The Effects of Ferulic Acid on the Growth Performance, Immune Function, Antioxidant Capacity, and Intestinal Microbiota of Broiler Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Grouping
2.2. Growth Performance
2.3. Sample Collection
2.4. Determination of Serum Biochemical Indexes
2.5. Determination of Antioxidant Index and Immune Factor Levels
2.6. Determination of Digestive Enzyme Activity in Duodenum
2.7. RT-qPCR
2.8. Bioinformatics Analysis of Intestinal Flora
2.9. Statistical Analysis
3. Results
3.1. Effects of Ferulic Acid on Production Performance of Broilers
3.2. Effect of Ferulic Acid on Serum Biochemical Indexes
3.3. Effects of Ferulic Acid on Duodenal Digestive Enzyme Activity
3.4. Effect of Ferulic Acid on Antioxidant Index and Immune Factor Levels
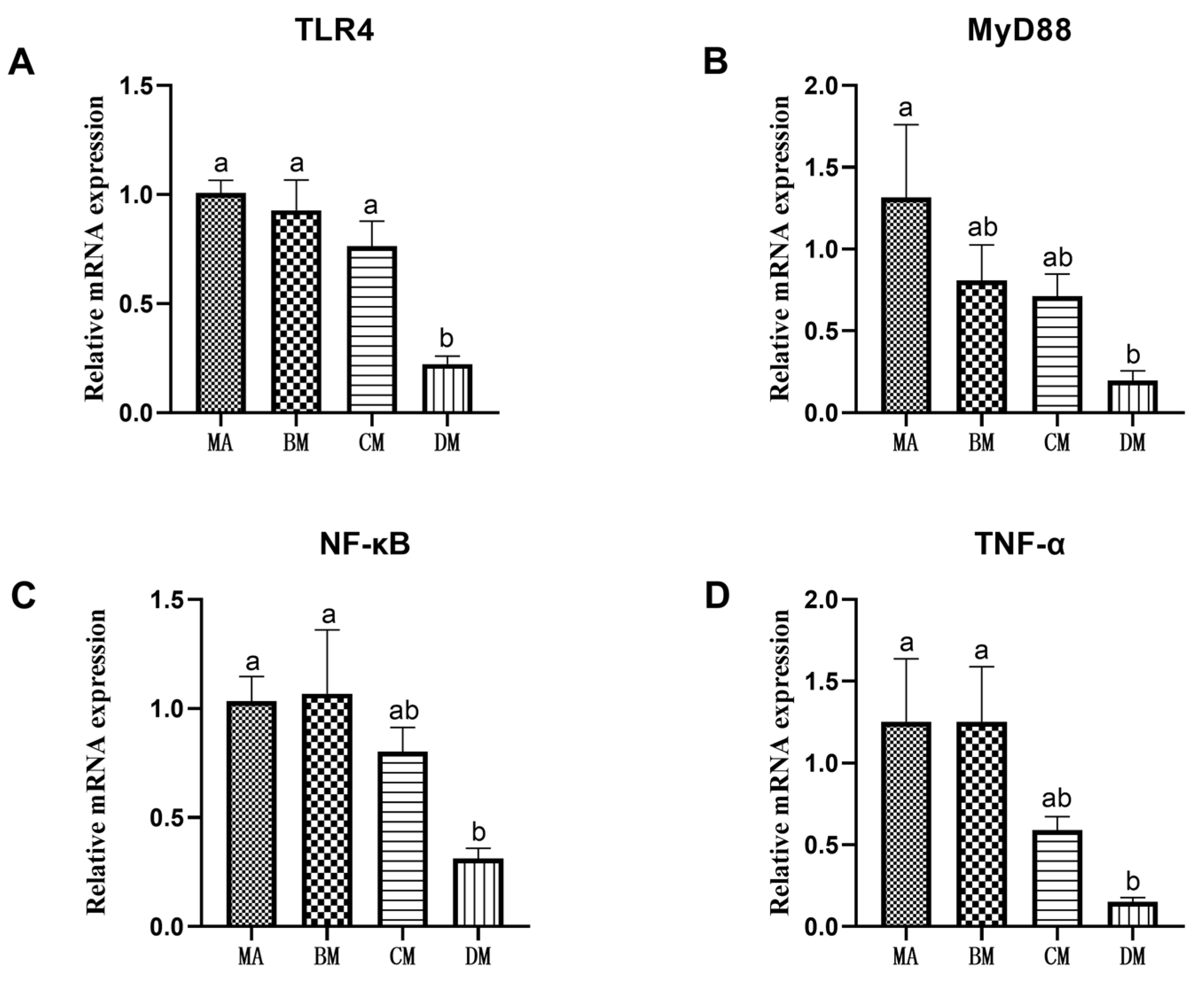
3.5. Effects of Ferulic Acid on Gene Expression of Jejunum Inflammation
3.6. Diversity Analysis of Intestinal Flora in Broilers
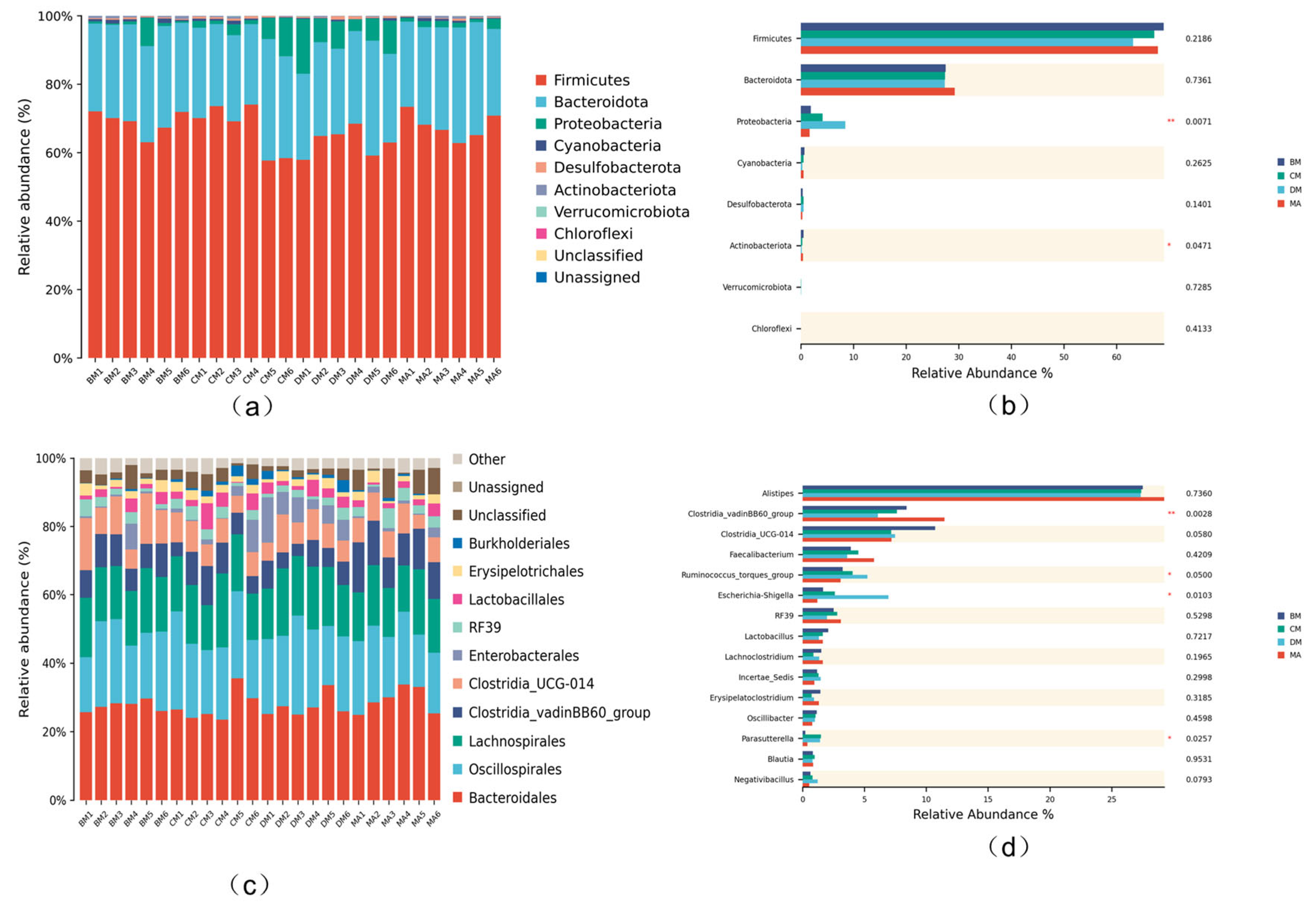
3.7. Analysis of Intestinal Microflora Structure in Broilers
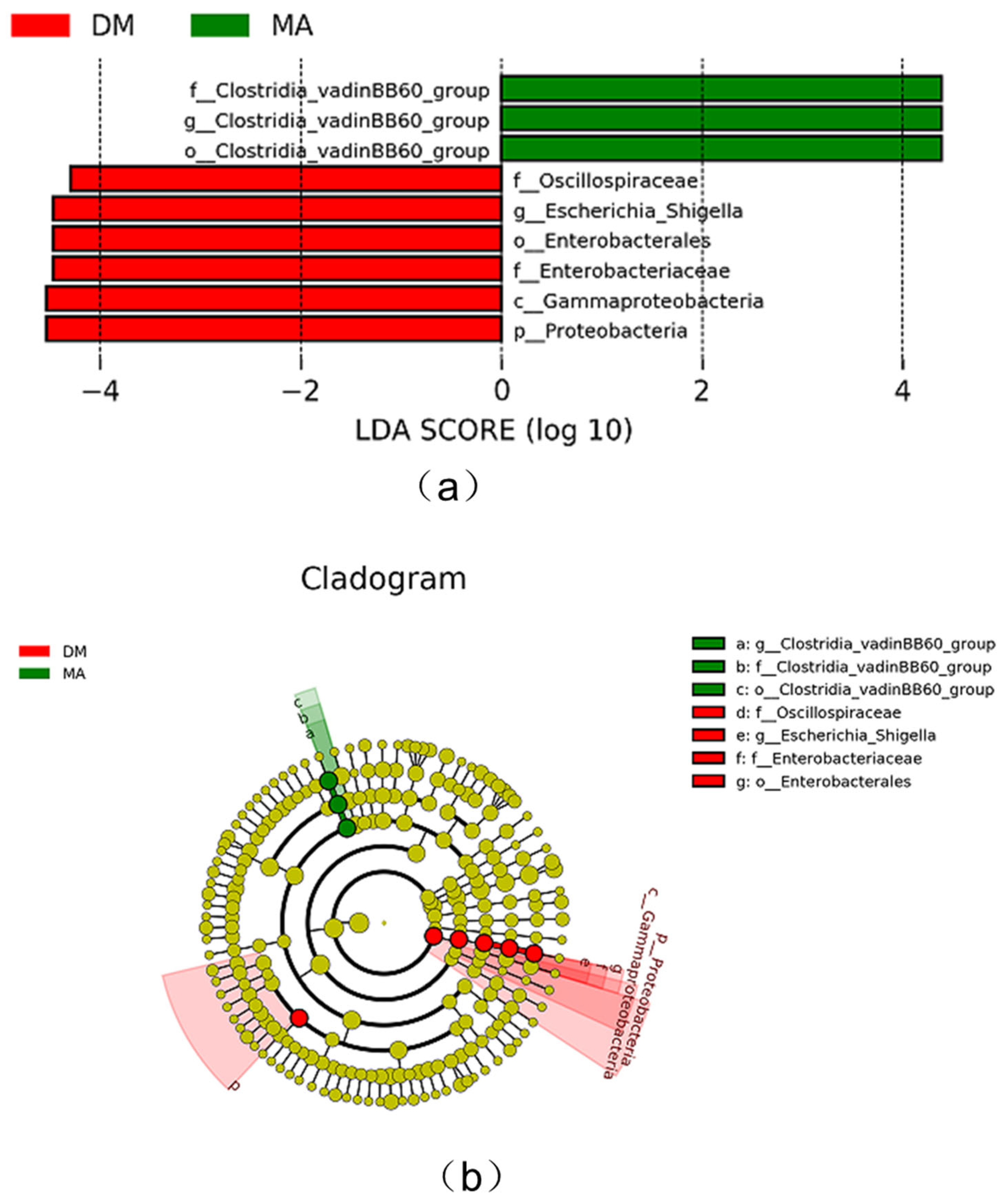
3.8. Comparison of Different Species of Intestinal Microflora in Broiler Chickens
3.9. Function Prediction Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, J.; Yang, Z.; Zhao, C.; Tang, X.; Jiang, Q.; Yin, Y. A comprehensive review on natural phenolic compounds as alternatives to in-feed antibiotics. Sci. China Life Sci. 2023, 66, 1518–1534. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Sri Balasubashini, M.; Rukkumani, R.; Menon, V.P. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003, 40, 118–122. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Olofinsan, K.A.; Msomi, N.Z.; Ijomone, O.K.; Islam, M.S. Ferulic acid mitigates diabetic cardiomyopathy via modulation of metabolic abnormalities in cardiac tissues of diabetic rats. Fundam. Clin. Pharmacol. 2023, 37, 44–59. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, J.; Zhao, L.; Zhao, Z.; Wang, S.; Zhang, L.; Zhou, F. Ferulic acid supplementation alleviates hyperuricemia in high-fructose/fat diet-fed rats via promoting uric acid excretion and mediating the gut microbiota. Food Funct. 