Barriers, Limitations, and Experiences with Clinical Trials—Treatment in Rare Diseases with Prader–Willi Syndrome as an Example
Abstract
1. Introduction
1.1. Clinical Barriers to Treatment Discovery in Rare Diseases
1.2. Clinical Findings in Prader–Willi Syndrome
1.3. Genetics of Prader–Willi Syndrome
1.4. Clinical Trial Experiences in Prader–Willi Syndrome
1.4.1. Non-Medication Interventions
1.4.2. Medication Interventions
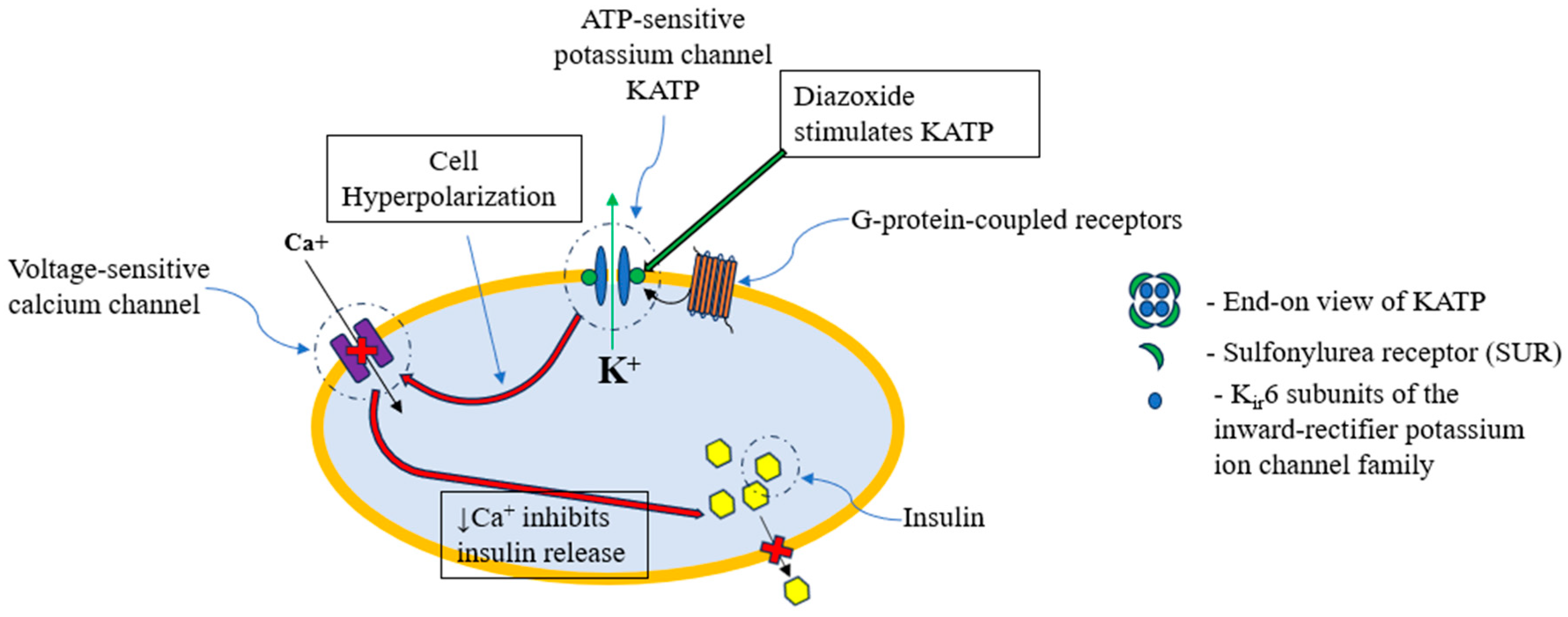
1.5. Diazoxide Choline Controlled Release (DCCR) (VYKAT XR)
2. Materials and Methods
2.1. Searchable Web-Based Programs and Databases Queried
2.1.1. STRING Web-Based Program and Database for Protein–Protein Interactions
2.1.2. Biological General Repository for Interaction Datasets (BioGRID) for Protein–Protein Interactions
2.1.3. PathwayCommons for Gene–Gene Interactions
3. Results
3.1. String Protein–Protein Interactions and Functional Analysis
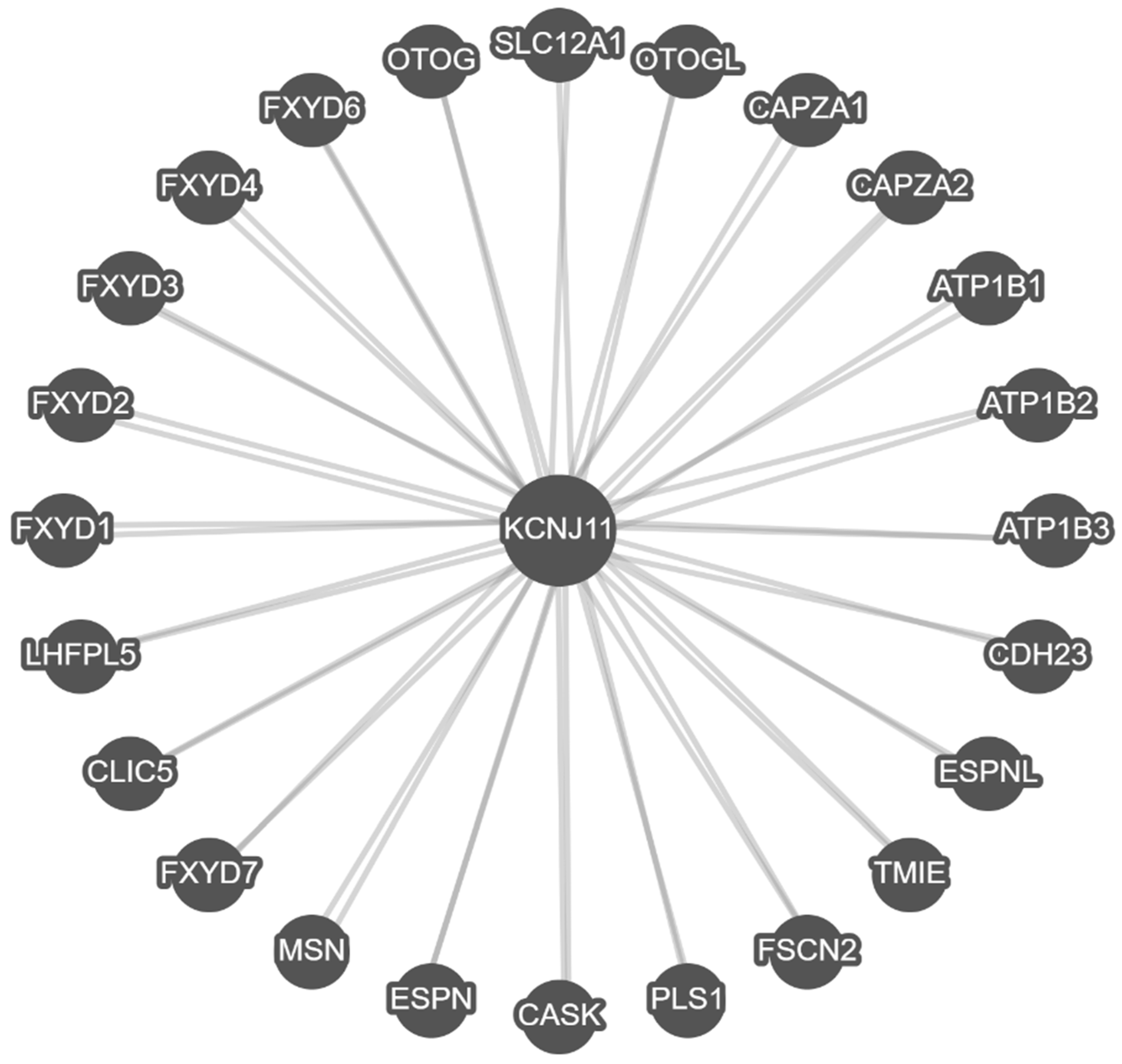
3.2. BioGRID Protein-Protein Interactions and Analysis
3.3. PathwayCommons Gene–Gene Interactions and Analysis
4. Discussion
4.1. Background in Prader–Willi Syndrome and Clinical Trials
4.2. Computational Biology and Assessment of KCNJ11
4.3. Study Limitations
4.4. Future Studies in Prader–Willi Syndrome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Food and Drug Administration. Rare Diseases at FDA. 2024. Available online: https://www.fda.gov/patients/rare-diseases-fda (accessed on 15 May 2005).
- Wang, C.M.; Whiting, A.H.; Rath, A.; Anido, R.; Ardigò, D.; Baynam, G.; Dawkins, H.; Hamosh, A.; Le Cam, Y.; Malherbe, H.; et al. Operational description of rare diseases: A reference to improve the recognition and visibility of rare diseases. Orphanet J. Rare Dis. 2024, 19, 334. [Google Scholar] [CrossRef]
- Butler, M.; Lee, P.; Whitman, B. (Eds.) Management of Prader-Willi Syndrome, 4th ed.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Butler, M.G. Single Gene and Syndromic Causes of Obesity: Illustrative Examples. Prog. Mol. Biol. Transl. Sci. 2016, 140, 1–45. [Google Scholar]
- Database.Earth. Annual Population Births by Country in 2024. 2024. Available online: https://database.earth/population/births/2024 (accessed on 14 May 2025).
