Identification and Expression Profiling of the Cytokinin Synthesis Gene Family IPT in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome-Wide Identification and Physicochemical Properties Analysis of the Maize IPT Gene Family Members
2.2. Plant Material and Stress Treatments
2.3. Total RNA Extraction, cDNA Reverse Transcription, and Quantitative Real-Time PCR Analysis
2.4. Statistics and Analysis
3. Result
3.1. Identification and Physicochemical Property Analysis of the Maize IPT Gene Family
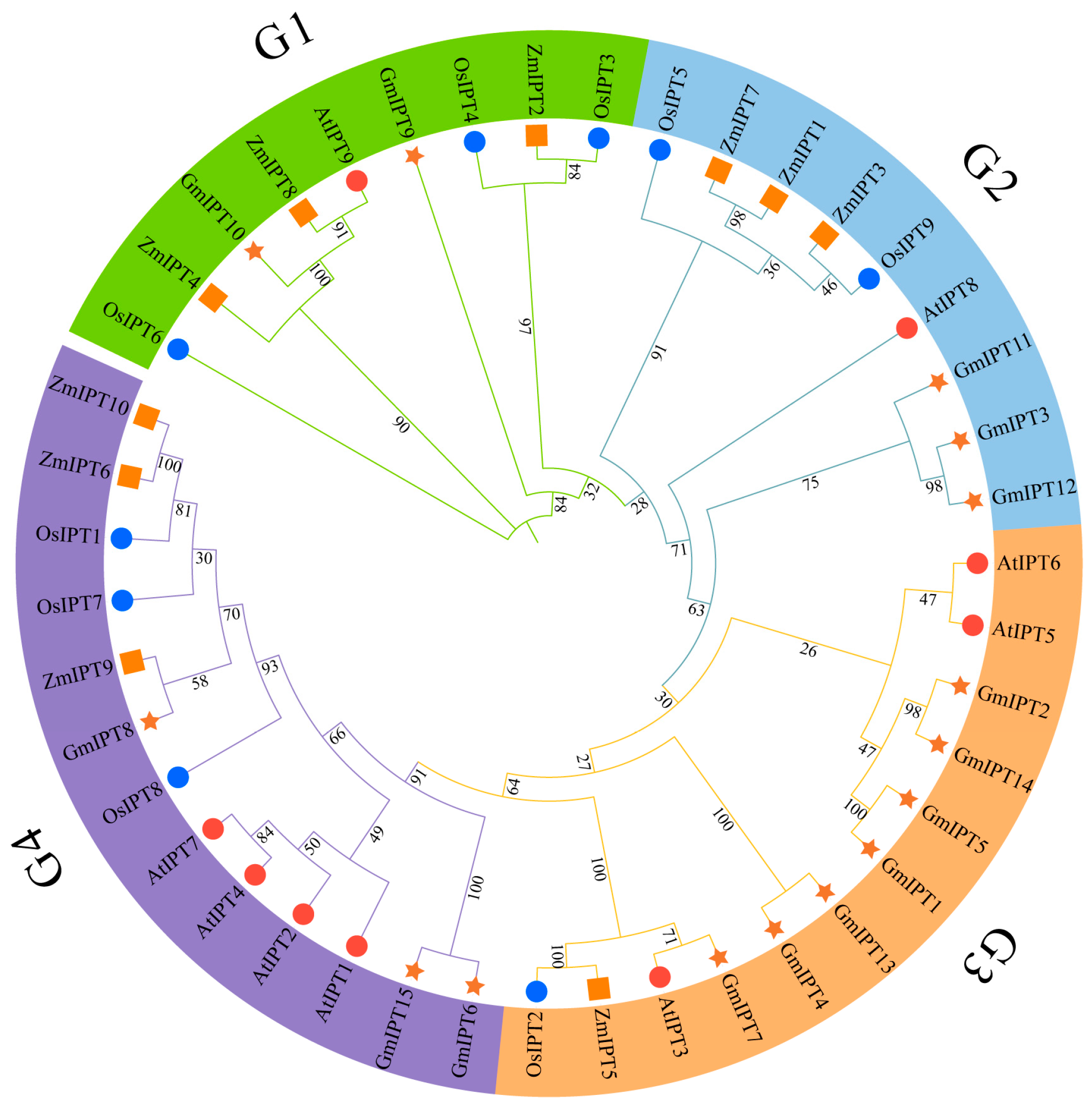
3.2. Phylogenetic Analysis of the ZmIPT Gene Family
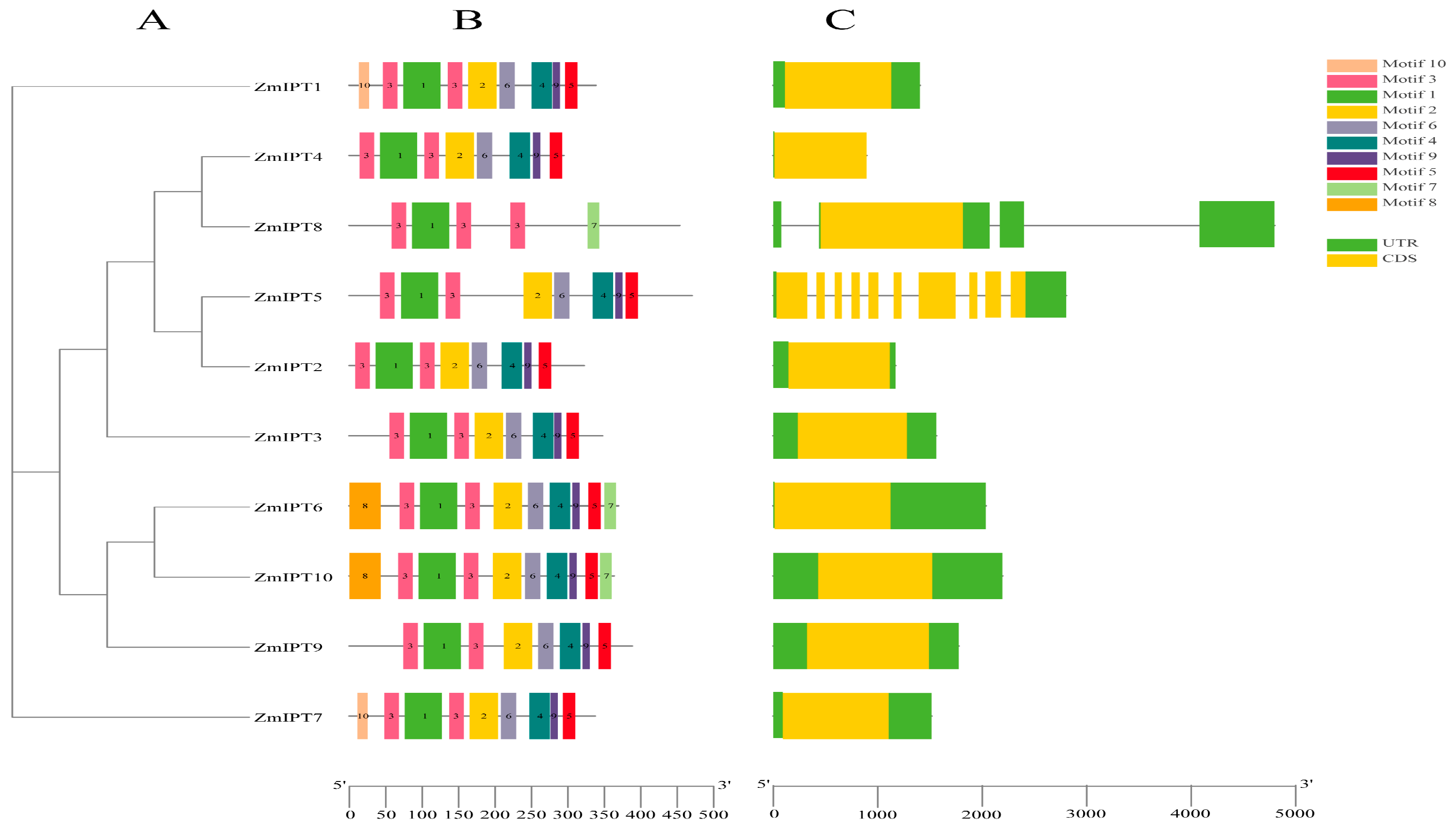
3.3. Analysis of Conserved Motifs in the ZmIPT Gene Family
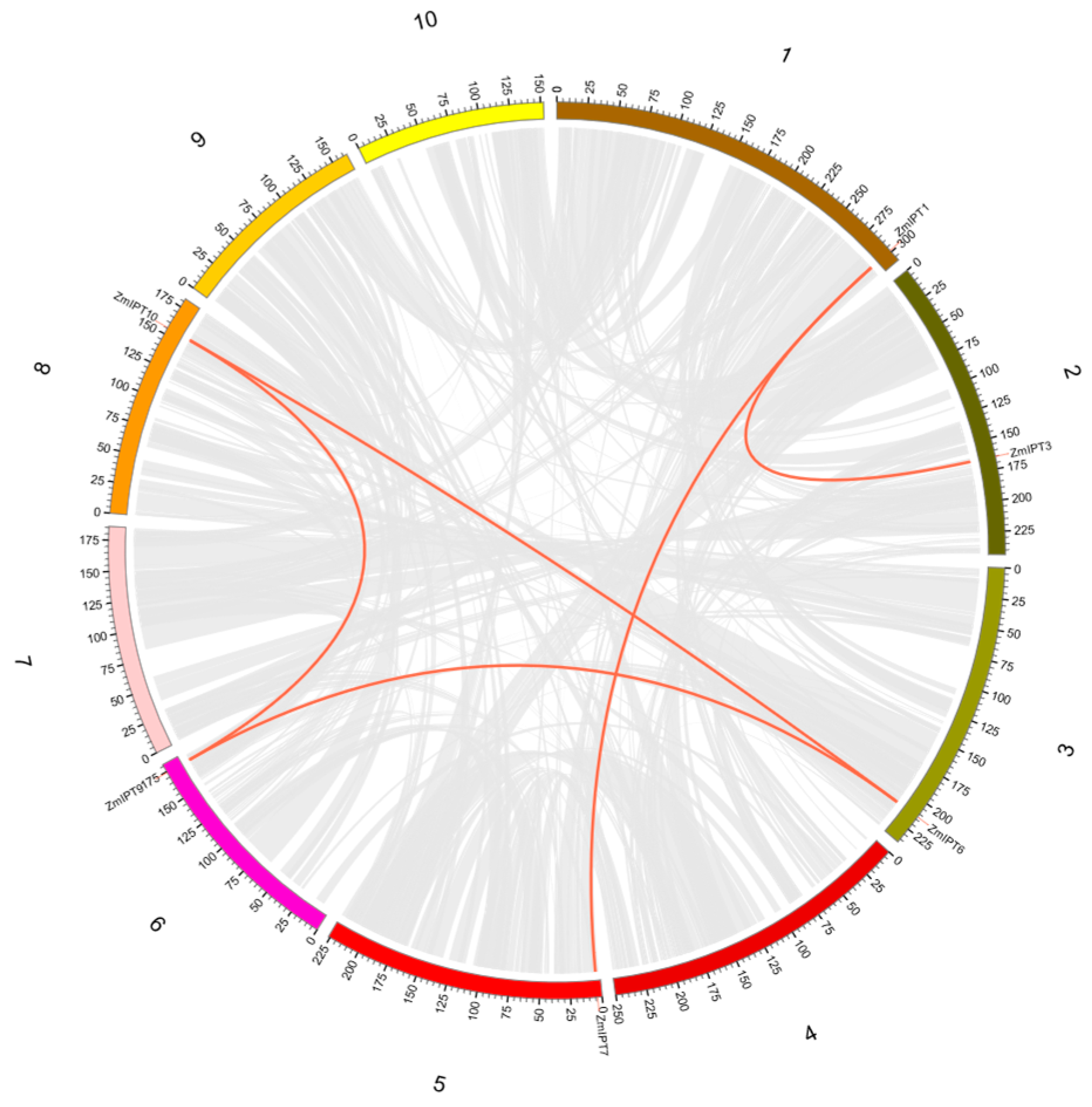
3.4. Synteny Analysis of the ZmIPT Gene Family
3.5. Analysis of Cis-Acting Elements in the Promoters of the ZmIPT Gene Family
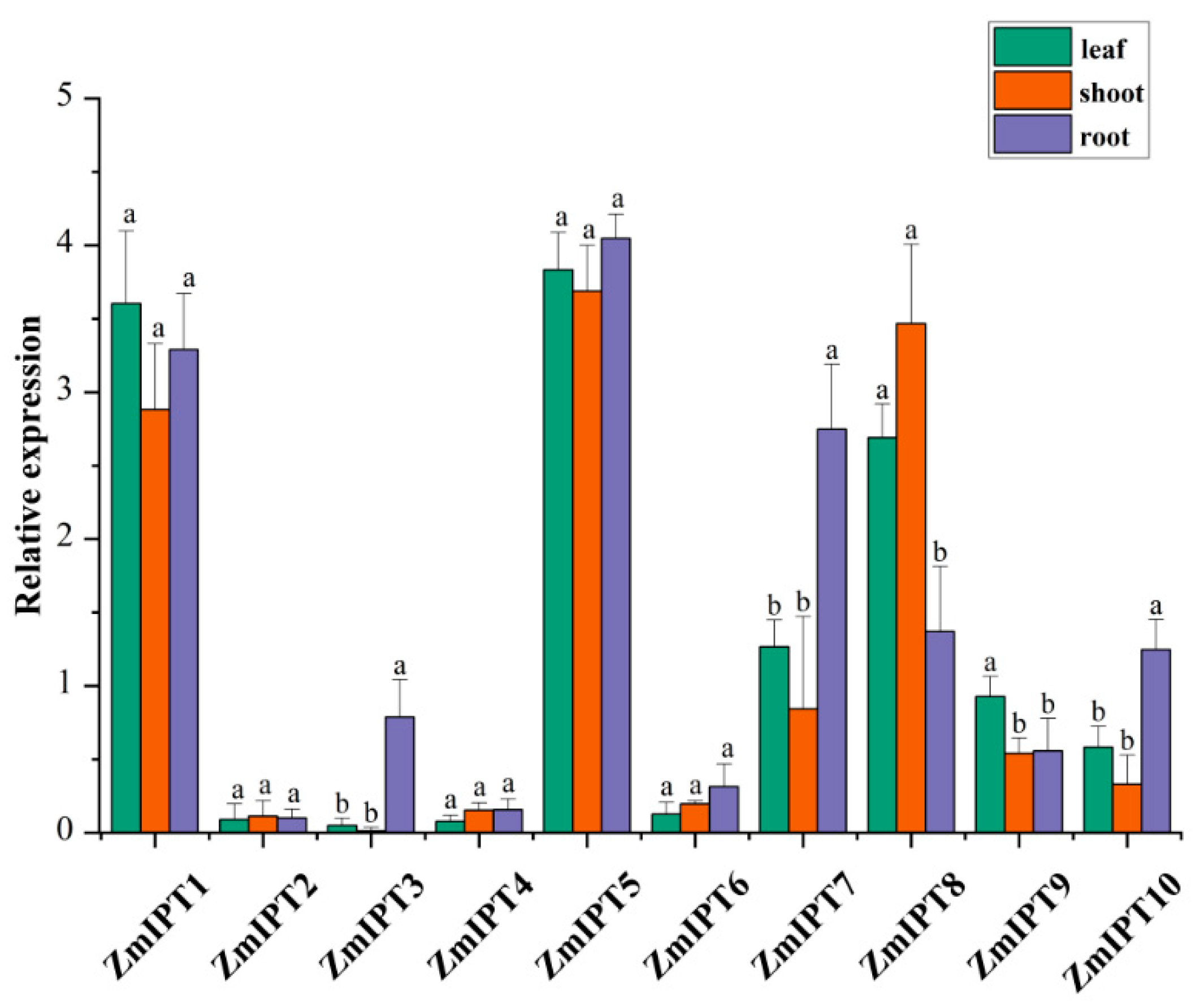
3.6. Expression Analysis of ZmIPT Genes in Different Tissues
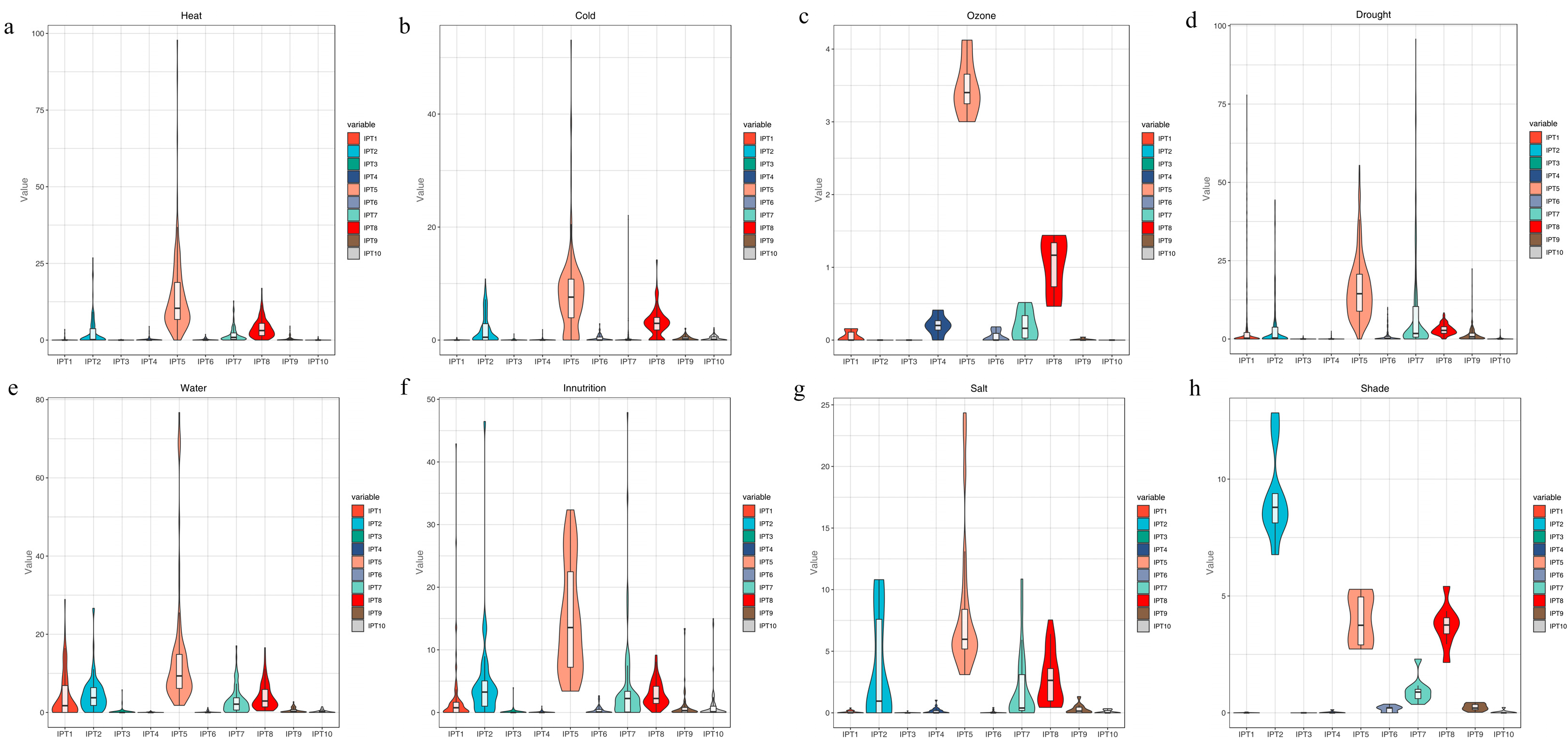
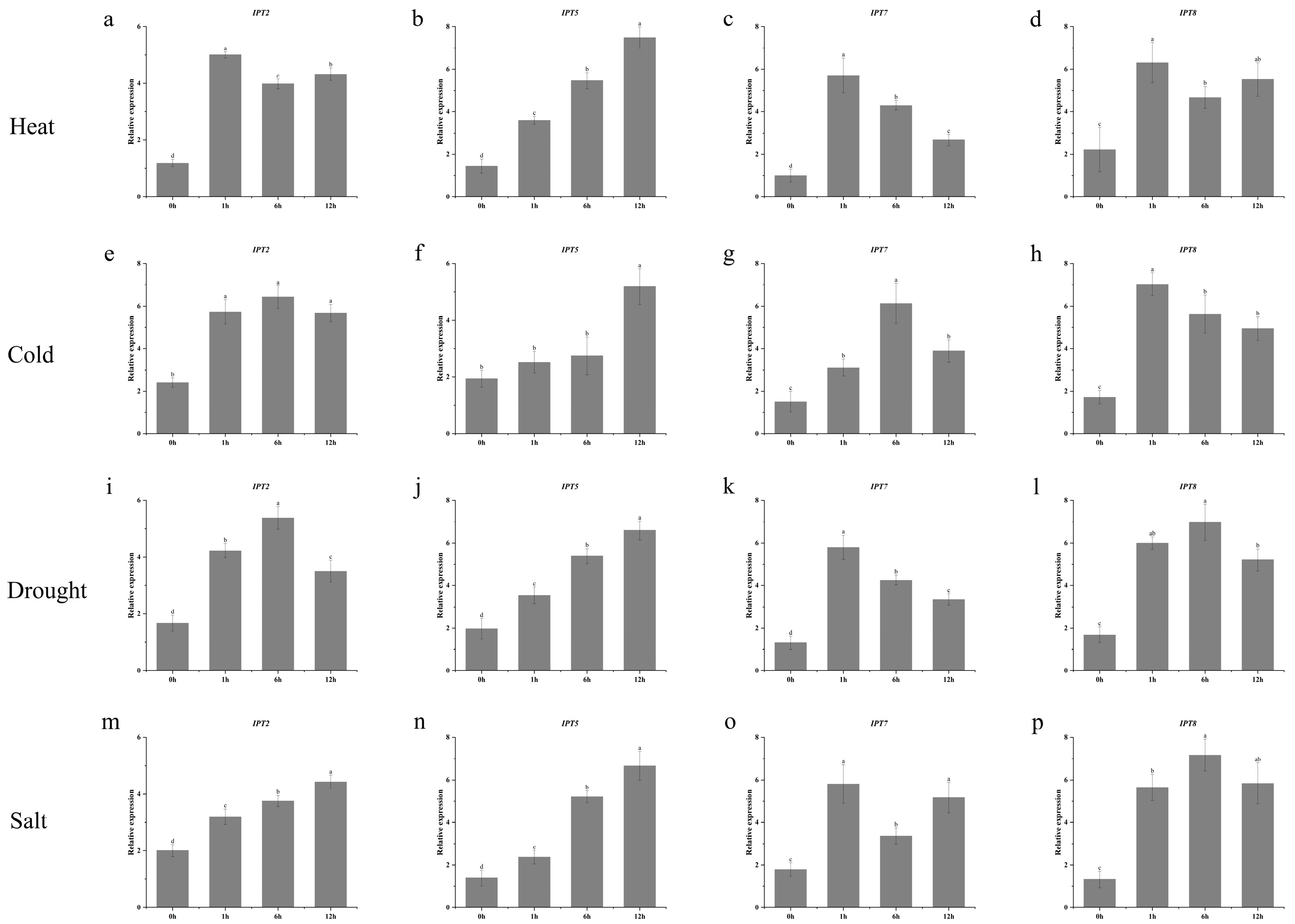
3.7. Expression Analysis of IPT Gene in Maize Under Abiotic Stress
3.8. Prediction of Interacting Proteins of IPT5 in Maize
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Argueso, C.T.; Ferreira, F.J.T.E.; Epple, P.; To, J.P.C.; Hutchison, C.E.; Schäller, G.; Dangl, J.L.; Kieber, J.J. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012, 8, e1002448. [Google Scholar]
- Glanz-Idan, N.; Lach, M.; Tarkowski, P.; Vrobel, O.; Wolf, S. Delayed leaf senescence by upregulation of cytokinin biosynthesis specifically in tomato roots. Front. Plant Sci. 2022, 13, 922106. [Google Scholar]