2023, 14, 1710–1725. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Che, Q.; Luo, T.; Shi, J.; He, Y.; Xu, D.L. Mechanisms by Which Traditional Chinese Medicines Influence the Intestinal Flora and Intestinal Barrier. Front. Cell. Infect. Microbiol. 2022, 12, 863779. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Corrigendum: Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Mafra, D.; Barros, A.F.; Fouque, D. Dietary protein metabolism by gut microbiota and its consequences for chronic kidney disease patients. Future Microbiol. 2013, 8, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Said, H.M.; Supuran, C.T.; Hageman, C.; Staab, A.; Polat, B.; Katzer, A.; Scozzafava, A.; Anacker, J.; Flentje, M.; Vordermark, D. Modulation of carbonic anhydrase 9 (CA9) in human brain cancer. Curr. Pharm. Des. 2010, 16, 3288–3299. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B.; et al. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Fasina, Y.O.; Newman, M.M.; Stough, J.M.; Liles, M.R. Effect of Clostridium perfringens infection and antibiotic administration on microbiota in the small intestine of broiler chickens. Poult. Sci. 2016, 95, 247–260. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Vieira, B.S.; Hofacre, C.; Applegate, T.J. Effect of different challenge models to induce necrotic enteritis on the growth performance and intestinal microbiota of broiler chickens. Poult. Sci. 2019, 98, 2800–2812. [Google Scholar] [CrossRef]
- Guo, H.; Zuo, Z.; Wang, F.; Gao, C.; Chen, K.; Fang, J.; Cui, H.; Ouyang, P.; Geng, Y.; Chen, Z.; et al. Attenuated Cardiac oxidative stress, inflammation and apoptosis in Obese Mice with nonfatal infection of Escherichia coli. Ecotoxicol. Environ. Saf. 2021, 225, 112760. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, X.; Luo, H.; Zhu, K. Therapeutic effect of polysaccharide of large yellow croaker swim bladder on lupus nephritis of mice. Nutrients 2014, 6, 1223–1235. [Google Scholar] [CrossRef]