- Butler, M.G.; Thompson, T. Prader-Willi Syndrome: Clinical and Genetic Findings. Endocrinologist 2000, 10, 3S–16S. [Google Scholar] [CrossRef]
- Butler, M.G. Prader-Willi syndrome: Current understanding of cause and diagnosis. Am. J. Med. Genet. 1990, 35, 319–332. [Google Scholar] [CrossRef]
- Bittel, D.C.; Butler, M.G. Prader–Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev. Mol. Med. 2005, 7, 1–20. [Google Scholar] [CrossRef]
- Butler, M.G. Prader–Willi Syndrome and Chromosome 15q11.2 BP1-BP2 Region: A Review. Int. J. Mol. Sci. 2023, 24, 4271. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Godler, D.E.; Singh, D.; Butler, M.G. Genetics of Prader–Willi and Angelman syndromes: 2024 update. Curr. Opin. Psychiatry 2025, 38, 95–100. [Google Scholar] [CrossRef]
- Butler, M.G. Management of obesity in Prader-Willi syndrome. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Manzardo, A.M.; Heinemann, J.; Loker, C.; Loker, J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet. Med. 2017, 19, 635–642. [Google Scholar] [CrossRef]
- Butler, M.G.; Duis, J. Chromosome 15 Imprinting Disorders: Genetic Laboratory Methodology and Approaches. Front. Pediatr. 2020, 8, 154. [Google Scholar] [CrossRef]
- Butler, M.G.; Hartin, S.N.; A Hossain, W.; Manzardo, A.M.; Kimonis, V.; Dykens, E.; Gold, J.A.; Kim, S.-J.; Weisensel, N.; Tamura, R.; et al. Molecular genetic classification in Prader-Willi syndrome: A multisite cohort study. J. Med. Genet. 2019, 56, 149–153. [Google Scholar] [CrossRef]
- Butler, M.G.; Bittel, D.C.; Kibiryeva, N.; Talebizadeh, Z.; Thompson, T. Behavioral Differences Among Subjects with Prader-Willi Syndrome and Type I or Type II Deletion and Maternal Disomy. Pediatrics 2004, 113, 565–573. [Google Scholar] [CrossRef]
- Butler, M.G.; Matthews, N.A.; Patel, N.; Surampalli, A.; Gold, J.-A.; Khare, M.; Thompson, T.; Cassidy, S.B.; Kimonis, V.E. Impact of genetic subtypes of Prader–Willi syndrome with growth hormone therapy on intelligence and body mass index. Am. J. Med. Genet. Part A 2019, 179, 1826–1835. [Google Scholar] [CrossRef]
- Butler, M.G.; Cowen, N.; Bhatnagar, A. Prader–Willi syndrome, deletion subtypes, and magnesium: Potential impact on clinical findings. Am. J. Med. Genet. Part A 2022, 188, 3278–3286. [Google Scholar] [CrossRef]
- Burnside, R.D.; Pasion, R.; Mikhail, F.M.; Carroll, A.J.; Robin, N.H.; Youngs, E.L.; Gadi, I.K.; Keitges, E.; Jaswaney, V.L.; Papenhausen, P.R.; et al. Microdeletion/microduplication of proximal 15q11.2 between BP1 and BP2: A susceptibility region for neurological dysfunction including developmental and language delay. Hum. Genet. 2011, 130, 517–528. [Google Scholar] [CrossRef]
- Butler, M.G. Clinical and genetic aspects of the 15q11.2 BP1–BP2 microdeletion disorder. J. Intellect. Disabil. Res. 2017, 61, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.K.; Butler, M.G.; Hartin, S.N.; Ling, L.; Bui, M.; Francis, D.; Rogers, C.; Field, M.J.; Slee, J.; Gamage, D.; et al. Relationships between UBE3A and SNORD116 expression and features of autism in chromosome 15 imprinting disorders. Transl. Psychiatry 2020, 10, 362. [Google Scholar] [CrossRef]
- Hartley, S.L.; MacLean, W.E., Jr.; Butler, M.G.; Zarcone, J.; Thompson, T. Maladaptive behaviors and risk factors among the genetic subtypes of Prader-Willi syndrome. Am. J. Med. Genet. Part A 2005, 136A, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Hossain, W.A.; Cowen, N.; Bhatnagar, A. Chromosomal Microarray Study in Prader-Willi Syndrome. Int. J. Mol. Sci. 2023, 24, 1220. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Miller, J.L.; Forster, J.L. Prader-Willi Syndrome—Clinical Genetics, Diagnosis and Treatment Approaches: An Update. Curr. Pediatr. Rev. 2019, 15, 207–244. [Google Scholar] [CrossRef]
- Duis, J.; Butler, M.G. Syndromic and Nonsyndromic Obesity: Underlying Genetic Causes in Humans. Adv. Biol. 2022, 6, e2101154. [Google Scholar] [CrossRef]
- Mahmoud, R.; Kimonis, V.; Butler, M.G. Clinical Trials in Prader–Willi Syndrome: A Review. Int. J. Mol. Sci. 2023, 24, 2150. [Google Scholar] [CrossRef]
