen, J. A new chicken 55K SNP genotyping array. BMC Genom. 2019, 20, 410. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Ma, J.E.; Xiong, X.W.; Zhou, M.; Wu, S.Q.; Han, T.; Rao, Y.S.; Wang, Z.F.; Xu, J.G. Full-Length Transcriptomic Analysis of Chicken Pituitary Reveals Candidate Genes for Testicular Trait. Sci. Agric. Sin. 2024, 57, 4130–4147.8. (In Chinese) [Google Scholar]
- Ma, J.E.; Wu, S.Q.; Wan, S.M.; Xiong, X.W.; Xu, J.G.; Xu, J.; Rao, Y.S.; Zhou, M. Proteomic study of large and small testes in Ningdu Yellow chicken based on TMT technology. Acta Agric. Zhejiangensis 2025, 8, 1–20. (In Chinese) [Google Scholar]
- Levi, B.P.; Yilmaz, O.H.; Duester, G.; Morrison, S.J. Aldehyde dehydrogenase 1a1 is dispensable for stem cell function in the mouse hematopoietic and nervous systems. Blood 2009, 113, 1670–1680. [Google Scholar] [CrossRef]
- Drag, M.; Hansen, M.B.; Kadarmideen, H.N. Systems genomics study reveals expression quantitative trait loci, regulator genes and pathways associated with boar taint in pigs. PLoS ONE 2018, 13, e0192673. [Google Scholar] [CrossRef]
- Duritahala, M.; Sakase; Harayama, H. Involvement of Ca(2+)-ATPase in suppressing the appearance of bovine helically motile spermatozoa with intense force prior to cryopreservation. J. Reprod. Dev. 2022, 68, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Abril-Parreño, L.; Carthy, T.R.; Keogh, K.; Štiavnická, M.; O’Meara, C.; Lonergan, P.; Kenny, D.A.; Fair, S. Genome-wide association study reveals candidate markers related to field fertility and semen quality traits in Holstein-Friesian bulls. Animal 2023, 17, 100841. [Google Scholar] [CrossRef]
- Tokuda, M.; Kadokawa, Y.; Kurahashi, H.; Marunouchi, T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol. Reprod. 2007, 76, 130–141. [Google Scholar] [CrossRef]
- Piprek, R.P.; Kolasa, M.; Podkowa, D.; Kloc, M.; Kubiak, J.Z. Tissue-specific knockout of E-cadherin (Cdh1) in developing mouse gonads causes germ cells loss. Reproduction 2019, 158, 147–157. [Google Scholar] [CrossRef]
- Nitzsche, B.; Gloesenkamp, C.; Schrader, M.; Hoffmann, B.; Zengerling, F.; Balabanov, S.; Honecker, F.; Höpfner, M. Anti-tumour activity of two novel compounds in cisplatin-resistant testicular germ cell cancer. Br. J. Cancer 2012, 107, 1853–1863. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Li, H.; Zhao, X.; Ding, Y.; Yao, Y.; Wang, L. Association between Yili goose sperm motility and expression profiles of mRNA and miRNA in testis. BMC Genom. 2023, 24, 640. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, P.; Di Giacomo, D.; Geremia, R. The endocannabinoid system and spermatogenesis. Front. Endocrinol. 2013, 4, 192. [Google Scholar] [CrossRef]
- Jiao, Z.J.; Yi, W.; Rong, Y.W.; Kee, J.D.; Zhong, W.X. MicroRNA-1285 Regulates 17β-Estradiol-Inhibited Immature Boar Sertoli Cell Proliferation via Adenosine Monophosphate-Activated Protein Kinase Activation. Int. J. Endocrinol. 2015, 156, 4059–4070. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, X.Z.; Do, H.L.; Chandimali, N.; Kang, T.Y.; Kim, N.; Ghosh, M.; Lee, S.B.; Mok, Y.S.; Kim, S.B.; et al. MicroRNA-7450 regulates non-thermal plasma-induced chicken Sertoli cell apoptosis via adenosine monophosphate-activated protein kinase activation. Sci. Rep. 2018, 8, 8761. [Google Scholar] [CrossRef]