- Han, K.; Ma, J.; Dou, J.; Hao, D.; Zhu, W.; Yu, X.; Zheng, W.; Song, Y.; Shi, F.; Li, Q. A Clinical Trial of the Effects of a Dietary Pattern on Health Metrics and Fecal Metabolites in Volunteers With Risk of Cardiovascular Disease. Front. Nutr. 2022, 9, 853365. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Wang, Y.; Guo, Y.; Zhu, P.; Li, G.; Zhang, J.; Ma, Q.; Zhao, L. Correction: Dietary ellagic acid ameliorated Clostridium perfringens-induced subclinical necrotic enteritis in broilers via regulating inflammation and cecal microbiota. J. Anim. Sci. Biotechnol. 2022, 13, 66. [Google Scholar] [CrossRef]
- Shu, G.; Tang, Z.; Du, H.; Zheng, Y.; Chang, L.; Li, H.; Xu, F.; Fu, H.; Zhang, W.; Lin, J. Effects of Dietary Ferulic Acid Supplementation on Hepatic Injuries in Tianfu Broilers Challenged with Lipopolysaccharide. Toxins 2022, 14, 227. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Wang, R.; Ji, X.; Zhang, J.; Guo, T.; Hu, Y.; An, X.; Gao, A.; Qi, J. Effect of ferulic acid on growth, digestibility, digestive enzyme activity, immunity and antioxidant status of broilers. S. Afr. J. Anim. Sci. 2022, 52, 280–290. [Google Scholar]
- Li, C.; Zhang, B.; Wang, X.; Pi, X.; Wang, X.; Zhou, H.; Mai, K.; He, G. Improved utilization of soybean meal through fermentation with commensal Shewanella sp. MR-7 in turbot (Scophthalmus maximus L.). Microb. Cell Factories 2019, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tang, L.; Bai, X.; Du, K.; Wang, H.; Jia, X.; Lai, S. Heat Stress Altered the Vaginal Microbiome and Metabolome in Rabbits. Front. Microbiol. 2022, 13, 813622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.; Huang, H.; Ma, Y.; Wang, R.; Hu, Y.; Zheng, X.; Chen, C.; Tang, H. Squid Ink Polysaccharides Protect Human Fibroblast Against Oxidative Stress by Regulating NADPH Oxidase and Connexin43. Front. Pharmacol. 2019, 10, 1574. [Google Scholar] [CrossRef]