- Miller, J.L.; Strong, T.V.; Heinemann, J. Medication Trials for Hyperphagia and Food-Related Behaviors in Prader–Willi Syndrome. Diseases 2015, 3, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Triador, L.; Field, C.J.; Tun, H.M.; Han, J.C.; Müller, T.D.; Haqq, A.M. Current and emerging therapies for managing hyperphagia and obesity in Prader-Willi syndrome: A narrative review. Obes. Rev. 2020, 21, e12992. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Wong, S.K.; Lam, C.C.; Ng, E.K. Bariatric surgery for Prader-Willi syndrome was ineffective in producing sustainable weight loss: Long term results for up to 10 years. Pediatr. Obes. 2020, 15, e12575. [Google Scholar] [CrossRef] [PubMed]
- Scheimann, A.; Butler, M.; Gourash, L.; Cuffari, C.; Klish, W. Critical Analysis of Bariatric Procedures in Prader-Willi Syndrome. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 80–83. [Google Scholar] [CrossRef]
- Bravo, G.L.; Poje, A.B.; Perissinotti, I.; Marcondes, B.F.; Villamar, M.F.; Manzardo, A.M.; Luque, L.; LePage, J.F.; Stafford, D.; Fregni, F.; et al. Transcranial direct current stimulation reduces food-craving and measures of hyperphagia behavior in participants with Prader-Willi syndrome. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 266–275. [Google Scholar] [CrossRef]
- Gabrielli, A.; Poje, A.B.; Manzardo, A.; Butler, M.G. Startle Response Analysis of Food-Image Processing in Prader-Willi Syndrome. J. Rare Disord. 2018, 6, 18–27. [Google Scholar]
- Poje, A.B.; Manzardo, A.; Gustafson, K.M.; Liao, K.; Martin, L.E.; Butler, M.G. Effects of Transcranial Direct Current Stimulation (tDCS) on Go/NoGo Performance Using Food and Non-Food Stimuli in Patients with Prader–Willi Syndrome. Brain Sci. 2021, 11, 250. [Google Scholar] [CrossRef]
- Richer, L.P.; Tan, Q.; Butler, M.G.; Avedzi, H.M.; DeLorey, D.S.; Peng, Y.; Tun, H.M.; Sharma, A.M.; Ainsley, S.; Orsso, C.E.; et al. Evaluation of Autonomic Nervous System Dysfunction in Childhood Obesity and Prader–Willi Syndrome. Int. J. Mol. Sci. 2023, 24, 8013. [Google Scholar] [CrossRef]
- Fadel, M.G.; Fehervari, M.; Das, B.; Soleimani-Nouri, P.; Ashrafian, H. Vagal Nerve Therapy in the Management of Obesity: A Systematic Review and Meta-Analysis. Eur. Surg. Res. 2023, 64, 365–375. [Google Scholar] [CrossRef]
- Holland, A.; Manning, K. t-VNS to treat disorders of behaviour in Prader-Willi Syndrome and in people with other neurodevelopmental conditions. Auton. Neurosci. 2022, 239, 102955. [Google Scholar] [CrossRef]
- Manning, K.E.; Beresford-Webb, J.A.; Aman, L.C.S.; Ring, H.A.; Watson, P.C.; Porges, S.W.; Oliver, C.; Jennings, S.R.; Holland, A.J. Transcutaneous vagus nerve stimulation (t-VNS): A novel effective treatment for temper outbursts in adults with Prader-Willi Syndrome indicated by results from a non-blind study. PLoS ONE 2019, 14, e0223750. [Google Scholar] [CrossRef]
- McCandless, S.E.; Yanovski, J.A.; Miller, J.; Fu, C.; Bird, L.M.; Salehi, P.; Chan, C.L.; Stafford, D.; Abuzzahab, M.J.; Viskochil, D.; et al. Effects of MetAP2 inhibition on hyperphagia and body weight in Prader–Willi syndrome: A randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2017, 19, 1751–1761. [Google Scholar] [CrossRef]
- Talebizadeh, Z.; Kibiryeva, N.; Bittel, D.C.; Butler, M.G. Ghrelin, peptide YY and their receptors: Gene expression in brain from subjects with and without Prader-Willi syndrome. Int. J. Mol. Med. 2005, 15, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Allas, S.; Caixàs, A.; Poitou, C.; Coupaye, M.; Thuilleaux, D.; Lorenzini, F.; Diene, G.; Crinò, A.; Illouz, F.; Grugni, G.; et al. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: A randomized placebo-controlled trial. PLoS ONE 2018, 13, e0190849. [Google Scholar] [CrossRef] [PubMed]
- Millendo Therapeutics, SAS. Effects of Livoletide (AZP-531) on Food-Related Behaviors in Patients with Prader-Willi Syndrome (ZEPHYR). ClinicalTrials.Gov. 2021. Available online: https://clinicaltrials.gov/study/NCT03790865 (accessed on 1 November 2022).