- Li, Y.Y.; Hao, Z.G.; Miao, S.; Zhang, X.; Li, J.Q.; Guo, S.X.; Lee, Y.I. Profiles of cytokinins metabolic genes and endogenous cytokinins dynamics during shoot multiplication in vitro of Phalaenopsis. Int. J. Mol. Sci. 2022, 23, 3755. [Google Scholar] [CrossRef] [PubMed]
- Antoniadi, I.; Mateo-Bonmatí, E.; Pernisová, M.; Brunoni, F.; Antoniadi, M.; Villalonga Garcia-Atance, M.; Ament, A.; Karády, M.; Turnbull, C.; Doležal, K.; et al. IPT9, a cis-zeatin cytokinin biosynthesis gene, promotes root growth. Front. Plant Sci. 2022, 13, 932008. [Google Scholar]
- Zhang, J.; Wang, T.; Jia, Z.; Jia, X.; Liu, Y.; Xuan, J.; Wang, G.; Zhang, F. Transcriptome analysis reveals a comprehensive virus resistance response mechanism in pecan infected by a novel badnavirus pecan virus. Int. J. Mol. Sci. 2022, 23, 13576. [Google Scholar] [CrossRef]
- Miyawaki, K.; Tarkowski, P.; Matsumoto-Kitano, M.; Kato, T.; Sato, S.; Tarkowská, D.; Tabata, S.; Sandberg, G.; Kakimoto, T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 16598–16603. [Google Scholar]
- Aoki, M.M.; Kisiała, A.; Mathavarajah, S.; Schincaglia, A.; Treverton, J.; Habib, E.; Dellaire, G.; Emery, R.J.N.; Brunetti, C.R.; Huber, R.J. From biosynthesis and beyond—Loss or overexpression of the cytokinin synthesis gene, iptA, alters cytokinesis and mitochondrial and amino acid metabolism in Dictyostelium discoideum. FASEB J. 2023, 38, e23366. [Google Scholar]
- Joshi, S.; Choukimath, A.; Isenegger, D.; Panozzo, J.; Spangenberg, G.; Kant, S. Improved wheat growth and yield by delayed leaf senescence using developmentally regulated expression of a cytokinin biosynthesis gene. Front. Plant Sci. 2019, 10, 1285. [Google Scholar]
- Frébort, I.; Kowalska, M.; Hluska, T.; Frébortová, J.; Galuszka, P. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar]