- Yan, J.; Chen, L.; Zhang, L.; Zhang, Z.; Zhao, Y.; Wang, Y.; Ou, J. New Insights Into the Persistent Effects of Acute Exposure to AFB1 on Rat Liver. Front. Microbiol. 2022, 13, 911757. [Google Scholar] [CrossRef]
- Wei, Z.; Xue, Y.; Xue, Y.; Cheng, J.; Lv, G.; Chu, L.; Ma, Z.; Guan, S. Ferulic acid attenuates non-alcoholic steatohepatitis by reducing oxidative stress and inflammation through inhibition of the ROCK/NF-κB signaling pathways. J. Pharmacol. Sci. 2021, 147, 72–80. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Jia, F.; Li, H.; Sun, M.; Fu, Z.; Zhou, H.; Guo, W.; Gao, Y. Effects of ferulic acid on growth performance and intestinal oxidation indexes of Jilin white geese under lipopolysaccharide-induced oxidative stress. PLoS ONE 2023, 18, e0291955. [Google Scholar] [CrossRef]
- Li, F.; Zhang, B.; Zhang, Y.; Zhang, X.; Usman, S.; Ding, Z.; Hao, L.; Guo, X. Probiotic effect of ferulic acid esterase-producing Lactobacillus plantarum inoculated alfalfa silage on digestion, antioxidant, and immunity status of lactating dairy goats. Anim. Nutr. 2022, 11, 38–47. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, N.; Zhou, L.; Wang, J.; Zhou, Y.; Zhang, T.; Fang, Y.; Deng, J.; Gao, Y.; Liang, X.; et al. IL-2 regulates tumor-reactive CD8+ T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 2021, 22, 358–369. [Google Scholar] [CrossRef]
- Peng, P.; Lou, Y.; Wang, J.; Wang, S.; Liu, P.; Xu, L.X. Th1-Dominant CD4+ T Cells Orchestrate Endogenous Systematic Antitumor Immune Memory After Cryo-Thermal Therapy. Front. Immunol. 2022, 13, 944115. [Google Scholar] [CrossRef]
- Liu, B.; Yan, Y.; Wang, X.; Chen, N.; Wu, J. Locally generated C3 regulates the clearance of Toxoplasma gondii by IFN-γ-primed macrophage through regulation of xenophagy. Front. Microbiol. 2022, 13, 944006. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Park, J.; Joe, Y.; Park, H.J.; Jekal, S.J.; Sato, D.; Hamada, H.; Chung, H.T. Pterostilbene 4′-β-Glucoside Protects against DSS-Induced Colitis via Induction of Tristetraprolin. Oxidative Med. Cell. Longev. 2017, 2017, 9427583. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wu, D.; Ma, Y.; Cao, Y.; Liu, Q.; Tang, M.; Pu, Y.; Zhang, T. Reactive oxygen species trigger NF-κB-mediated NLRP3 inflammasome activation involvement in low-dose CdTe QDs exposure-induced hepatotoxicity. Redox Biol. 2021, 47, 102157. [Google Scholar] [CrossRef]
- Kuang, Z.S.; Leng, Y.X.; Yang, N.; Li, Z.Q.; Zong, Y.N.; Han, D.Y.; Li, Y.; He, J.D.; Mi, X.N.; Cong, Z.K.; et al. Inhibition of visfatin alleviates sepsis-induced intestinal damage by inhibiting Hippo signaling pathway. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2022, 71, 911–922. [Google Scholar] [CrossRef]
- Zhou, Z.; He, W.; Tian, H.; Zhan, P.; Liu, J. Thyme (Thymus vulgaris L.) polyphenols ameliorate DSS-induced ulcerative colitis of mice by mitigating intestinal barrier damage, regulating gut microbiota, and suppressing TLR4/NF-κB-NLRP3 inflammasome pathways. Food Funct. 2023, 14, 1113–1132. [Google Scholar] [CrossRef]
- Kinra, M.; Ranadive, N.; Nampoothiri, M.; Arora, D.; Mudgal, J. Involvement of NLRP3 inflammasome pathway in the protective mechanisms of ferulic acid and p-coumaric acid in LPS-induced sickness behavior and neuroinflammation in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 1829–1839. [Google Scholar] [CrossRef]
- Zhang, D.; Jing, B.; Chen, Z.N.; Li, X.; Shi, H.M.; Zheng, Y.C.; Chang, S.Q.; Gao, L.; Zhao, G.P. Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-κB pathway. CNS Neurosci. Ther. 2023, 29, 1000–1011. [Google Scholar] [CrossRef]
- Yu, S.; Qian, H.; Zhang, D.; Jiang, Z. Ferulic acid relieved ulcerative colitis by inhibiting the TXNIP/NLRP3 pathway in rats. Cell Biol. Int. 2023, 47, 417–427. [Google Scholar] [CrossRef]
- Clavel, T.; Gomes-Neto, J.C.; Lagkouvardos, I.; Ramer-Tait, A.E. Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunol. Rev. 2017, 279, 8–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, K.; Chen, H.; Su, Y.; Zhu, W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb. Biotechnol. 2018, 11, 859–868. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, X.; Tao, G.; Hao, W.; Wang, L.; Lan, Z.; Song, Y.; Wu, M.; Huang, J.Q. Ferulic acid and feruloylated oligosaccharides alleviate anxiety and depression symptom via regulating gut microbiome and microbial metabolism. Food Res. Int. 2022, 162, 111887. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Naskar, M.; Bera, A.; Mukhopadhyay, B. Chemical synthesis of the pentasaccharide repeating unit of the O-specific polysaccharide from Ruminococcus gnavus. Carbohydr. Res. 2021, 507, 108384. [Google Scholar] [CrossRef] [PubMed]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]



| Group | MA | BM | CM | DM |
|---|---|---|---|---|
| average daily feed intake | 40.36 ± 0.48 a | 39.47 ± 0.44 a | 39.47 ± 0.44 a | 32.84 ± 0.51 a |
| average daily gain | 35.92 ± 0.52 a | 32.03 ± 0.30 a | 29.71 ± 0.20 a | 31.67 ± 0.41 a |
| feed–gain ratio | 1.12 ± 0.07 b | 1.27 ± 0.14 a | 1.32 ± 0.10 a | 1.05 ± 0.03 b |
| Group | Urea Nitrogen | Creatinine | Total Cholesterol | Triglyceride |
|---|---|---|---|---|
| MA | 0.76 ± 0.07 b | 1.50 ± 1.00 b | 3.43 ± 0.39 b | 0.47 ± 0.14 b |
| BM | 0.82 ± 0.09 b | 1.75 ± 1.25 b | 4.62 ± 0.77 b | 0.53 ± 0.13 b |
| CM | 1.66 ± 0.01 a | 133.00 ± 27.38 a | 7.46 ± 0.26 a | 2.7 ± 0.12 a |
| DM | 1.74 ± 0.51 a | 144.25 ± 21.53 a | 7.54 ± 0.29 a | 2.82 ± 0.22 a |
| Group | Chymotrypsin | Lipase | Trypsin | Amylase |
|---|---|---|---|---|
| MA | 0.23 ± 0.15 b | 5.16 ± 1.07 b | 167.52 ± 40.57 b | 3343.33 ± 465.93 b |
| BM | 0.31 ± 0.32 b | 5.14 ± 1.10 b | 460.71 ± 27.03 b | 4051.33 ± 302.93 a |
| CM | 2.11 ± 0.01 a | 6.04 ± 2.46 b | 544.39 ± 39.37 b | 2940.00 ± 238.16 b |
| DM | 3.09 ± 0.24 a | 9.55 ± 1.42 a | 1241.91 ± 17.76 a | 3045.00 ± 318.56 b |
| Item | MA | BM | CM | DM |
|---|---|---|---|---|
| SOD (U/mL) | 13.18 ± 0.90 b | 13.68 ± 1.72 b | 16.98 ± 1.38 a | 18.31 ± 1.64 a |
| MDA (nmol/mL) | 4.46 ± 0.18 a | 3.9 ± 0.26 b | 3.8 ± 0.13 b | 3.52 ± 0.22 b |
| GSH-Px (U/mL) | 210.07 ± 15.46 b | 214.82 ± 19.65 b | 360.79 ± 16.58 a | 330.50 ± 17.26 a |