- Jin, H.J.; Ruan, T.; Dai, S.; Geng, X.Y.; Yang, Y.; Shen, Y.; Chen, S.R. Identification of CFAP52 as a novel diagnostic target of male infertility with defects of sperm head-tail connection and flagella development. eLife 2023, 12, RP92769. [Google Scholar] [CrossRef] [PubMed]
- Maqdasy, S.; El Hajjaji, F.Z.; Baptissart, M.; Viennois, E.; Oumeddour, A.; Brugnon, F.; Trousson, A.; Tauveron, I.; Volle, D.; Lobaccaro, J.M.; et al. Identification of the Functions of Liver X Receptor-β in Sertoli Cells Using a Targeted Expression-Rescue Model. Int. J. Endocrinol. 2015, 156, 4545–4557. [Google Scholar] [CrossRef]
- Mignani, L.; Facchinello, N.; Varinelli, M.; Massardi, E.; Tiso, N.; Ravelli, C.; Mitola, S.; Schu, P.; Monti, E.; Finazzi, D.; et al. Deficiency of AP1 Complex Ap1g1 in Zebrafish Model Led to Perturbation of Neurodevelopment, Female and Male Fertility; New Insight to Understand Adaptinopathies. Int. J. Mol. Sci. 2023, 24, 7108. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.R.; Gagnon, L.H.; Chang, B. A hypomorphic mutation of the γ-1 adaptin gene (Ap1g1) causes inner ear, retina, thyroid, and testes abnormalities in mice. Mamm. Genome 2016, 27, 200–212. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhu, Y.; Lo, L.J. Loss-of-function of zebrafish cdt1 causes retarded body growth and underdeveloped gonads resembling human Meier-Gorlin syndrome. J. Zhejiang Univ. Sci. B 2023, 24, 1037–1046. [Google Scholar] [CrossRef]
- Nishijima, Y.; Hagiya, Y.; Kubo, T.; Takei, R.; Katoh, Y.; Nakayama, K. RABL2 interacts with the intraflagellar transport-B complex and CEP19 and participates in ciliary assembly. Mol. Biol. Cell 2017, 28, 1652–1666. [Google Scholar] [CrossRef]
- Dubé, E.; Chan, P.T.; Hermo, L.; Cyr, D.G. Gene expression profiling and its relevance to the blood-epididymal barrier in the human epididymis. Biol. Reprod. 2007, 76, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Gomes, A.S.; Paul, D.L.; Goodenough, D.A. Study of claudin function by RNA interference. J. Biol. Chem. 2006, 281, 36117–36123. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, H.; Zeng, J.; Li, W.; Zhang, S.; Zhang, L.; Song, S.; Zhou, T.; Sutovsky, M.; Sutovsky, P.; et al. COP9 signalosome complex subunit 5, an IFT20 binding partner, is essential to maintain male germ cell survival and acrosome biogenesis†. Biol. Reprod. 2020, 102, 233–247. [Google Scholar] [CrossRef]
- Lv, M.; Liu, W.; Chi, W.; Ni, X.; Wang, J.; Cheng, H.; Li, W.Y.; Yang, S.; Wu, H.; Zhang, J.; et al. Homozygous mutations in DZIP1 can induce asthenoteratospermia with severe MMAF. J. Med. Genet. 2020, 57, 445–453. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.; Yang, L.; Ma, C.; Fei, Y.; Ding, J.; Song, W.; Tong, W.M.; Niu, Y.; Li, H. ALKBH5 in mouse testicular Sertoli cells regulates Cdh2 mRNA translation to maintain blood-testis barrier integrity. Cell. Mol. Biol. Lett. 2022, 27, 101. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Korrodi-Gregório, L.; Sinha, N.; Bhutada, S.; Bhattacharjee, R.; Kline, D.; Vijayaraghavan, S. Regulators of the protein phosphatase PP1γ2, PPP1R2, PPP1R7, and PPP1R11 are involved in epididymal sperm maturation. J. Cell. Physiol. 2019, 234, 3105–3118. [Google Scholar] [CrossRef]
