Reduction in Liver Cancer Risk by Quercetin via Modulation of Urate Levels: Insights from Drug-Target Mendelian Randomization
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Preprocessing
2.2. Drug-Target Mendelian Randomization Analysis
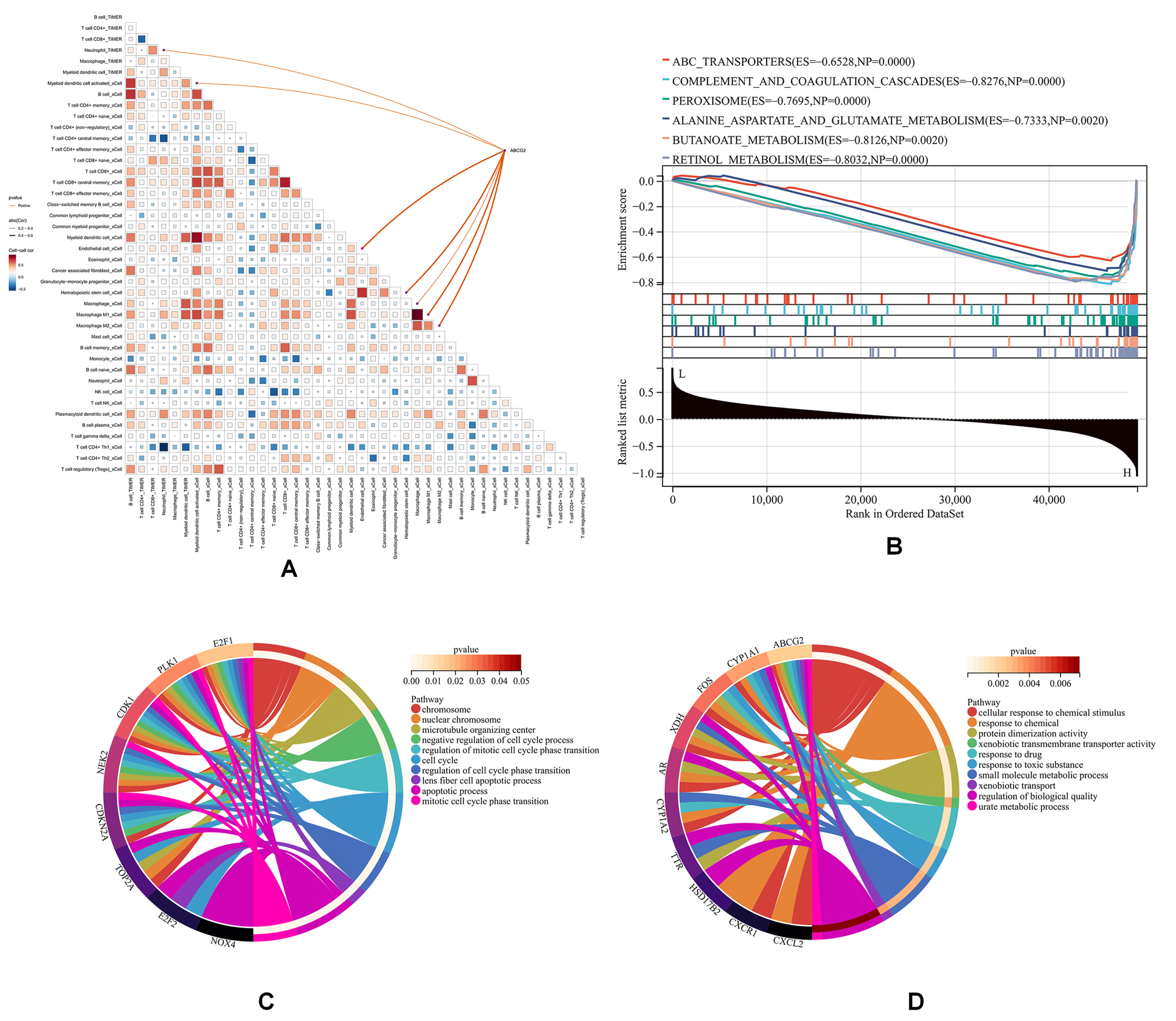
2.3. Bioinformatics Analysis
3. Results
3.1. MR Analysis Results Show That ABCG2 Has a Causal Relationship with Urate
3.2. MR Analysis Results Show That ABCG2 (Urate Related) Has a Causal Relationship with Liver Cancer
3.3. Heterogeneity and Pleiotropy Analysis
3.4. ABCG2 Expression Is Elevated in CCA Endothelial Cells by Single-Cell Analysis
3.5. ABCG2 Is Associated with Immune Infiltration in CCA
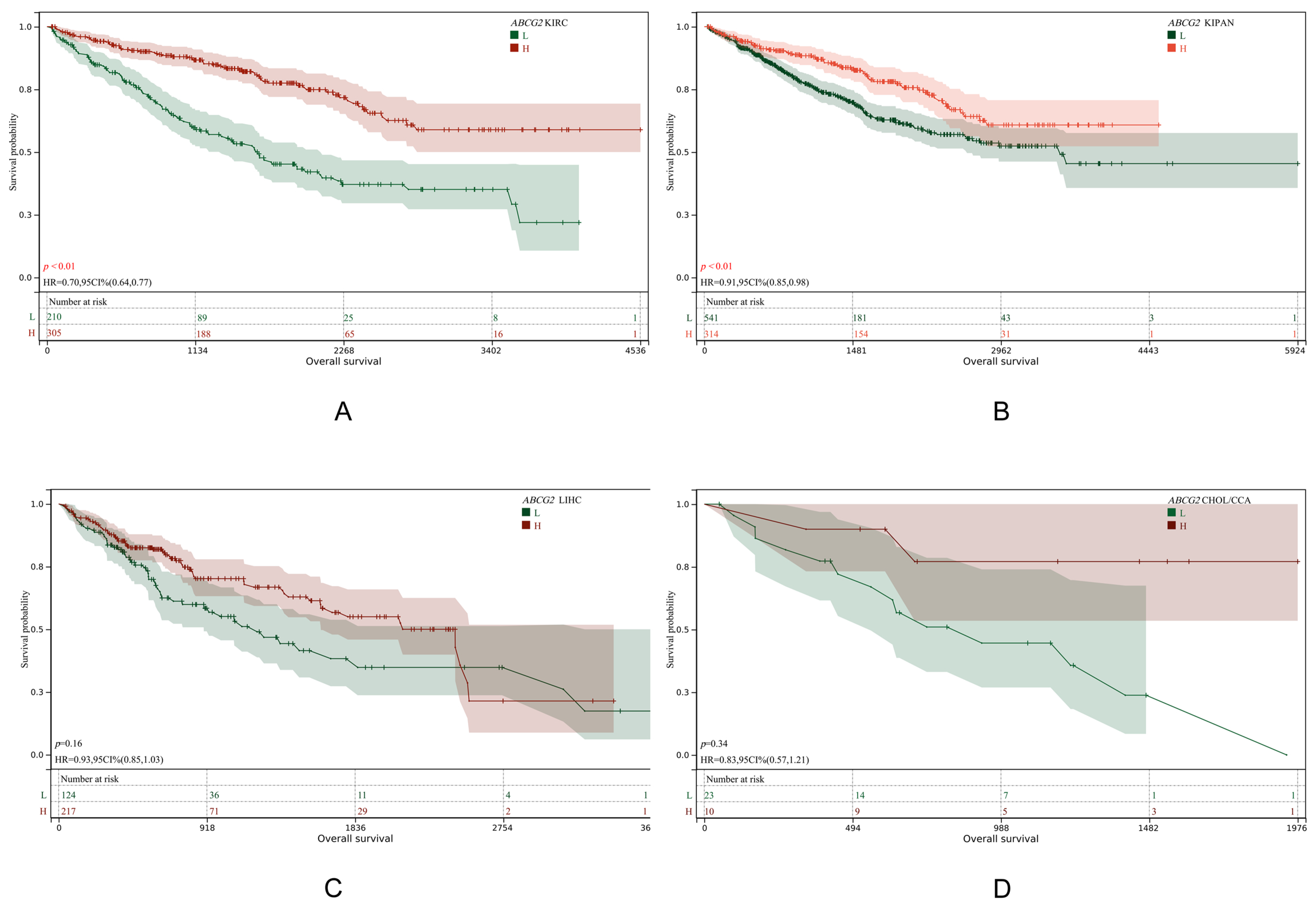
3.6. ABCG2 Is Not Associated with Poor Prognosis in CCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Wang, Y.; Yao, L.; Gu, W.; Zhao, S.; Shen, Z.; Lin, Z.; Liu, W.; Yan, T. Pharmacological activity of quercetin: An updated review. Evid.-Based Complement. Altern. Med. 2022, 2022, 3997190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Chen, H.-Y.; Tang, Q.-Q.; Li, Y.-F.; Liu, X.-S.; Lu, F.-H.; Gu, Y.-Y. Protective effect of quercetin on kidney diseases: From chemistry to herbal medicines. Front. Pharmacol. 2022, 13, 968226. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; McGlynn, K.A. The changing epidemiology of primary liver cancer. Curr. Epidemiol. Rep. 2019, 6, 104–111. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Ladd, A.D.; Duarte, S.; Sahin, I.; Zarrinpar, A. Mechanisms of drug resistance in HCC. Hepatology 2024, 79, 926–940. [Google Scholar] [CrossRef]
