Heterologous Expression of Bacterial Dehydrin Promotes Arabidopsis Tolerance to Cadmium and Arsenic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Assessment of Root and Shoot Length
2.3. Fresh Weight and Dry Weight
2.4. Relative Water Content Determination
2.5. Assessment of Secondary Metabolism and Radical Scavenging Activity
2.5.1. Secondary Metabolite Extraction
2.5.2. Quantification of Total Phenolic Content
2.5.3. Determination of Radical Scavenging Assay
2.6. Assessment of Free Proline Content
2.7. Quantification of Photosynthetic Pigments
2.8. Total Protein Quantification
2.9. Statistical Analysis
3. Results
3.1. Transgenic Plants Expressing Bacterial Dehydrin Gene BG757 Grow Better Under Cd and as Stress
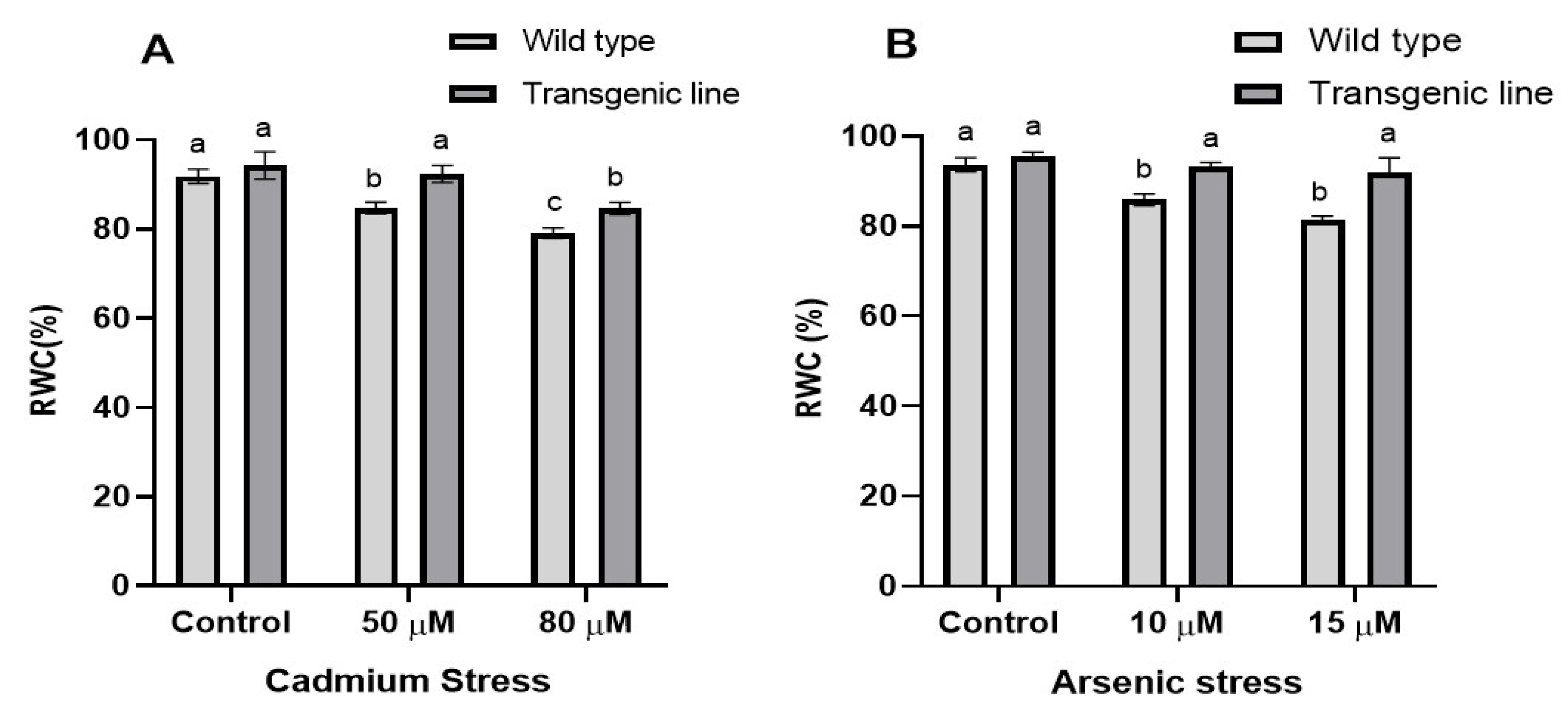
3.2. Transgenic Plants Retain Higher Relative Water Content in the Leaf Tissue Under Cd and as Stress
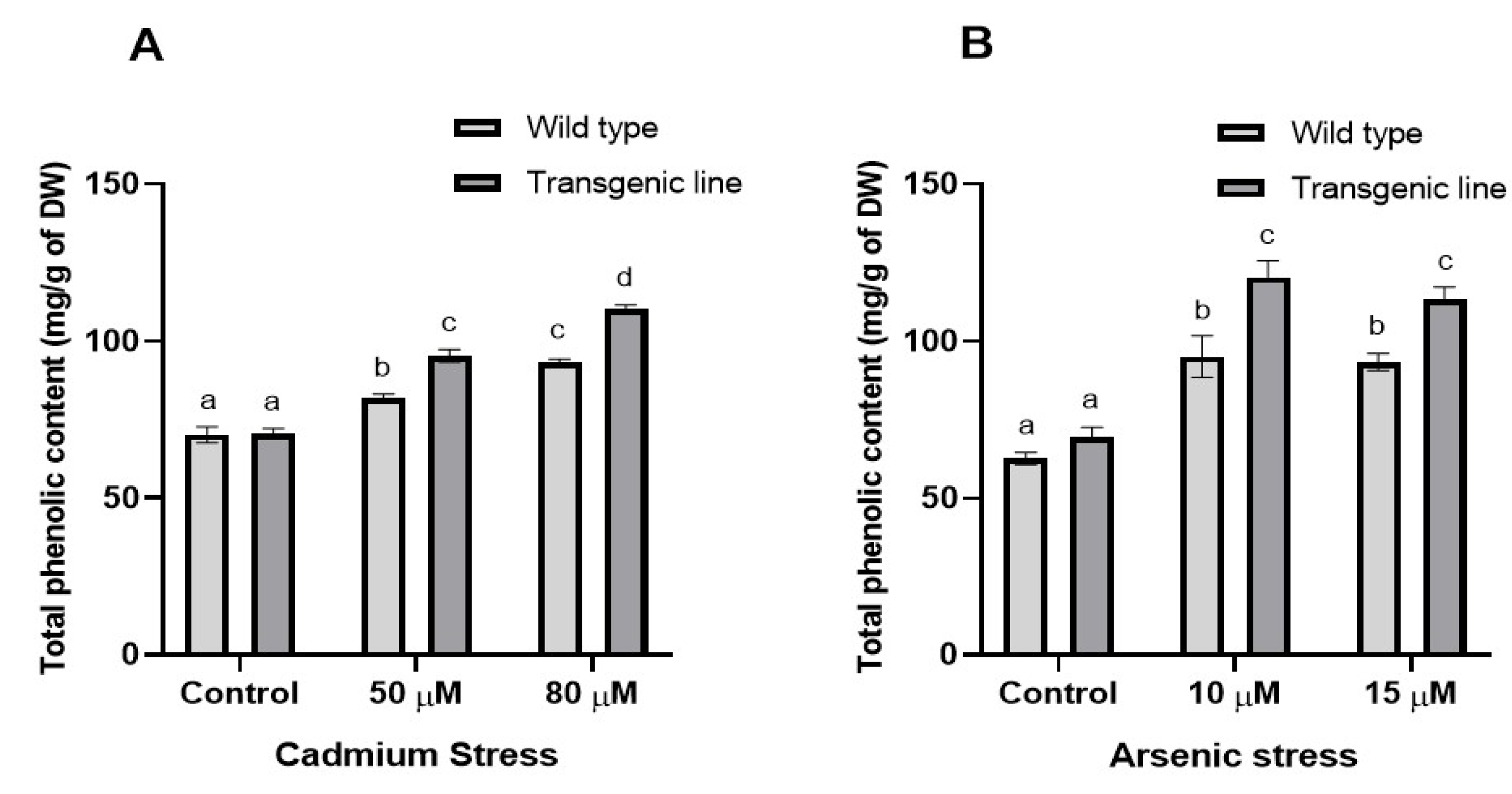