- Damen, L.; Grootjen, L.N.; Juriaans, A.F.; Donze, S.H.; Huisman, T.M.; Visser, J.A.; Delhanty, P.J.; Hokken-Koelega, A.C. Oxytocin in young children with Prader-Willi syndrome: Results of a randomized, double-blind, placebo-controlled, crossover trial investigating 3 months of oxytocin. Clin. Endocrinol. 2020, 94, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Dykens, E.M.; Miller, J.; Angulo, M.; Roof, E.; Reidy, M.; Hatoum, H.T.; Willey, R.; Bolton, G.; Korner, P. Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome. J. Clin. Investig. 2018, 3, e98333. [Google Scholar] [CrossRef]
- Einfeld, S.L.; Smith, E.; McGregor, I.S.; Steinbeck, K.; Taffe, J.; Rice, L.J.; Horstead, S.K.; Rogers, N.; Hodge, M.A.; Guastella, A.J. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am. J. Med. Genet. Part A 2014, 164, 2232–2239. [Google Scholar] [CrossRef]
- Hollander, E.; Levine, K.G.; Ferretti, C.J.; Freeman, K.; Doernberg, E.; Desilva, N.; Taylor, B.P. Intranasal oxytocin versus placebo for hyperphagia and repetitive behaviors in children with Prader-Willi Syndrome: A randomized controlled pilot trial. J. Psychiatr. Res. 2021, 137, 643–651. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Miller, J.L.; Tamura, R.; Butler, M.G.; Kimonis, V.; Sulsona, C.; Gold, J.A.; Driscoll, D.J. Oxytocin treatment in children with Prader-Willi syn-drome: A double-blind, placebo-controlled, crossover study. Am. J. Med. Genet. A 2017, 173, 1243–1250. [Google Scholar] [CrossRef]
- Tauber, M.; Mantoulan, C.; Copet, P.; Jauregui, J.; Demeer, G.; Diene, G.; Rogé, B.; Laurier, V.; Ehlinger, V.; Arnaud, C.; et al. Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader-Willi syndrome: A randomised placebo-controlled trial in 24 patients. Orphanet J. Rare Dis. 2011, 6, 47. [Google Scholar] [CrossRef]
- Knani, I.; Earley, B.J.; Udi, S.; Nemirovski, A.; Hadar, R.; Gammal, A.; Cinar, R.; Hirsch, H.J.; Pollak, Y.; Gross, I.; et al. Targeting the endocannabinoid/CB1 receptor system for treating obesity in Prader–Willi syndrome. Mol. Metab. 2016, 5, 1187–1199. [Google Scholar] [CrossRef]
- Nagappan, A.; Shin, J.; Jung, M.H. Role of Cannabinoid Receptor Type 1 in Insulin Resistance and Its Biological Implications. Int. J. Mol. Sci. 2019, 20, 2109. [Google Scholar] [CrossRef] [PubMed]
- Duis, J.; van Wattum, P.J.; Scheimann, A.; Salehi, P.; Brokamp, E.; Fairbrother, L.; Childers, A.; Shelton, A.R.; Bingham, N.C.; Shoemaker, A.H.; et al. A multidisciplinary approach to the clinical management of Prader–Willi syndrome. Mol. Genet. Genom. Med. 2019, 7, e514. [Google Scholar] [CrossRef] [PubMed]
- Rhythm Pharmaceuticals Inc. Phase 2 Trial to Evaluate Safety and Efficacy of Setmelanotide (RM-493) in Obese Participants With Prader-Willi Syndrome. ClinicalTrials.Gov. 2023. Available online: https://clinicaltrials.gov/study/NCT02311673 (accessed on 1 November 2023).
- Mahmoud, R.; Kimonis, V.; Butler, M.G. Genetics of Obesity in Humans: A Clinical Review. Int. J. Mol. Sci. 2022, 23, 11005. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, B.H.; Grunnet, M.; Hyveled-Nielsen, L.; Sundgreen, C.; Lassen, J.B.; Hansen, H.H. Anti-hypertensive treatment preserves appetite suppression while preventing cardiovascular adverse effects of tesofensine in rats. Obesity 2012, 21, 985–992. [Google Scholar] [CrossRef]
- Anandhakrishnan, A.; Korbonits, M. Glucagon-like peptide 1 in the pathophysiology and pharmacotherapy of clinical obesity. World J. Diabetes 2016, 7, 572–598. [Google Scholar] [CrossRef]
- Garber, A.J. Long-acting glucagon-like peptide 1 receptor agonists: A review of their efficacy and tolerability. Diabetes Care 2011, 34, S279–S284. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Hsu, I.; Azen, C.G.; Mittelman, S.D.; Geffner, M.E.; Jeandron, D. Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader-Willi syndrome. Pediatr. Obes. 2016, 12, 221–228. [Google Scholar] [CrossRef]
- Ng, N.B.H.; Low, Y.W.; Rajgor, D.D.; Low, J.M.; Lim, Y.Y.; Loke, K.Y.; Lee, Y.S. The effects of glucagon-like peptide (GLP)-1 receptor agonists on weight and glycaemic control in Prader–Willi syndrome: A systematic review. Clin. Endocrinol. 2021, 96, 144–154. [Google Scholar] [CrossRef]