- Sadagopan, N.; He, A.R. Recent progress in systemic therapy for advanced hepatocellular carcinoma. Int. J. Mol. Sci. 2024, 25, 1259. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Aboona, M.B.; Sukphutanan, B.; Kongarin, S.; Duangsonk, K.; Ng, C.H.; Muthiah, M.D.; Huang, D.Q.; Seko, Y.; Díaz, L.A. Incidence of liver cancer in young adults according to the global burden of disease database 2019. Hepatology 2024, 80, 828–843. [Google Scholar] [CrossRef]
- Hisaka, T.; Sakai, H.; Sato, T.; Goto, Y.; Nomura, Y.; Fukutomi, S.; Fujita, F.; Mizobe, T.; Nakashima, O.; Tanigawa, M. Quercetin suppresses proliferation of liver cancer cell lines in vitro. Anticancer Res. 2020, 40, 4695–4700. [Google Scholar] [CrossRef]
- Sethi, G.; Rath, P.; Chauhan, A.; Ranjan, A.; Choudhary, R.; Ramniwas, S.; Sak, K.; Aggarwal, D.; Rani, I.; Tuli, H.S. Apoptotic mechanisms of quercetin in liver cancer: Recent trends and advancements. Pharmaceutics 2023, 15, 712. [Google Scholar] [CrossRef]
- Wu, L.; Yang, W.; Zhang, Y.; Du, X.; Jin, N.; Chen, W.; Li, H.; Zhang, S.; Xie, B. Elevated serum uric acid is associated with poor survival in advanced HCC patients and febuxostat improves prognosis in HCC rats. Front. Pharmacol. 2021, 12, 778890. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yamada, S.; Tanabe, H.; Takami, H.; Inokawa, Y.; Sonohara, F.; Shimizu, D.; Hattori, N.; Kanda, M.; Tanaka, C. High serum uric acid levels could be a risk factor of hepatocellular carcinoma recurrences. Nutr. Cancer 2021, 73, 996–1003. [Google Scholar] [CrossRef]