- Mazaheri Moghaddam, M.; Moghaddam, M.M.; Amini, M.; Bahramzadeh, B.; Baghbanzadeh, A.; Biglari, A.; Sakhinia, E. Evaluation of SEPT2 and SEPT4 transcript contents in spermatozoa from men with asthenozoospermia and teratozoospermia. Health Sci. Rep. 2021, 4, e436. [Google Scholar] [CrossRef]
- Schirmer, S.U.; Eckhardt, I.; Lau, H.; Klein, J.; DeGraaf, Y.C.; Lips, K.S.; Pineau, C.; Gibbins, I.L.; Kummer, W.; Meinhardt, A.; et al. The cholinergic system in rat testis is of non-neuronal origin. Reproduction 2011, 142, 157–166. [Google Scholar] [CrossRef]
- Chakravarthi, V.P.; Ratri, A.; Masumi, S.; Borosha, S.; Ghosh, S.; Christenson, L.K.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. Granulosa cell genes that regulate ovarian follicle development beyond the antral stage: The role of estrogen receptor β. Mol. Cell. Endocrinol. 2021, 528, 111212. [Google Scholar] [CrossRef]
- Mbarek, H.; Gordon, S.D.; Duffy, D.L.; Hubers, N.; Mortlock, S.; Beck, J.J.; Hottenga, J.J.; Pool, R.; Dolan, C.V.; Actkins, K.V. Genome-wide association study meta-analysis of dizygotic twinning illuminates genetic regulation of female fecundity. Hum. Reprod. 2024, 39, 240–257. [Google Scholar] [CrossRef]
- Gao, N.; Chen, Y.; Liu, X.; Zhao, Y.; Zhu, L.; Liu, A.; Jiang, W.; Peng, X.; Zhang, C.; Tang, Z.; et al. Weighted single-step GWAS identified candidate genes associated with semen traits in a Duroc boar population. BMC Genom. 2019, 20, 797. [Google Scholar] [CrossRef] [PubMed]
- Sieper, M.H.; Gaikwad, A.S.; Fros, M.; Weber, P.; Di Persio, S.; Oud, M.S.; Kliesch, S.; Neuhaus, N.; Stallmeyer, B.; Tüttelmann, F.; et al. Scrutinizing the human TEX genes in the context of human male infertility. Andrology 2024, 12, 570–584. [Google Scholar] [CrossRef]
- Laqqan, M.; Hammadeh, M.E. Alterations in DNA methylation patterns and gene expression in spermatozoa of subfertile males. Andrologia 2018, 50. [Google Scholar] [CrossRef]
- De Gendt, K.; Denolet, E.; Willems, A.; Daniels, V.W.; Clinckemalie, L.; Denayer, S.; Wilkinson, M.F.; Claessens, F.; Swinnen, J.V.; Verhoeven, G. Expression of Tubb3, a β-tubulin isotype, is regulated by androgens in mouse and rat Sertoli cells. Biol. Reprod. 2011, 85, 934–945. [Google Scholar] [CrossRef]
- Tanaka, N.; Goto, M.; Kawasaki, A.; Sasano, T.; Eto, K.; Nishi, R.; Sugasawa, K.; Abe, S.; Saitoh, H. An EF-hands protein, centrin-1, is an EGTA-sensitive SUMO-interacting protein in mouse testis. Cell Biochem. Funct. 2010, 28, 604–612. [Google Scholar] [CrossRef]
- Yang, G.; Li, S.; Zhao, Q.; Chu, J.; Zhou, B.; Fan, S.; Shi, F.; Wei, X.; Hu, X.; Zheng, X.; et al. Transcriptomic and metabolomic insights into the variety of sperm storage in oviduct of egg layers. Poultry Sci. 2021, 100, 101087. [Google Scholar] [CrossRef]