- Nguyen, H.N.; Lai, N.; Kisiała, A.; Emery, R.J.N. Isopentenyltransferases as master regulators of crop performance: Their function, manipulation, and genetic potential for stress adaptation and yield improvement. Plant Biotechnol. J. 2021, 19, 1297–1313. [Google Scholar] [PubMed]
- Belintani, N.G.; Guerzoni, J.T.S.; Moreira, R.M.P.; Vieira, L.G.E. Improving low-temperature tolerance in sugarcane by expressing the IPT gene under a cold inducible promoter. Biol. Plant. 2012, 56, 71–77. [Google Scholar]
- Xiao, X.M.; Zeng, Y.L.; Cao, B.H.; Liu, J.; Chen, Q.H.; Meng, C.; Cheng, Y.B. PSAG12-IPT overexpression in eggplant delays leaf senescence and induces abiotic stress tolerance. J. Hortic. Sci. Biotechnol. 2017, 92, 349–357. [Google Scholar]
- Nishiyama, R.; Watanabe, Y.; Fujita, Y.; Le, D.T.; Kojima, M.; Werner, T.; Vaňková, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Kakimoto, T.; et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt, and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 2011, 23, 2169–2183. [Google Scholar]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar]
- Hussain, S.B.; Jaspal, M.H.; Shoaib, M.; Zubair, M. Genome-wide Identification and Characterization of Cytokinin Metabolic Gene Families in Chickpea (Cicer arietinum). Asian Plant Res. J. 2023, 11, 98–118. [Google Scholar]
- Qin, H.; Gu, Q.; Zhang, J.L.; Sun, L.; Kuppu, S.; Zhang, Y.Z.; Burow, M.D.; Payton, P.; Blumwald, E.; Zhang, H. Regulated expression of an isopentenyltransferase gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 2011, 52, 1904–1914. [Google Scholar]