- Niethammer, A.G.; Zheng, Z.; Timmer, A.; Lee, T. First-in-Human Evaluation of Oral Denatonium Acetate (ARD-101), a Potential Bitter Taste Receptor Agonist: A Randomized, Double-Blind, Placebo-Controlled Phase 1 Trial in Healthy Adults. Clin. Pharmacol. Drug Dev. 2022, 11, 997–1006. [Google Scholar] [CrossRef]
- Dubern, B.; Faccioli, N.; Poitou, C.; Clément, K. Novel therapeutics in rare genetic obesities: A narrative review. Pharmacol. Res. 2023, 191, 106763. [Google Scholar] [CrossRef]
- Faccioli, N.; Poitou, C.; Clément, K.; Dubern, B. Current Treatments for Patients with Genetic Obesity. J. Clin. Res. Pediatr. Endocrinol. 2023, 15, 108–119. [Google Scholar] [CrossRef]
- Butler, M.G. New drug approved for hyperphagia in Prader–Willi syndrome. Lancet Diabetes Endocrinol. 2025, 13, 547–549. [Google Scholar] [CrossRef]
- Miller, J.L.; Gevers, E.; Bridges, N.; A Yanovski, J.; Salehi, P.; Obrynba, K.S.; I Felner, E.; Bird, L.M.; Shoemaker, A.H.; Angulo, M.; et al. Diazoxide Choline Extended-Release Tablet in People with Prader-Willi Syndrome: A Double-Blind, Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2023, 108, 1676–1685. [Google Scholar] [CrossRef]
- Chen, X.; Feng, L.; Yao, H.; Yang, L.; Qin, Y. Efficacy and safety of diazoxide for treating hyperinsulinemic hypoglycemia: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0246463. [Google Scholar] [CrossRef]
- Cowen, N.; Bhatnagar, A. The Potential Role of Activating the ATP-Sensitive Potassium Channel in the Treatment of Hyperphagic Obesity. Genes 2020, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Kharade, S.V.; Sanchez-Andres, J.V.; Fulton, M.G.; Shelton, E.L.; Blobaum, A.L.; Engers, D.W.; Hofmann, C.S.; Dadi, P.K.; Lantier, L.; Jacobson, D.A.; et al. Structure-Activity Relationships, Pharmacokinetics, and Pharmacodynamics of the Kir6.2/SUR1-Specific Channel Opener VU0071063. J. Pharmacol. Exp. Ther. 2019, 370, 350–359. [Google Scholar] [CrossRef]
- Mannhold, R. KATP channel openers: Structure-activity relationships and therapeutic potential. Med. Res. Rev. 2004, 24, 213–266. [Google Scholar] [CrossRef]
- Dodd, C.J.; Chronister, K.S.; Rathnayake, U.; Parr, L.C.; Li, K.; Chang, S.; Mi, D.; Days, E.L.; Bauer, J.A.; Cho, H.P.; et al. Synthesis and SAR of a novel Kir6.2/SUR1 channel opener scaffold identified by HTS. Bioorganic Med. Chem. Lett. 2023, 87, 129256. [Google Scholar] [CrossRef]
- Shyng, S.-L.; Nichols, C. Octameric Stoichiometry of the KATP Channel Complex. J. Gen. Physiol. 1997, 110, 655–664. [Google Scholar] [CrossRef]
- Wang, M.; Wu, J.X.; Ding, D.; Chen, L. Structural insights into the mechanism of pancreatic KATP channel regulation by nucle-otides. Nat. Commun. 2022, 13, 2770. [Google Scholar]
- Bienengraeber, M.; Alekseev, A.E.; Abraham, M.R.; Carrasco, A.J.; Moreau, C.; Vivaudou, M.; Dzeja, P.P.; Terzic, A. ATPase activity of the sulfonylurea receptor: A catalytic function for the KATP channel complex. FASEB J. 2000, 14, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.B. Towards Selective Kir6.2/SUR1 Potassium Channel Openers, Medicinal Chemistry and Therapeutic Perspectives. Curr. Med. Chem. 2006, 13, 361–376. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Li, S.; Zhang, J.; Zheng, J.; Hou, W.; Zhao, H.; Guo, Y.; Liu, X.; Dou, K.; et al. N-myc Downstream-regulated Gene 2, a Novel Estrogen-targeted Gene, Is Involved in the Regulation of Na+/K+-ATPase. J. Biol. Chem. 2011, 286, 32289–32299. [Google Scholar] [CrossRef] [PubMed]
- Pipatpolkai, T.; Usher, S.; Stansfeld, P.J.; Ashcroft, F.M. New insights into KATP channel gene mutations and neonatal diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 378–393. [Google Scholar] [CrossRef]
- Tacer, K.F.; Potts, P.R. Cellular and disease functions of the Prader-Willi Syndrome gene MAGEL2. Biochem. J. 2017, 474, 2177–2190. [Google Scholar] [CrossRef]
- Forster, J.; Duis, J.; Butler, M.G. Pharmacogenetic Testing of Cytochrome P450 Drug Metabolizing Enzymes in a Case Series of Patients with Prader-Willi Syndrome. Genes 2021, 12, 152. [Google Scholar] [CrossRef]