- Allegrini, S.; Garcia-Gil, M.; Pesi, R.; Camici, M.; Tozzi, M.G. The good, the bad and the new about uric acid in cancer. Cancers 2022, 14, 4959. [Google Scholar] [CrossRef]
- Mi, S.; Gong, L.; Sui, Z. Friend or foe? An unrecognized role of uric acid in cancer development and the potential anticancer effects of uric acid-lowering drugs. J. Cancer 2020, 11, 5236. [Google Scholar] [CrossRef]
- Bahriz, H.A.; Abdelaziz, R.R.; El-Kashef, D.H. Allopurinol abates hepatocellular carcinoma in rats via modulation of NLRP3 inflammasome and NF-κB pathway. Naunyn-Schmiedebergs Arch. Pharmacol. 2024, 398, 6043–6058. [Google Scholar] [CrossRef]
- Rao, H.; Wang, Q.; Zeng, X.; Wen, X.; Huang, L. Analysis of the prognostic value of uric acid on the efficacy of immunotherapy in patients with primary liver cancer. Clin. Transl. Oncol. 2024, 26, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Ma, Y.; Tang, M.; Yuan, X.; Shen, L. Elevated serum uric acid is associated with the risk of advanced staging and vascular involvement in patients with hepatoblastoma: A 14-year retrospective study. Front. Oncol. 2023, 13, 1144349. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Li, F.; Huiyao, Z.; Song, Z.; Li, D. Quercetin ameliorates hyperuricemic nephropathy by repressing uric acid synthesis and reabsorption in mice and cells. eFood 2024, 5, e139. [Google Scholar] [CrossRef]