- Kuppu, S.; Mishra, N.; Hu, R.; Sun, L.; Zhu, X.; Shen, G.; Zhang, H. Water-deficit inducible expression of a cytokinin biosynthetic gene IPT improves drought tolerance in cotton. PLoS ONE 2013, 8, e64190. [Google Scholar]
- Oneto, C.D.; Otegui, M.E.; Baroli, I.; Beznec, A.; Faccio, P.; Bossio, E.; Blumwald, E.; Lewi, D.M. Water deficit stress tolerance in maize conferred by expression of an isopentenyltransferase (IPT) gene driven by a stress- and maturation-induced promoter. J. Biotechnol. 2016, 220, 66–77. [Google Scholar]
- Bedada, L.T.; Seth, M.S.; Runo, S.; Teffera, W.; Mugoya, C.; Masiga, C.W.; Oduor, R.; Blumwald, E.; Wachira, F.N. Drought tolerant tropical maize (Zea mays L.) developed through genetic transformation with isopentenyltransferase gene. Afr. J. Biotechnol. 2016, 15, 2447–2464. [Google Scholar]
- Zhang, Y.; Liang, C.J.; Xu, Y.; Gianfagna, T.J. Effects of IPT Gene Expression on Leaf Senescence Induced by Nitrogen or Phosphorus Deficiency in Creeping Bentgrass. J. Am. Soc. Hortic. Sci. 2010, 135, 108–115. [Google Scholar]
- Jackai, L.E.N. Laboratory and screenhouse assays for evaluating cowpea resistance to the legume pod borer. Crop Prot. 1991, 10, 48–52. [Google Scholar]
- Li, H.L.; Zhao, D.G. IPT gene expression increased the aphid resistance of transgenic plant in oilseed. Mol. Plant Breed. 2012, 9, 343–349. [Google Scholar]
- Schnable, J.C. Genome evolution in maize: From genomes back to genes. Annu. Rev. Plant Biol. 2015, 66, 329–343. [Google Scholar]
- Huang, H.; He, W. Application of exogenous cytokinin regulates cytokinin oxidase and antioxidant activity to maintain chlorophyll pigment during ripening of banana fruit. Food Biosci. 2023, 55, 102998. [Google Scholar]