- Crisà, A.; Claps, S.; Moioli, B.; Marchitelli, C. Identification of the complete coding cDNAs and expression analysis of B4GALT1, LALBA, ST3GAL5, ST6GAL1 in the colostrum and milk of the Garganica and Maltese goat breeds to reveal possible implications for oligosaccharide biosynthesis. BMC Vet. Res. 2019, 15, 457. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Li, Z.D.; Zheng, L.Q.; Zhang, T.; Shen, W.; Lei, C.Z. Genome-wide detection of selective signals for fecundity traits in goats (Capra hircus). Genes 2022, 818, 146221. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Feng, B.; Du, C.; Hou, C.; Jin, S.; Tang, D.; Zhu, J.; Lv, Y. Expression dynamics indicate the involvement of SPG7 in the reproduction and spermiogenesis of Phascolosoma esculenta. Genes 2024, 895, 148028. [Google Scholar] [CrossRef]
- Hoffmann, H.M.; Meadows, J.D.; Breuer, J.A.; Yaw, A.M.; Nguyen, D.; Tonsfeldt, K.J.; Devries, B.M.; Trang, C.; Oosterhouse, H.J.; Lee, J.S.; et al. The transcription factors SIX3 and VAX1 are required for suprachiasmatic nucleus circadian output and fertility in female mice. J. Neurosci. Res. 2021, 99, 2625–2645. [Google Scholar] [CrossRef]
- Qian, Y.C.; Xie, Y.X.; Wang, C.S.; Shi, Z.M.; Jiang, C.F.; Tang, Y.Y.; Qian, X.; Wang, L.; Jiang, B.H. Mkrn2 deficiency induces teratozoospermia and male infertility through p53/PERP-mediated apoptosis in testis. Asian J. Androl. 2020, 22, 414–421. [Google Scholar] [PubMed]
- Iyer, H.; Collins, J.J., 3rd; Newmark, P.A. NF-YB Regulates Spermatogonial Stem Cell Self-Renewal and Proliferation in the Planarian Schmidtea mediterranea. PLoS Genet. 2016, 12, e1006109. [Google Scholar] [CrossRef]
- Lavanya, M.; Archana, S.S.; Swathi, D.; Ramya, L.; Arangasamy, A.; Binsila, B.; Dhali, A.; Krishnaswamy, N.; Singh, S.K.; Kumar, H.; et al. Sperm preparedness and adaptation to osmotic and pH stressors relate to functional competence of sperm in Bos taurus. Sci. Rep. 2021, 11, 22563. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wang, Y.; Liu, C.; Jiang, Y.; Kang, L. A Novel Single-Nucleotide Polymorphism in WNT4 Promoter Affects Its Transcription and Response to FSH in Chicken Follicles. Genes 2022, 13, 1174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Chu, M. miRNA-mRNA analysis of sheep adrenal glands reveals the network regulating reproduction. BMC Genom. 2022, 23, 44. [Google Scholar] [CrossRef]
- Lee, H.H.; An, S.M.; Ye, B.J.; Lee, J.H.; Yoo, E.J.; Jeong, G.W.; Kang, H.J.; Alfadda, A.A.; Lim, S.W.; Park, J.; et al. TonEBP/NFAT5 promotes obesity and insulin resistance by epigenetic suppression of white adipose tissue beiging. Nat. Commun. 2019, 10, 3536. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, W.; Du, L.; He, Z. OIP5 Interacts with NCK2 to Mediate Human Spermatogonial Stem Cell Self-Renewal and Apoptosis through Cell Cyclins and Cycle Progression and Its Abnormality Is Correlated with Male Infertility. Research 2023, 6, 0162. [Google Scholar] [CrossRef]
- Bolino, A.; Bolis, A.; Previtali, S.C.; Dina, G.; Bussini, S.; Dati, G.; Amadio, S.; Del Carro, U.; Mruk, D.D.; Feltri, M.L.; et al. Disruption of Mtmr2 produces CMT4B1-like neuropathy with myelin outfolding and impaired spermatogenesis. J. Cell Biol. 2004, 167, 711–721. [Google Scholar] [CrossRef]