- Amaral, M.D.; Pankonien, I. Theranostics vs theratyping or theranostics plus theratyping? J. Cyst. Fibros. 2025, 24, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.J.; Downey, D.G. Theratyping in cystic fibrosis. Curr. Opin. Pulm. Med. 2018, 24, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.; Ramsey, B.W. New Therapeutic Approaches to Modulate and Correct Cystic Fibrosis Transmembrane Conductance Regulator. Pediatr. Clin. North Am. 2016, 63, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]

| Protein and/or Gene Symbol | Description |
|---|---|
| ABCC9 | ATP-binding cassette sub-family C member 9, a subunit of ATP-sensitive potassium channel (KATP) that forms the channel pore belonging to the ABC transporter superfamily. Encodes sulfonylurea receptor 2 (SUR2). |
| ABCC8 | ATP-binding cassette sub-family C member 8, a subunit of beta-cell ATP-sensitive potassium channel (KATP) that forms the channel pore belonging to the ABC transporter superfamily and acts as a regulator for insulin release. Encodes sulfonylurea receptor 1 (SUR1). |
| KCNJ8 | ATP-sensitive inward rectifier potassium channel 8 controlled by G proteins regulating potassium flow with inward rectification mainly due to the blockage of outward current by internal magnesium. Encodes the transmembrane potassium inward-rectifying Kir6.1. |
| DIABLO or DIABLO-2 | Diablo IAP-binding mitochondrial protein promotes apoptosis by activating caspases in the cytochrome c/Apaf-1/caspase-9 pathway by opposing the inhibitory activity of the inhibitor of apoptosis proteins (IAP). |
| GABBR1 | Gamma-aminobutyric acid type B receptor subunit 1 as a component of a heterodimeric G protein-coupled receptor for GABA, formed by GABBR1 and GABBR2, but GABBR1 only binds agonists. Ligand binding causes a conformational change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of downstream effectors such as adenylate cyclase. |
| GABBR2 | Gamma-aminobutyric acid type B receptor subunit 2 as a component of a heterodimeric G protein-coupled receptor for GABA, formed by GABBR1 and GABBR2, but GABBR1 only binds agonists while GABBR2 mediates coupling to G proteins. Ligand binding causes a conformational change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of downstream effectors such as adenylate cyclase. |
| GCK | Hexokinase-4, or glucokinase, catalyzes the phosphorylation of hexose, such as D-glucose, D-fructose, and D-mannose to hexose 6-phosphate and D-mannose 6-phosphate, respectively, and involves the first step in glycolysis. |
| XIAP | E3 ubiquitin-protein ligase, a multifunctional protein which regulates not only caspases and apoptosis but also modulates inflammatory signaling and immunity, copper homeostasis, mitogenic kinase signaling, cell proliferation and invasion, and metastasis. It acts as a direct caspase inhibitor and targets proteins for degradation. |
| INS | Insulin A chain which decreases blood glucose concentration and increases cell permeability to monosaccharides, amino acids, and fatty acids with the acceleration of glycolysis and glycogen synthesis in the liver. |
| Biological Process | CIN A | Strength B | Signal C | FDR D |
|---|---|---|---|---|
| Neuron-glial cell signaling (GO:0150099) | 2 of 5 | 2.86 | 1.25 | 0.0054 |
| Potassium ion import across plasma membrane (GO:1990573) | 4 of 44 | 2.21 | 1.97 | 0.00016 |
| Negative regulation of protein secretion (GO:0050709) | 3 of 62 | 1.94 | 1.17 | 0.0054 |
| Negative regulation of secretion by cell (GO:1903531) | 4 of 141 | 1.71 | 1.32 | 0.0016 |
| Regulation of peptide hormone secretion (GO:0090276) | 5 of 185 | 1.68 | 1.60 | 0.00027 |
| Molecular Function | CIN | Strength | Signal | FDR |
| Sulfonylurea receptor activity (GO:0008281) | 2 of 2 | 3.25 | 1.60 | 0.0013 |
| G protein-coupled GABA receptor activity (GO:0004965) | 2 of 3 | 3.08 | 1.55 | 0.0016 |
| ATP-activated inward rectifier potassium channel activity (GO:0015272) | 3 of 5 | 3.03 | 2.45 | 3.57 × 10−5 |