- Liu, Z.N.; Lv, Y.X.; Zhang, M.; Liu, Y.P.; Kong, L.G.; Zou, M.H.; Lü, G.; Cao, J.S.; Xu, X.L. Identification, expression, and comparative genomic analysis of the IPT and CKX gene families in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genom. 2013, 14, 594. [Google Scholar]
- Azzam, C.R.; Zaki, S.-N.S.; Bamagoos, A.A.; Rady, M.M.; Alharby, H.F. Soaking maize seeds in zeatin-type cytokinin biostimulators improves salt tolerance by enhancing the antioxidant system and photosynthetic efficiency. Plants 2022, 11, 1004. [Google Scholar] [CrossRef]
- Suliman, A.A.; Elkhawaga, F.A.; Zargar, M.; Zargar, M.; Pakina, E.; Abdelkader, M. Boosting resilience and efficiency of tomato fields to heat stress tolerance using cytokinin (6-benzylaminopurine). Horticulturae 2024, 10, 170. [Google Scholar] [CrossRef]
- Macková, H.; Hronková, M.; Dobrá, J.; Turečková, V.; Novák, O.; Lubovská, Z.; Motyka, V.; Haisel, D.; Hájek, T.; Prášil, I.T.; et al. Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J. Exp. Bot. 2013, 64, 2805–2815. [Google Scholar]
- Hirose, N.; Takei, K.; Kuroha, T.; Kamada-Nobusada, T.; Hayashi, H.; Sakakibara, H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. J. Exp. Bot. 2007, 59, 75–83. [Google Scholar]
- Sakamoto, T.; Sakakibara, H.; Kojima, M.; Yamamoto, Y.; Nagasaki, H.; Inukai, Y.; Satō, Y.; Matsuoka, M. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiol. 2006, 142, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Li, G.F.; Qi, S.H.; Liu, X.J.; Chen, X.L.; Ma, J.S.; Zhang, D. Identification and expression analysis of the IPT and CKX gene families during axillary bud outgrowth in apple (Malus domestica Borkh.). Gene 2018, 651, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.J.; Shu, W.; Kong, F.N.; Zhou, C.J.; Yang, Q.; Sun, Y.; Wang, B. Identification and characterization of an isopentenyltransferase (IPT) gene in soybean (Glycine max L.). Plant Sci. 2006, 170, 542–550. [Google Scholar] [CrossRef]