- He, K.; Qu, H.; Wang, H.; Zhang, S.; Qian, X.H.; Li, W. Regulated and Functional Expression of the Corepressor MTA3 in Rodent Testis. Int. J. Endocrinol. 2016, 157, 4400–4410. [Google Scholar] [CrossRef]
- Qu, M.; Zhao, Y.; Qing, X.; Zhang, X.; Li, H. Androgen-dependent miR-125a-5p targets LYPLA1 and regulates global protein palmitoylation level in late-onset hypogonadism males. J. Cell. Physiol. 2021, 236, 4738–4749. [Google Scholar] [CrossRef]
- Nagae, M.; Yamada, K.; Enomoto, Y.; Kometani, M.; Tsuchida, H.; Panthee, A.; Nonogaki, M.; Matsunaga, N.; Takizawa, M.; Matsuzaki, S.; et al. Conditional Oprk1-dependent Kiss1 deletion in kisspeptin neurons caused estrogen-dependent LH pulse disruption and LH surge attenuation in female rats. Sci. Rep. 2023, 13, 20495. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Gupta, M.K. Transcriptome Analysis Reveals Spermatogenesis-Related CircRNAs and LncRNAs in Goat Spermatozoa. Biochem. Genet. 2023, 62, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Kherraf, Z.E.; Barbotin, A.L.; Martinez, G.; Mazet, A.; Cazin, C.; Coutton, C.; Arnoult, C.; Thierry-Mieg, N.; Rives, N.; Rives-Feraille, A.; et al. A splice donor variant of GAS8 induces structural disorganization of the axoneme in sperm flagella and leads to nonsyndromic male infertility. Clin. Genet. 2024, 105, 220–225. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, C.; Mo, C.; Liu, M.; Wan, Y.; Li, J.; Wang, Y. Single-Cell RNA Sequencing Analysis of Chicken Anterior Pituitary: A Bird’s-Eye View on Vertebrate Pituitary. Front. Physiol. 2021, 12, 562817. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, D.; Ye, Z.; Jiang, Z.; Ge, L.; Ren, Y.; Wang, J.; Xu, X.; Yang, J.; Wang, T. Comparative transcriptome analysis reveals key genes and pathways related to gonad development in the sea cucumber Apostichopus japonicus. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2023, 48, 101144. [Google Scholar] [CrossRef]
- Li, C.; Tan, Y.P.; Ma, X.S.; Wang, Z.B.; Meng, T.G.; Sun, Q.Y. CDT1 is the major functional regulatory subunit of the pre-replication complex in zygotes. Cell Prolif. 2023, 56, e13377. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.S.; Lin, F.; Wang, Z.W.; Hu, M.W.; Huang, L.; Meng, T.G.; Jiang, Z.Z.; Schatten, H.; Wang, Z.B.; Sun, Q.Y. Geminin deletion in mouse oocytes results in impaired embryo development and reduced fertility. Mol. Biol. Cell 2016, 27, 768–775. [Google Scholar] [CrossRef]
- Sun, X.; Niu, Q.; Jiang, J.; Wang, G.; Zhou, P.; Li, J.; Chen, C.; Liu, L.; Xu, L.; Ren, H. Identifying Candidate Genes for Litter Size and Three Morphological Traits in Youzhou Dark Goats Based on Genome-Wide SNP Markers. Genes 2023, 14, 1183. [Google Scholar] [CrossRef]
- Pérez Baca, M.D.R.; Jacobs, E.Z.; Vantomme, L.; Leblanc, P.; Bogaert, E.; Dheedene, A.; De Cock, L.; Haghshenas, S.; Foroutan, A.; Levy, M.A. Callewaert. Haploinsufficiency of ZFHX3, encoding a key player in neuronal development, causes syndromic intellectual disability. Am. J. Hum. Genet. 2024, 111, 509–528. [Google Scholar] [CrossRef]