| ATPase-coupled cation transmembrane transporter activity (GO:0019829) | 4 of 53 | 2.13 | 2.17 | 5.04 × 10−5 |
| Potassium channel activity (GO:0005267) | 4 of 126 | 1.75 | 1.52 | 0.00058 |
| Cellular Component | CIN | Strength | Signal | FDR |
| Inward rectifying potassium channel (GO:0008282) | 4 of 4 | 3.25 | 4.47 | 7.51 × 10−9 |
| Potassium ion-transporting ATPase complex (GO:0031004) | 3 of 3 | 3.25 | 3.18 | 1.76 × 10−6 |
| G protein-coupled GABA receptor complex (GO:1902712) | 2 of 2 | 3.25 | 1.92 | 0.00035 |
| G protein-coupled receptor heterodimeric complex (GO:0038039) | 2 of 3 | 3.08 | 1.81 | 0.00053 |
| Plasma membrane protein complex (GO:0098797) | 8 of 589 | 1.39 | 2.01 | 1.05 × 10−7 |
| KEGG Pathway | CIN | Strength | Signal | FDR |
| Type II diabetes mellitus (hsa04930) | 4 of 45 | 2.20 | 2.73 | 3.69 × 10−6 |
| Maturity onset diabetes of the young (hsa04950) | 2 of 25 | 2.16 | 1.11 | 0.0083 |
| Insulin secretion (hsa04911) | 4 of 82 | 1.94 | 2.25 | 1.83 × 10−5 |
| GnRH secretion (hsa04929) | 3 of 63 | 1.93 | 1.58 | 0.00065 |
| Apoptosis—multiple species (hsa04215) | 2 of 30 | 2.08 | 1.07 | 0.0094 |
| Reactome Pathway | CIN | Strength | Signal | FDR |
| ATP-sensitive potassium channels (HSA-1296025) | 4 of 4 | 3.25 | 4.61 | 4.19 × 10−9 |
| Inwardly rectifying K+ channels (HSA-1296065) | 6 of 35 | 2.49 | 5.15 | 5.78 × 10−11 |
| Defective ABCC8 can cause hypo- and hyperglycemias (HSA-5683177) | 2 of 2 | 3.25 | 1.72 | 0.00078 |
| Defective ABCC9 causes CMD10, ATFB12, and Cantu syndrome (HSA-5678420) | 2 of 2 | 3.25 | 1.72 | 0.00078 |
| Disorders of transmembrane transporters (HSA-5619115) | 4 of 176 | 1.61 | 1.39 | 0.00078 |
| Disease-Gene Association | CIN | Strength | Signal | FDR |
| Hypertrichotic osteochondrodysplasia Cantu type (DOID:0060569) | 3 of 3 | 3.25 | 3.11 | 2.37 × 10−6 |
| Permanent neonatal diabetes mellitus (DOID:0060639) | 4 of 6 | 3.08 | 3.99 | 5.06 × 10−8 |
| Gestational diabetes (DOID:11714) | 3 of 9 | 2.78 | 2.54 | 2.17 × 10−5 |
| Maturity-onset diabetes of the young (DOID:0050524) | 4 of 14 | 2.71 | 3.54 | 2.45 × 10−7 |
| Hypoglycemia (DOID:9993) | 4 of 22 | 2.51 | 3.18 | 8.96 × 10−7 |
| Biological Processes | Molecular Functions | Cellular Components |
|---|---|---|
| Energy reserve metabolic process | ATP binding | ATP-sensitive potassium channel complex |
| Glucose metabolic process | ATP-activated inward rectifier potassium channel activity | Muscle T-tubule |
| Negative regulation of insulin secretion | G protein-activated inward rectifier potassium channel activity | Integral component of plasma membrane |
| Neurological system process | Ankyrin binding | Plasma membrane |
| Potassium ion import | Ion channel binding | Voltage-gated potassium channel complex |
| Potassium ion transmembrane transport | Potassium ion binding | |
| Regulation of membrane potential | Voltage-gated potassium channel activity | |
| Response to ATP | ||
| Response to drug | ||
| Small molecule metabolic process | ||
| Synaptic transmission | ||
| Regulation of insulin secretion | ||
| Regulation of ion transmembrane transport |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler, M.G.; Silvey, S.; van Bosse, H.J.P. Barriers, Limitations, and Experiences with Clinical Trials—Treatment in Rare Diseases with Prader–Willi Syndrome as an Example. Genes 2025, 16, 1436. https://doi.org/10.3390/genes16121436
Butler MG, Silvey S, van Bosse HJP. Barriers, Limitations, and Experiences with Clinical Trials—Treatment in Rare Diseases with Prader–Willi Syndrome as an Example. Genes. 2025; 16(12):1436. https://doi.org/10.3390/genes16121436
Chicago/Turabian StyleButler, Merlin G., Spencer Silvey, and Harold J. P. van Bosse. 2025. "Barriers, Limitations, and Experiences with Clinical Trials—Treatment in Rare Diseases with Prader–Willi Syndrome as an Example" Genes 16, no. 12: 1436. https://doi.org/10.3390/genes16121436
APA StyleButler, M. G., Silvey, S., & van Bosse, H. J. P. (2025). Barriers, Limitations, and Experiences with Clinical Trials—Treatment in Rare Diseases with Prader–Willi Syndrome as an Example. Genes, 16(12), 1436. https://doi.org/10.3390/genes16121436