- Chen, J.D.; Wan, H.P.; Zhu, W.H.; Dai, X.H.; Chen, Y.; Zeng, C.L. Identification and expression analysis of the isopentenyl transferase (IPT) gene family under lack of nitrogen stress in oilseed (Brassica napus L.). Plants 2023, 12, 2166. [Google Scholar] [CrossRef]
- Merewitz, E.; Xu, Y.; Huang, B. Differentially expressed genes associated with improved drought tolerance in creeping bentgrass overexpressing a gene for cytokinin biosynthesis. PLoS ONE 2016, 11, e0166676. [Google Scholar] [CrossRef]
- Sakakibara, H.; Kasahara, H.; Ueda, N.; Kojima, M.; Takei, K.; Hishiyama, S.; Asami, T.; Okada, K.; Kamiya, Y.; Yamaya, T.; et al. Agrobacterium tumefaciens increases cytokinin production in plastids by modifying the biosynthetic pathway in the host plant. Proc. Natl. Acad. Sci. USA 2005, 102, 9972–9977. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhao, F.; Geng, S.W.; Li, S.M.; Su, Z.L.; Chen, Q.J.; Yu, Y.; Qu, Y.Y. Genome-wide and expression pattern analysis of the DVL gene family reveals GhM_A05G1032 is involved in fuzz development in G. hirsutum. Int. J. Mol. Sci. 2024, 25, 1346. [Google Scholar] [CrossRef]
- Pang, Y.-J.; Cao, L.R.; Ye, F.Y.; Ma, C.C.; Liang, X.H.; Song, Y.H.; Lu, X.-S. Identification of the Maize PP2C Gene Family and Functional Studies on the Role ofZmPP2C15 in Drought Tolerance. Plants 2024, 13, 340. [Google Scholar]