- Shi, Y.; Williamson, G. Quercetin lowers plasma uric acid in pre-hyperuricaemic males: A randomised, double-blinded, placebo-controlled, cross-over trial. Br. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef]
- Xie, R.; Zhang, Y. Associations between dietary flavonoid intake with hepatic steatosis and fibrosis quantified by VCTE: Evidence from NHANES and FNDDS. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1179–1189. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef]
- Fatima, T.; Dehlin, M.; Burgess, S.; Mason, A.M.; Nilsson, P.M.; Melander, O.; Jacobsson, L.T.; Kapetanovic, M.C. Genetically Predicted Serum Urate and the Risk of All-Cause and Site-Specific Cancer: A Mendelian Randomization Study; eLife Sciences Publications Ltd.: Cambridge, UK, 2024. [Google Scholar]
- Schmidt, A.F.; Finan, C.; Gordillo-Marañón, M.; Asselbergs, F.W.; Freitag, D.F.; Patel, R.S.; Tyl, B.; Chopade, S.; Faraway, R.; Zwierzyna, M. Genetic drug target validation using Mendelian randomisation. Nat. Commun. 2020, 11, 3255. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, W.; Zhang, D.; Mi, Y.; Zhang, J.; He, G. The causal relationship between PCSK9 inhibitors and malignant tumors: A mendelian randomization study based on drug targeting. Genes 2024, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Sun, K.; Li, L.; Zu, X.; Han, T.; Huang, H. Mechanism of quercetin therapeutic targets for Alzheimer disease and type 2 diabetes mellitus. Sci. Rep. 2021, 11, 22959. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Kang, D.-H.; Ha, S.-K. Uric acid puzzle: Dual role as anti-oxidantand pro-oxidant. Electrolytes Blood Press. E BP 2014, 12, 1. [Google Scholar] [CrossRef]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E. Soluble uric acid activates the NLRP3 inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef]
- Yen, P.-C.; Chou, Y.-T.; Li, C.-H.; Sun, Z.-J.; Wu, C.-H.; Chang, Y.-F.; Lu, F.-H.; Yang, Y.-C.; Chang, C.-J.; Wu, J.-S. Hyperuricemia is associated with significant liver fibrosis in subjects with nonalcoholic fatty liver disease, but not in subjects without it. J. Clin. Med. 2022, 11, 1445. [Google Scholar] [CrossRef]
- Eckenstaler, R.; Benndorf, R.A. The role of ABCG2 in the pathogenesis of primary hyperuricemia and gout—An update. Int. J. Mol. Sci. 2021, 22, 6678. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Schuetz, J. Role of ABCG2/BCRP in biology and medicine. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 381–410. [Google Scholar] [CrossRef]
- Hoque, K.M.; Dixon, E.E.; Lewis, R.M.; Allan, J.; Gamble, G.D.; Phipps-Green, A.J.; Halperin Kuhns, V.L.; Horne, A.M.; Stamp, L.K.; Merriman, T.R. The ABCG2 Q141K hyperuricemia and gout associated variant illuminates the physiology of human urate excretion. Nat. Commun. 2020, 11, 2767. [Google Scholar] [CrossRef]