| Item | MA | BM | CM | DM |
|---|---|---|---|---|
| IL-2 (pg/mL) | 389.08 ± 40.88 a | 343.23 ± 25.25 b | 356.51 ± 29.77 ab | 381.20 ± 36.70 ab |
| C-3 (µg/mL) | 627.13 ± 63.92 b | 629.70 ± 155.61 ab | 632.68 ± 121.64 ab | 787.56 ± 175.88 a |
| IFN-γ (pg/mL) | 106.84 ± 11.34 b | 109.07 ± 10.61 ab | 122.68 ± 12.63 a | 109.71 ± 11.60 ab |
| Group | Faith_PD Index | ACE Index | Chao1 Index | Shannon Index | Simpson Index |
|---|---|---|---|---|---|
| MA | 29.98 ± 3.22 b | 624.03 ± 42.80 | 619.71 ± 41.28 | 6.37 ± 0.33 | 0.94 ± 0.03 |
| BM | 32.71 ± 2.10 ab | 816.82 ± 34.94 | 811.75 ± 32.89 | 7.02 ± 0.12 | 0.97 ± 0.01 |
| CM | 32.81 ± 2.33 ab | 738.11 ± 21.55 | 734.50 ± 26.02 | 6.59 ± 0.13 | 0.96 ± 0.01 |
| DM | 34.47 ± 1.60 a | 682.91 ± 12.68 | 680.76 ± 13.73 | 6.57 ± 0.37 | 0.96 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, X.; Ma, Q.; Li, Z.; Hu, Y.; Wu, H.; Wang, R.; Sun, X.; Wang, E.; Ma, C.; Qin, Q. The Effects of Ferulic Acid on the Growth Performance, Immune Function, Antioxidant Capacity, and Intestinal Microbiota of Broiler Chickens. Genes 2025, 16, 572. https://doi.org/10.3390/genes16050572
Yi X, Ma Q, Li Z, Hu Y, Wu H, Wang R, Sun X, Wang E, Ma C, Qin Q. The Effects of Ferulic Acid on the Growth Performance, Immune Function, Antioxidant Capacity, and Intestinal Microbiota of Broiler Chickens. Genes. 2025; 16(5):572. https://doi.org/10.3390/genes16050572
Chicago/Turabian StyleYi, Xianguo, Quanchao Ma, Zhili Li, Yuli Hu, Haigang Wu, Rui Wang, Xuyang Sun, Enen Wang, Chaofeng Ma, and Qingmin Qin. 2025. "The Effects of Ferulic Acid on the Growth Performance, Immune Function, Antioxidant Capacity, and Intestinal Microbiota of Broiler Chickens" Genes 16, no. 5: 572. https://doi.org/10.3390/genes16050572
APA StyleYi, X., Ma, Q., Li, Z., Hu, Y., Wu, H., Wang, R., Sun, X., Wang, E., Ma, C., & Qin, Q. (2025). The Effects of Ferulic Acid on the Growth Performance, Immune Function, Antioxidant Capacity, and Intestinal Microbiota of Broiler Chickens. Genes, 16(5), 572. https://doi.org/10.3390/genes16050572