- Sun, W.J.; Ma, Z.T.; Chen, H.; Liu, M.Y. MYB gene family in potato (Solanum tuberosum L.): Genome-wide identification of hormone-responsive genes reveals their potential functions in growth and development. Int. J. Mol. Sci. 2019, 20, 4856. [Google Scholar]
- Wei, H.R.; Wang, P.P.; Chen, J.Q.; Li, C.J.; Wang, Y.Z.; Yuan, Y.; Fang, J.H.; Leng, X.P. Genome-wide identification and analysis of B-BOX gene family in grapevine reveals its potential functions in berry development. BMC Plant Biol. 2020, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Liu, Z.H.; Sun, J.H.; Wu, C.L.; Li, Y.; Zhang, C.Q.; Zhao, L.M. Genome-wide identification of maize aquaporin and functional analysis during seed germination and seedling establishment. Front. Plant Sci. 2022, 13, 831916. [Google Scholar]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Vaňková, R.; Tanaka, M.; Seki, M.; Hàm, L.H.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Identification and expression analysis of cytokinin metabolic genes in soybean under normal and drought conditions in relation to cytokinin levels. PLoS ONE 2012, 7, e42411. [Google Scholar]
- Ye, L.; Cao, L.; Zhao, X.M.; Guo, X.Y.; Ye, K.Z.; Jiao, S.B.; Wang, Y.; He, X.X.; Dong, C.X.; Hu, B.; et al. Investigation of the JASMONATE ZIM-DOMAIN gene family reveals the canonical JA-signaling pathway in pineapple. Biology 2022, 11, 338. [Google Scholar]
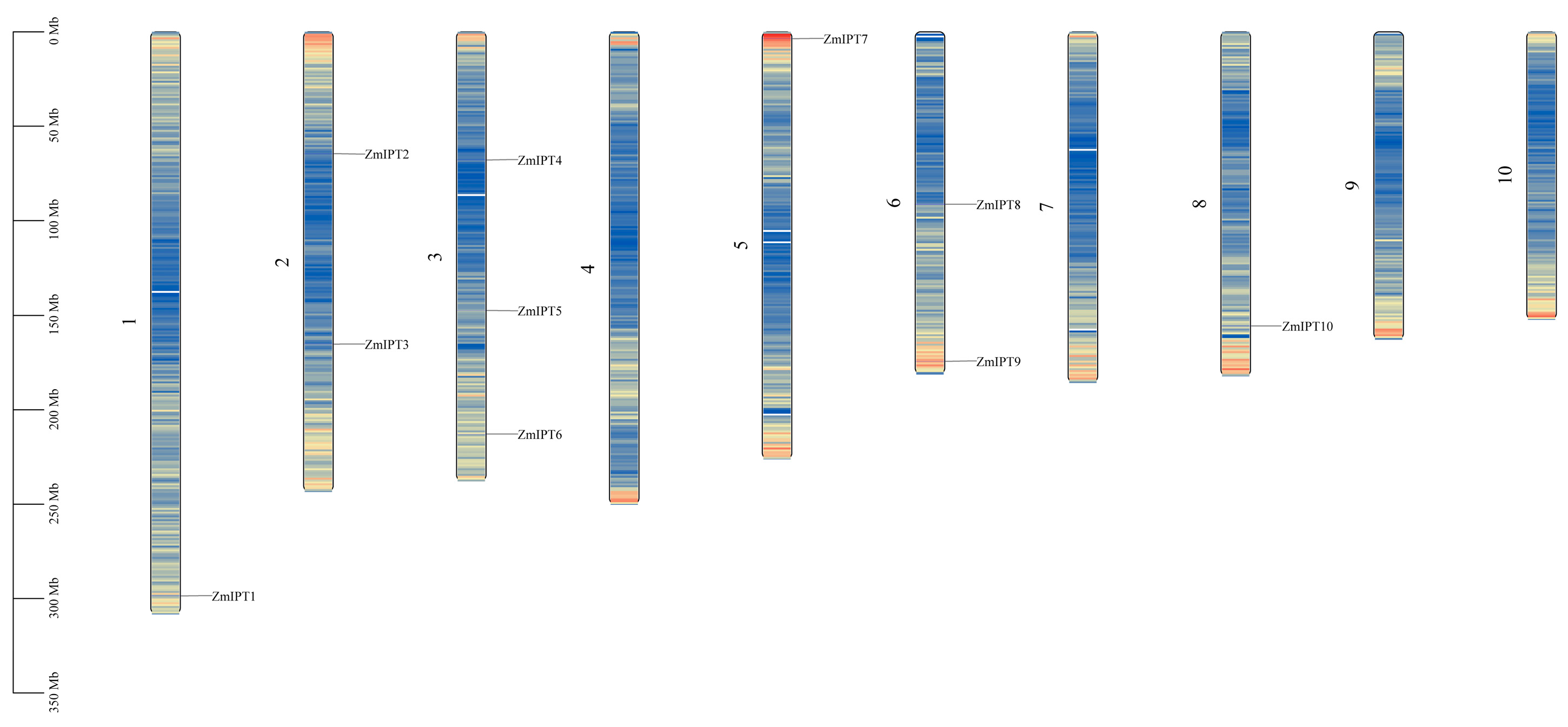


| Gene Name | Gene ID | Chromosomal Location | Protein Length | MW (kDa) | pI | Subcellular Localization Prediction |
|---|---|---|---|---|---|---|
| ZmIPT1 | Zm00001eb062030 | Chr1:298566258..298567661 (+) | 338 | 36,488.4 | 9.04 | chloroplast |
| ZmIPT2 | Zm00001eb084710 | Chr2:64584655..64585825 (+) | 322 | 34,485.8 | 4.88 | cytoplasm |