- Song, Y.-K.; Yoon, J.-H.; Woo, J.K.; Kang, J.-H.; Lee, K.-R.; Oh, S.H.; Chung, S.-J.; Maeng, H.-J. Quercetin is a flavonoid breast cancer resistance protein inhibitor with an impact on the oral pharmacokinetics of sulfasalazine in rats. Pharmaceutics 2020, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Xu, D.; Wu, J.; Jiang, Q.; Zeng, Y. Phyllanthi Fructus ameliorates hyperuricemia and kidney injure via inhibiting uric acid synthesis, modulating urate transporters, and alleviating inflammation. Sci. Rep. 2024, 14, 27605. [Google Scholar] [CrossRef]
- Mo, W.; Zhang, J.-T. Human ABCG2: Structure, function, and its role in multidrug resistance. Int. J. Biochem. Mol. Biol. 2011, 3, 1. [Google Scholar]
- Tan, S.; Radi, R.; Gaudier, F.; Evans, R.A.; Rivera, A.; Kirk, K.A.; Parks, D.A. Physiologic levels of uric acid inhibit xanthine oxidase in human plasma. Pediatr. Res. 1993, 34, 303–307. [Google Scholar] [CrossRef]
- Lin, Z.; Xie, Y.-Z.; Zhao, M.-C.; Hou, P.-P.; Tang, J.; Chen, G.-L. Xanthine dehydrogenase as a prognostic biomarker related to tumor immunology in hepatocellular carcinoma. Cancer Cell Int. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu JiXiao, Z.J.; Wang Ying, W.Y.; Kong LingDong, K.L.; Yang Cheng, Y.C.; Zhang Xin, Z.X. Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J. Ethnopharmacol. 2004, 93, 133–140. [Google Scholar]