- Ma, G.; Gao, A.; Yang, Y.; He, Y.; Zhang, X.; Zhang, B.; Zhang, Z.; Li, M.; Fu, X.; Zhao, D.; et al. Zfhx3 is essential for progesterone/progesterone receptor signaling to drive ductal side-branching and alveologenesis in mouse mammary glands. J. Genet. Genom. 2019, 46, 119–131. [Google Scholar] [CrossRef]
- Ito, S.; Liu, X.; Ishikawa, Y.; Conti, D.D.; Otomo, N.; Kote-Jarai, Z.; Suetsugu, H.; Eeles, R.A.; Koike, Y.; Hikino, K. Androgen receptor binding sites enabling genetic prediction of mortality due to prostate cancer in cancer-free subjects. Nat. Commun. 2023, 14, 4863. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.M.; Pandolfi, E.C.; Larder, R.; Mellon, P.L. Haploinsufficiency of Homeodomain Proteins Six3, Vax1, and Otx2 Causes Subfertility in Mice via Distinct Mechanisms. J. Neuroendocrinol. 2019, 109, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Martin, L.J. Classical cadherins in the testis: How are they regulated? Reprod. Fertil. Dev. 2023, 35, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Qin, X.L.; Ma, X.L.; Mo, H.Q.; Qin, S.; Zhang, C.X.; Wei, X.W.; Liu, X.Q.; Zhang, Y.; Tian, F.J.; et al. CLDN1 regulates trophoblast apoptosis and proliferation in preeclampsia. Reproduction 2021, 161, 623–632. [Google Scholar] [CrossRef]
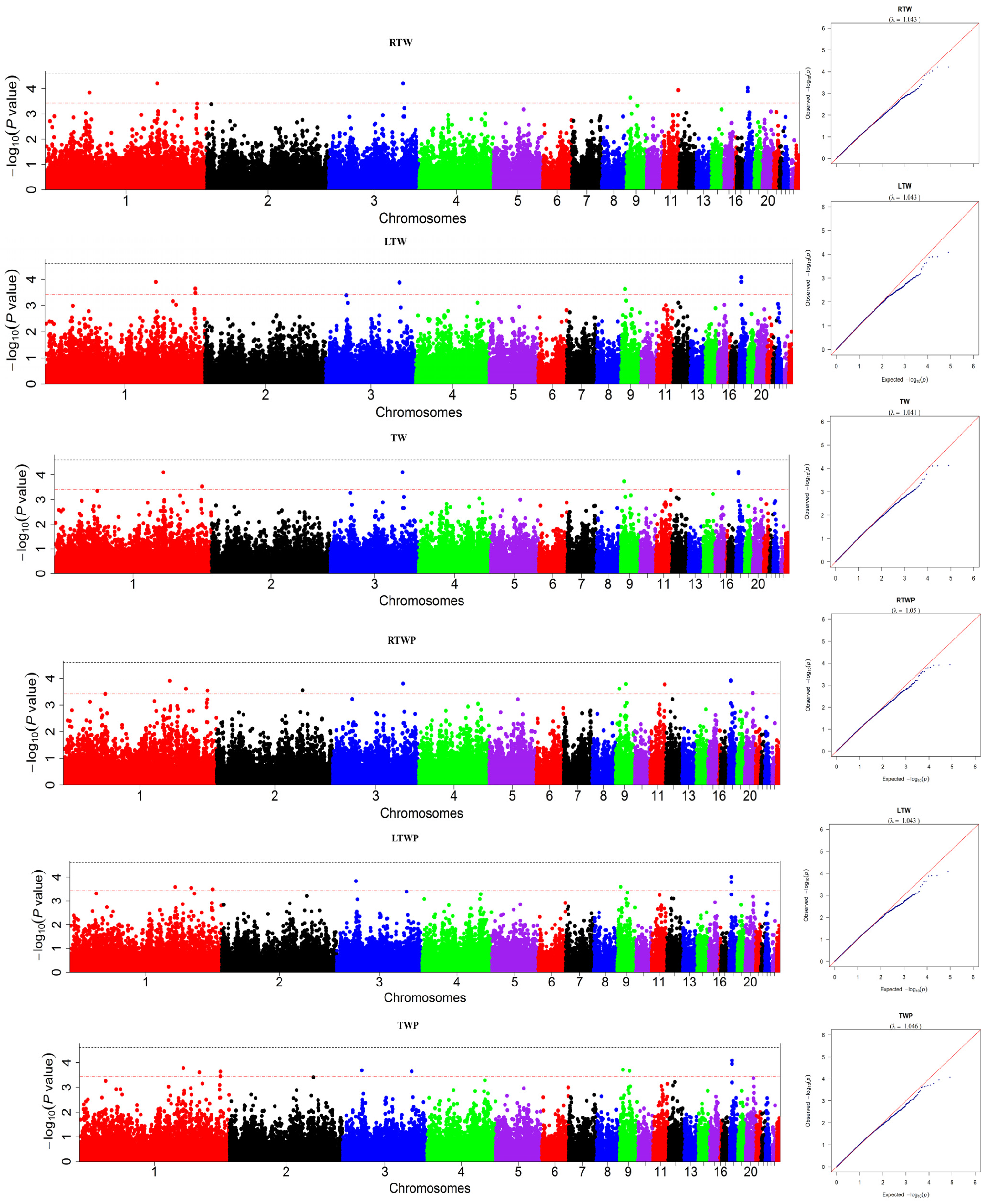

| Chr | Position | Trait | Candidate Genes Related to Testicle Traits |
|---|---|---|---|
| 1 | 136,871,054 | TW, LTW, RTW, TWP, LTWP, RTWP | ALDH1L2, APPL2, BPIFCB, C1H12ORF73, C1H2ORF40, C1H2ORF49, CCDC138, CCDC82, CKAP4, DACH1, DIS3, FAM76B, GCC2, GLT8D2, KLF12, LARGE1, LIMS1, MAML2, MIR1743, MIR6570, MIR7450, MRPS9, MTERF2, MTMR2, MZT1, NCK2, NFYB, NT5DC3, NUAK1, PGR, POU3F3, PRDM4, RTCB, SLC41A2, SLC5A7, SULT1C3, SYN3 |
| 185,660,571 | TW, LTW, TWP, LTWP, RTWP | ||
| 185,445,943 | TW (L) | ||
| 157,924,199 | TWP, LTWP, RTWP | ||
| 53,862,399 | RTW | ||
| 2 | 110,473,923 | RTWP | ATP6V1H, CHCHD7, ERD2L, FAM150A, KIF20AL, LYN, LYPLA1, MOS, MRPL15, NPBWR1, OPRK1, PCMTD1, PENK, PLAG1, RB1CC1, RGS20, RP1RP1-2, RPS20, SDR16C5, SOX17, ST18, TCEA1, TGS1, TMEM68, TMEM68L, XKR4 |
| 3 | 90,628,313 | TW, LTW, RTW, TWP, RTWP | ABCG8, CAMKMT, DYNC2LI1, MTA3, PLEKHH2, PPM1B, SIX3, SLC3A1, TRNAI-UAU |
| 25,006,145 | TWP, LTWP, | ||
| 9 | 5,124,976 | TW, LTW, RTW, TWP, LTWP, RTWP | A4GNT, ACAP2, AGXT, AMOTL2, APOD, ATP13A3, ATP13A4, ATP13A5, BDH1B, BOK, CEP19, CLDN1, COPS9, CPN2, DNAJB11, DTYMK, DZIP1L, FARP2, FGF12, GAL3ST4, GMNC, GP5, GPR35, GPR35L, GPR55, HES6, IGF2BP2, KLHL30, LRRC15, LSG1, MB21D2, MIR1577, MIR1608, MIR1612, MIR1704, MRPS22, OTOS, P3H2, PFKL, PPP1R2, PPP1R7, PTTG1IP, RNF168, RYK, SEPT2, SLCO2A1, SNED1, ST6GAL1, SUMO3, THAP4, TM4SF19, TMEM207, TRA2B, TSPEAR, UBE2G2, UBXN7, UTS2B, WDR53, XXYLT1 |
| 13,678,170 | TWP, RTWP | ||
| 11 | 18,863,652 | RTW, RTWP | ACSF3, AP1G1, BANP, CDH1, CDH15, CDH3, CDK10, CDT1, CHST4, CPNE7, CTU2, CYB5B, DEF8, DPEP1, GAS8, HAS3, IL17C, JPH3, MC1R, MIR140, MIR1571, MIR6667, NFAT5, NIP7, PHLPP2, PMFBP1, RPL13, SNTB2, SPG7, SPIRE2, TAT, TCF25, TMCO7, TRAPPC2L, TUBB3, UTP4, VPS4A, WWP2, ZC3H18, ZFHX3, ZFPM1, ZNF276, ZNF469, ZNF821 |
| 18 | 3,169,740, 3,170,246 | TW, LTW, RTW, TWP, LTWP, RTWP | B3GNTL1, C18H17orf62, CD7, CDRT1, CFAP52, FAM18B1, HS3ST3B1, OGFOD3, PMP22, RAB40B, SEPT9, TBC1D24L, TEKT3, TNRC6C, TRNAM-CAU, TRNAQ-CUG, TRNAQ-UUG, USP43, WDR45B, ZNF750 |