| ZmIPT3 | Zm00001eb095250 | Chr2:165161688..165163248 (−) | 347 | 37,238 | 8.22 | chloroplast |
| ZmIPT4 | Zm00001eb131660 | Chr3:67863267..67864159 (−) | 294 | 31,953 | 5.92 | cytoplasm |
| ZmIPT5 | Zm00001eb139980 | Chr3:147368450..147371548 (−) | 470 | 52,240.5 | 7.34 | chloroplast |
| ZmIPT6 | Zm00001eb156350 | Chr3:212809994..147371548 (−) | 369 | 39,117.1 | 9.56 | chloroplast |
| ZmIPT7 | Zm00001eb212350 | Chr5:3603911..3605426 (−) | 337 | 36,571.7 | 9.97 | chloroplast |
| ZmIPT8 | Zm00001eb271810 | Chr6:91352085..91357228 (−) | 453 | 50,884.2 | 7.22 | chloroplast |
| ZmIPT9 | Zm00001eb294970 | Chr6:174266460..174268235 (−) | 388 | 40,656.8 | 8.98 | chloroplast |
| ZmIPT10 | Zm00001eb360550 | Chr8:155530469..155532662 (−) | 363 | 38,146.4 | 10.17 | chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yan, Y.; Li, D.; Dong, W.; Zhang, Y.; Tao, P. Identification and Expression Profiling of the Cytokinin Synthesis Gene Family IPT in Maize. Genes 2025, 16, 415. https://doi.org/10.3390/genes16040415
Chen C, Yan Y, Li D, Dong W, Zhang Y, Tao P. Identification and Expression Profiling of the Cytokinin Synthesis Gene Family IPT in Maize. Genes. 2025; 16(4):415. https://doi.org/10.3390/genes16040415
Chicago/Turabian StyleChen, Congcong, Yujie Yan, Dongxiao Li, Weixin Dong, Yuechen Zhang, and Peijun Tao. 2025. "Identification and Expression Profiling of the Cytokinin Synthesis Gene Family IPT in Maize" Genes 16, no. 4: 415. https://doi.org/10.3390/genes16040415
APA StyleChen, C., Yan, Y., Li, D., Dong, W., Zhang, Y., & Tao, P. (2025). Identification and Expression Profiling of the Cytokinin Synthesis Gene Family IPT in Maize. Genes, 16(4), 415. https://doi.org/10.3390/genes16040415