- Cheng, M.; Yuan, C.; Ju, Y.; Liu, Y.; Shi, B.; Yang, Y.; Jin, S.; He, X.; Zhang, L.; Min, D. Quercetin Attenuates Oxidative Stress and Apoptosis in Brain Tissue of APP/PS1 Double Transgenic AD Mice by Regulating Keap1/Nrf2/HO-1 Pathway to Improve Cognitive Impairment. Behav. Neurol. 2024, 2024, 5698119. [Google Scholar] [CrossRef]
- Luo, X.; Weng, X.; Bao, X.; Bai, X.; Lv, Y.; Zhang, S.; Chen, Y.; Zhao, C.; Zeng, M.; Huang, J. A novel anti-atherosclerotic mechanism of quercetin: Competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 2022, 57, 102511. [Google Scholar] [CrossRef]
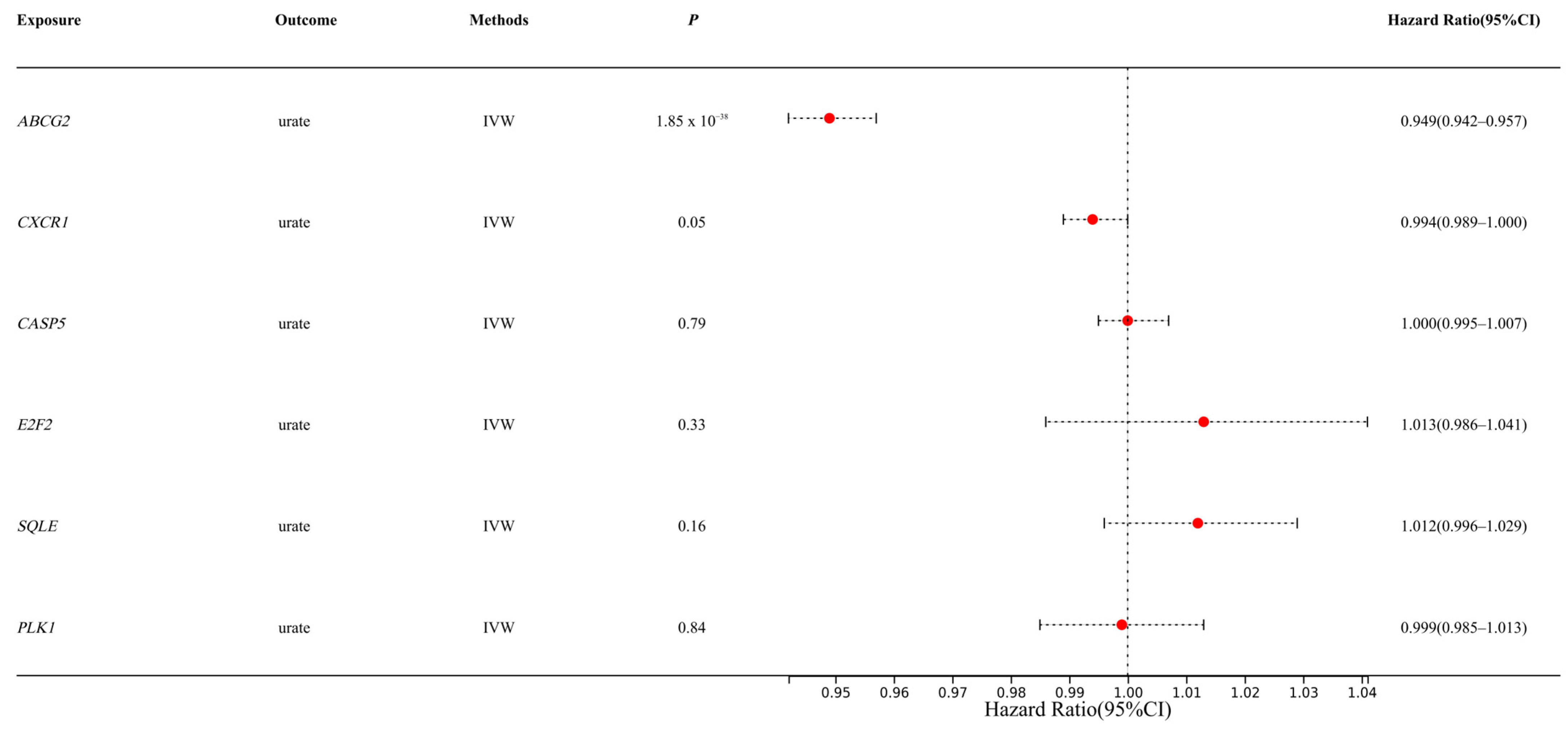
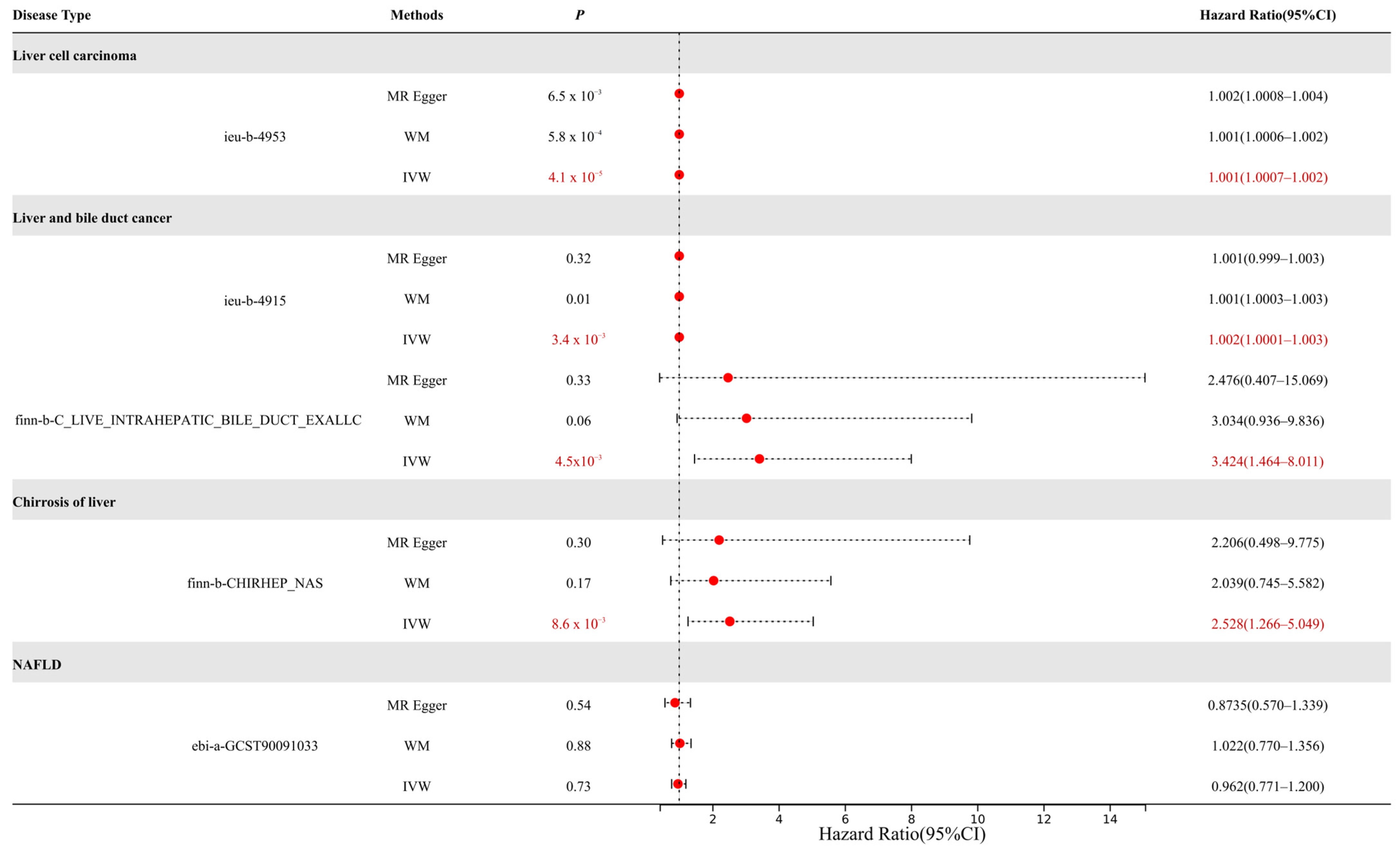
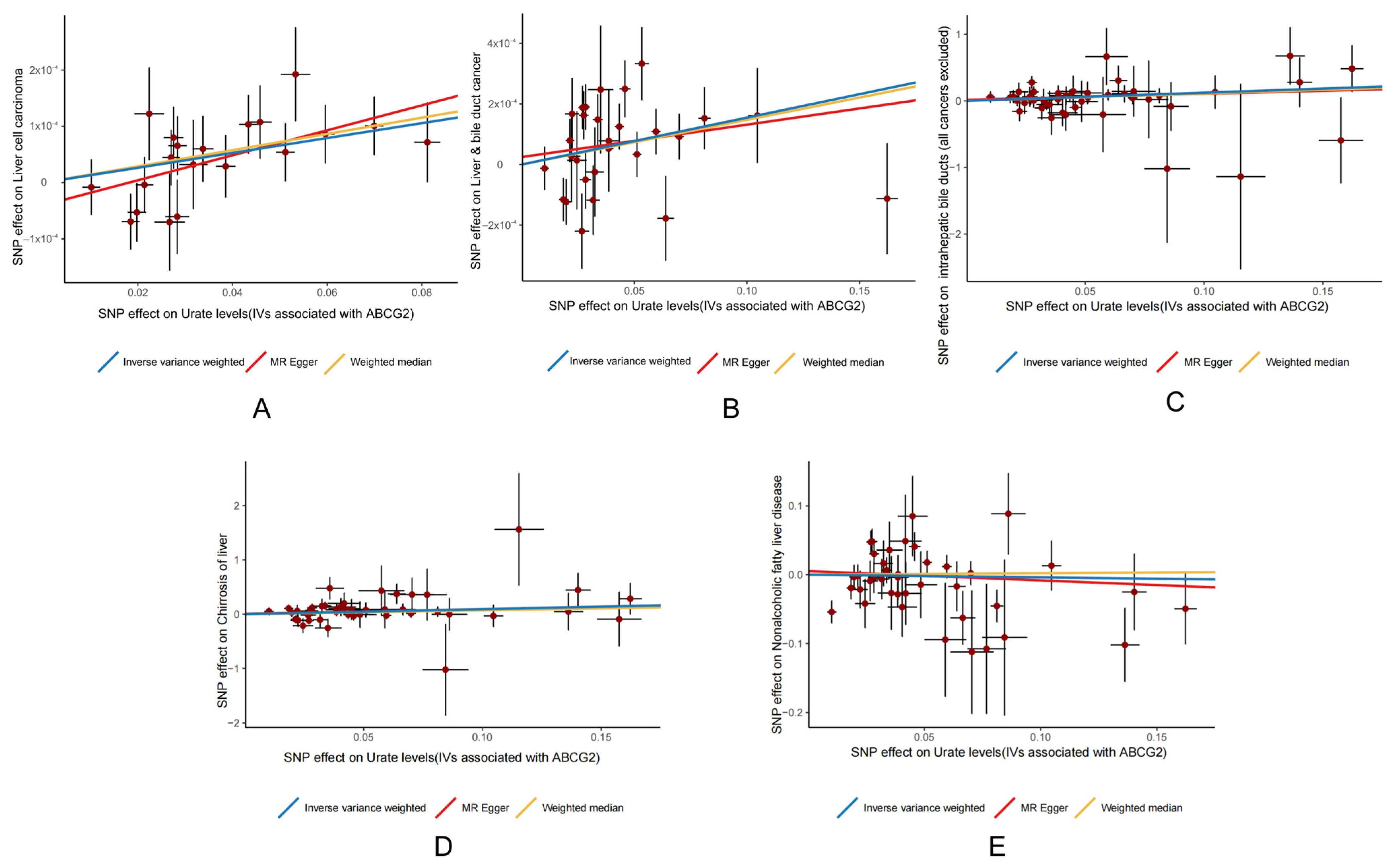

| Characteristics | GWAS-ID | Type | Sample Size | Number of SNPs |
|---|---|---|---|---|
| Uric acid (Urate) | ebi-a-GCST90014015 | Exposure | 389,404 | 10,783,684 |
| Liver cell carcinoma | ieu-b-4953 | Outcome | 372,184 | 6,304,034 |
| Liver and bile duct cancer | ieu-b-4915 | Outcome | 372,366 | 7,687,713 |
| Malignant neoplasm of liver and intrahepatic bile ducts | finn-b-C3_LIVER_INTRAHEPATIC_BILE_DUCTS_EXALLC | Outcome | 174,006 | 16,380,303 |
| Chirrosis of liver | finn-b-CHIRHEP_NAS | Outcome | 217,334 | 16,380,465 |
| NAFLD | ebi-a-GCST90091033 | Outcome | 778,164 | 6,784,388 |
| Disease Type | Methods | SNPs | Heterogeneity | p | Pleiotropy | p |
|---|---|---|---|---|---|---|
| Liver cell carcinoma (ieu-b-4953) | MR Egger | 20 | 14.474 | 0.698 | 2.9 × 10−5 | 0.181 |
| IVW | 20 | 16.409 | 0.630 | |||
| Liver and bile duct cancer (ieu-b-4915) | MR Egger | 28 | 46.584 | 7.8 × 10−3 | 4.6 × 10−5 | 0.600 |