| Tissue | Count | Gene Name | |
|---|---|---|---|
| Up-Regulated in L-TES | Down-Regulated in L-TES | ||
| Hypothalamus | 8 | OPRK1, SYN3, PENK, FGF12, ATP6V1H, MRPL15 | MAML2, PTTG1IP |
| Pituitary | 1 | WDR45B | - |
| Liver | 1 | - | CD7 |
| Testis | 30 | CAMKMT, CDK10, CDRT1, DIS3, ERICH1, GAS8, KIF20AL, PMFBP1, PRDM4, SPG7, TEKT3 | ACSF3, C18H17orf62, C1H12ORF73, CD7, CDH3, CDT1, DTYMK, FAM18B1, FARP2, GCC2, IGF2BP2, MRPL15, RNF168, ST6GAL1, TMEM68L, TRAPPC2L, USP43, XKR4, ZFPM1 |
| Candidate Gene | Gene Description | Gene Function |
|---|---|---|
| ALDH1L2 | Aldehyde dehydrogenase 1 family member L2 | Functions in meiosis and regulating mouse fertility [14] |
| ATP13A3 | ATPase 13A3 | Related to enone levels in pigs [15] |
| ATP13A4 | ATPase 13A4 | Associated with bovine sperm motility [16] |
| ATP13A5 | ATPase 13A5 | Related to the freezing of bull sperm [17] |
| CDH1 | Cadherin 1 | A specific marker for undifferentiated spermatogonia in mouse testes [18,19] |
| CDH3 | Cadherin 3 | Related to the number of germ cells in mice [20] |
| DNAJB11 | DnaJ heat shock protein family (Hsp40) member B11 | Regulated the production of germ cells and pre-Sertoli cells of the developing gonad [21] |
| MIR140 | MicroRNA 140 | Affected testicular function through oxidative stress pathway [22] |
| MIR7450 | MicroRNA 7450 | Regulation of testicular development and spermatogenesis in geese [22,23,24] |
| CFAP52 | Cilia and flagella associated protein 52 | Affected blood-testis barrier and sperm formation [25] |
| ABCG8 | ATP-binding cassette subfamily G member 8 | Determined sperm flagellum morphology [26] |
| AP1G1 | Adaptor-related protein complex 1 subunit γ 1 | Affected spermatogenesis [27,28] |
| CDT1 | Chromatin licensing and DNA replication factor 1 | After deletion, mouse and zebrafish testicular epithelial cells were abnormal [29] |
| CEP19 | Centrosomal protein 19 | Involved in the ciliary assembly of the human sperm [30] |
| CLDN1 | Claudin 1 | Affected sperm morphology [31,32] |
| COPS5 | COP9 signalosome subunit 5 | Ensured the normal formation of the blood epididymal barrier in mammals [33] |
| DZIP1L | DAZ-interacting zinc finger protein 1 like | Related to sperm count and infertility [34] |
| IGF2BP2 | Insulin-like growth factor 2 mRNA binding protein 2 | Its mutation led to abnormal sperm flagellum morphology and affected sperm motility [35] |
| PPP1R2 PPP1R7 | Protein phosphatase 1 regulatory inhibitor subunit 2 | In motile caudal sperm of mammalian, the association of PP1γ2 to PPP1R2 and PPP1R7 resembled immature caput sperm [36] |
| SEPT2 | Septin 2 | Associated with poor sperm motility in humans [37] |
| SLC5A7 | Solute carrier family 5 member 7 | Played an important role in mouse germ cell differentiation [38] |
| ZNF750 | Zinc finger protein 750 | Acted as a regulatory gene for the estrogen receptor in the rat [39] |
| ZFPM1 | Zinc finger protein, FOG family member 1 | Associated with regulation of testicular development and function in mice [40] |