| IVW | 28 | 47.088 | 9.7 × 10−3 | |||
| Liver and bile duct cancer (finn-b-C3_LIVER_INTRAHEPATIC_BILE_DUCTS_EXALLC) | MR Egger | 46 | 29.846 | 0.949 | 0.040 | 0.692 |
| IVW | 46 | 30.004 | 0.958 | |||
| Chirrosis of liver (finn-b-CHIRHEP_NAS) | MR Egger | 46 | 46.033 | 0.388 | 0.033 | 0.840 |
| IVW | 46 | 46.076 | 0.427 | |||
| NAFLD (ebi-a-GCST90091033) | MR Egger | 40 | 65.087 | 4.0 × 10−3 | 0.010 | 0.607 |
| IVW | 40 | 65.547 | 4.9 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, Y.; Yang, K.; Li, C.; Zhang, M. Reduction in Liver Cancer Risk by Quercetin via Modulation of Urate Levels: Insights from Drug-Target Mendelian Randomization. Genes 2025, 16, 449. https://doi.org/10.3390/genes16040449
Li Z, Wang Y, Yang K, Li C, Zhang M. Reduction in Liver Cancer Risk by Quercetin via Modulation of Urate Levels: Insights from Drug-Target Mendelian Randomization. Genes. 2025; 16(4):449. https://doi.org/10.3390/genes16040449
Chicago/Turabian StyleLi, Zhengwen, Yue Wang, Kaichuan Yang, Chujie Li, and Ming Zhang. 2025. "Reduction in Liver Cancer Risk by Quercetin via Modulation of Urate Levels: Insights from Drug-Target Mendelian Randomization" Genes 16, no. 4: 449. https://doi.org/10.3390/genes16040449
APA StyleLi, Z., Wang, Y., Yang, K., Li, C., & Zhang, M. (2025). Reduction in Liver Cancer Risk by Quercetin via Modulation of Urate Levels: Insights from Drug-Target Mendelian Randomization. Genes, 16(4), 449. https://doi.org/10.3390/genes16040449