| VPS4A | Vacuolar protein sorting 4 homolog A | Affected the progressive motility of spermatozoa in the Duroc boar population [41] |
| UTP4 | UTP4 small subunit processome component | Affected fertility in Drosophila [42] |
| UBE2G2 | Ubiquitin-conjugating enzyme E2 G2 | Affected fertility in men [43] |
| TUBB3 | Tubulin β 3 class III | Acted as a target of androgenic action on SCs from the initiation of meiosis to adult mice spermatogenesis [44] |
| SUMO3 | Small ubiquitin-like modifier 3 | Involvement of calcium in the regulation of centrin-1-SUMO-2/3 interaction in mouse testis [45] |
| ST6GAL1 | ST6 β-galactoside α-2,6-sialyltransferase 1 | As regulators and biomarkers of sperm storage duration in egg layer breeders [46,47] |
| SPIRE2 | Spire-type actin nucleation factor 2 | Associated with fertility traits in goats [48] |
| SPG7 | SPG7 matrix AAA peptidase subunit, paraplegin | Participated in spermiogenesis by functioning in the mitochondria in Phascolosoma esculenta [49] |
| SIX3 | SIX homeobox 3 | Be required for female fertility [50] |
| PMP22 | Peripheral myelin protein 22 | As a negative regulator of spermatogenesis in mice [51] |
| NFYB | Nuclear transcription factor Y subunit β | Played a role in the self-renewal and proliferation of planarian SSCs [52] |
| NFAT5 | Nuclear factor of activated T cells 5 | Having the strongest activity and greatest response to FSH stimulation [53,54,55,56] |
| NCK2 | NCK adaptor protein 2 | Interacted with NCK2 to modulate human SSC self-renewal and apoptosis via cell cyclins and cycle progression [57] |
| MTMR2 | Myotubularin-related protein 2 | Affected the depletion of spermatids and spermatocytes from the seminiferous epithelium in man [58] |
| MTA3 | Metastasis-associated 1 family member 3 | Associated with steroidogenic dysfunction in mammals [59] |
| LYPLA1 | Lysophospholipase 1 | Regulating testosterone secretion [60] |
| OPRK1 | Opioid receptor kappa 1 | Playing a role in maintaining normal GnRH pulse [61] |
| GAS8 | Growth arrest specific 8 | Involved in sperm motility [62,63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.-E.; Huang, K.; Gibril, B.A.A.; Xiong, X.; Wu, Y.; Wang, Z.; Xu, J. Identification of Novel Genetic Loci Involved in Testis Traits of the Jiangxi Local Breed Based on GWAS Analyses. Genes 2025, 16, 637. https://doi.org/10.3390/genes16060637
Ma J-E, Huang K, Gibril BAA, Xiong X, Wu Y, Wang Z, Xu J. Identification of Novel Genetic Loci Involved in Testis Traits of the Jiangxi Local Breed Based on GWAS Analyses. Genes. 2025; 16(6):637. https://doi.org/10.3390/genes16060637
Chicago/Turabian StyleMa, Jing-E, Ke Huang, Bahareldin Ali Abdalla Gibril, Xinwei Xiong, Yanping Wu, Zhangfeng Wang, and Jiguo Xu. 2025. "Identification of Novel Genetic Loci Involved in Testis Traits of the Jiangxi Local Breed Based on GWAS Analyses" Genes 16, no. 6: 637. https://doi.org/10.3390/genes16060637
APA StyleMa, J.-E., Huang, K., Gibril, B. A. A., Xiong, X., Wu, Y., Wang, Z., & Xu, J. (2025). Identification of Novel Genetic Loci Involved in Testis Traits of the Jiangxi Local Breed Based on GWAS Analyses. Genes, 16(6), 637. https://doi.org/10.3390/genes16060637