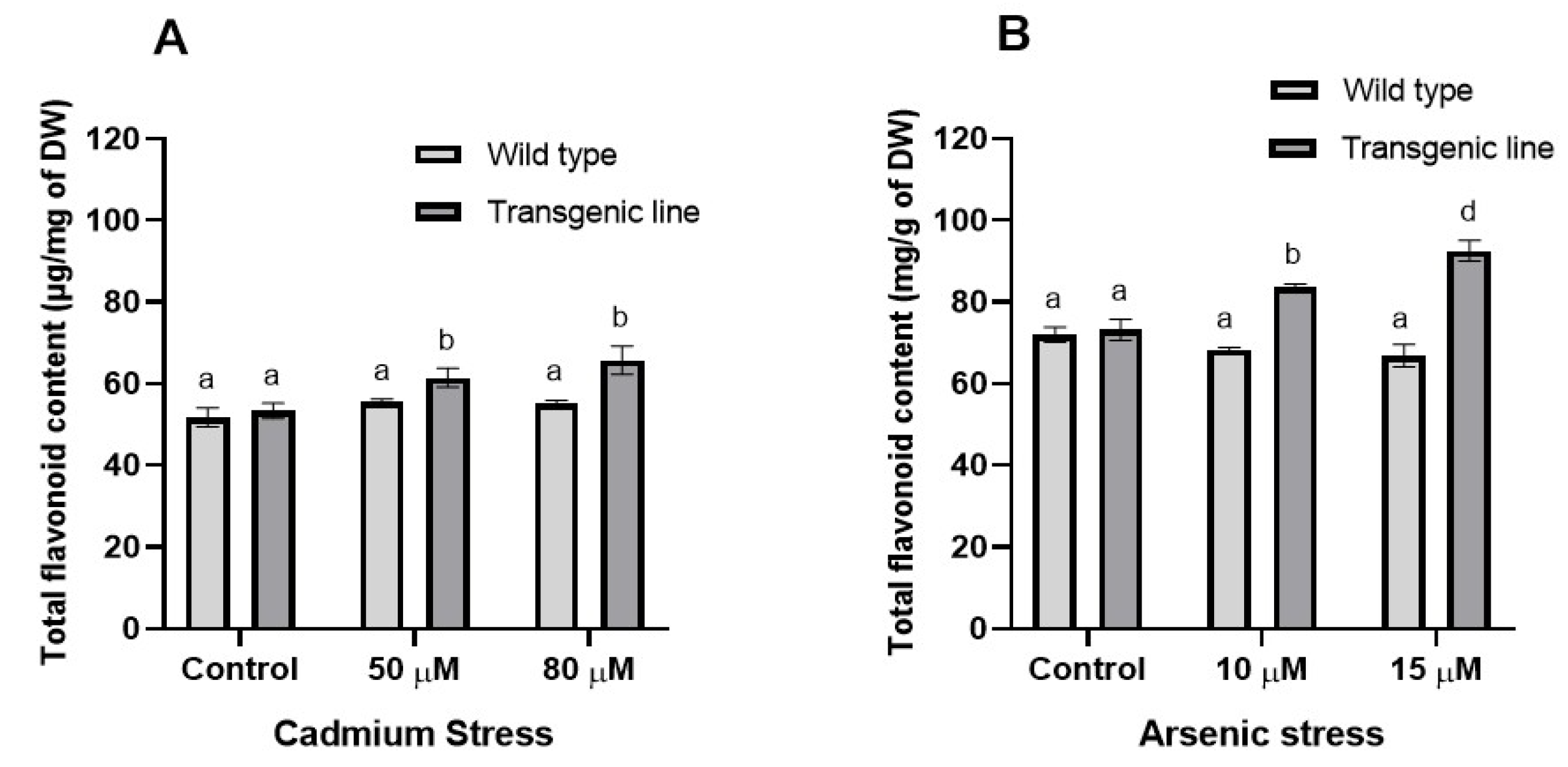
3.3. Transgenic Plants Accumulate More Proline, Phenolic and Flavonoid Content Under Cd and as Stress
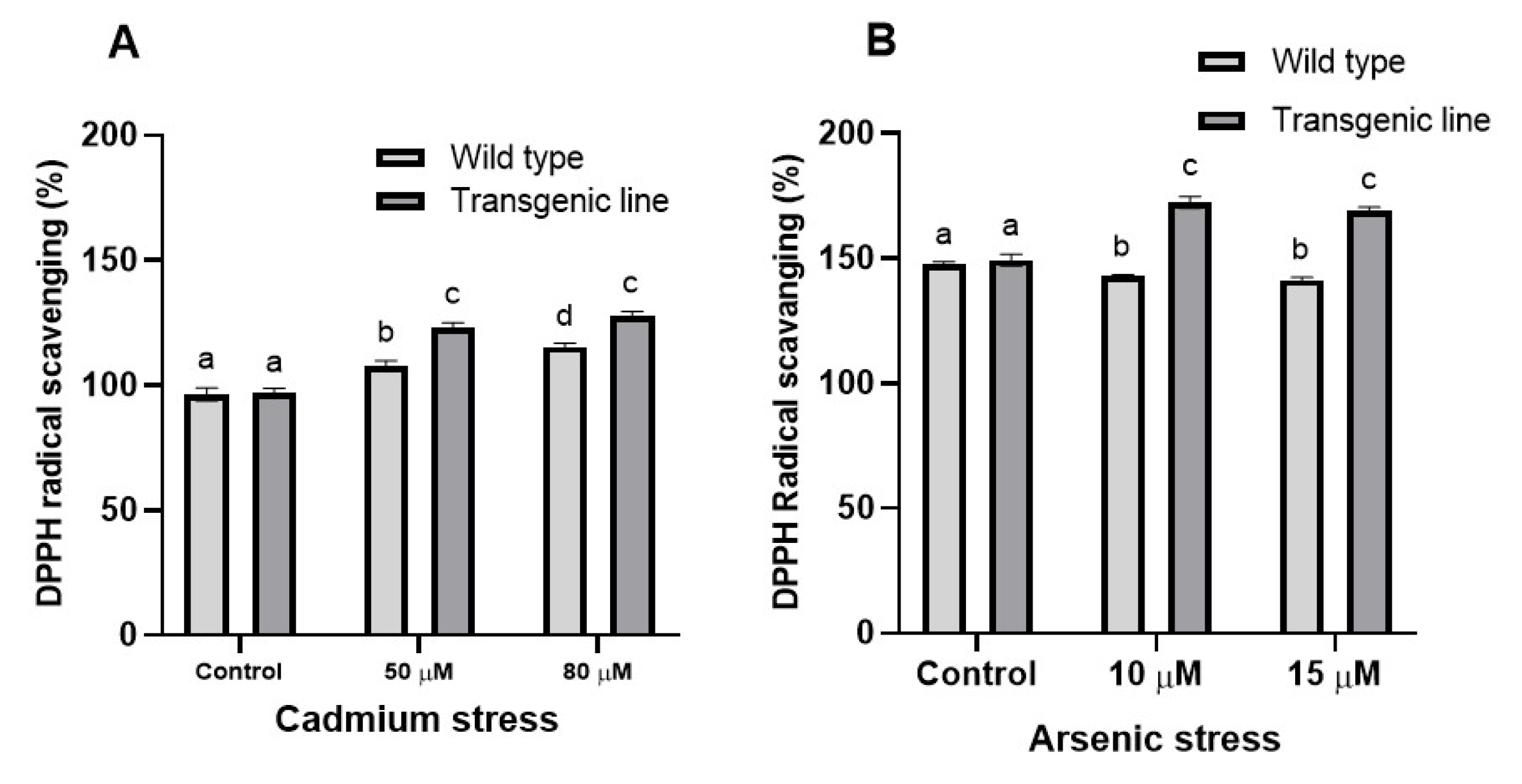
3.4. DPPH Free Radical Scavenging Activity Increases in Transgenic Line upon Cd and as Stress
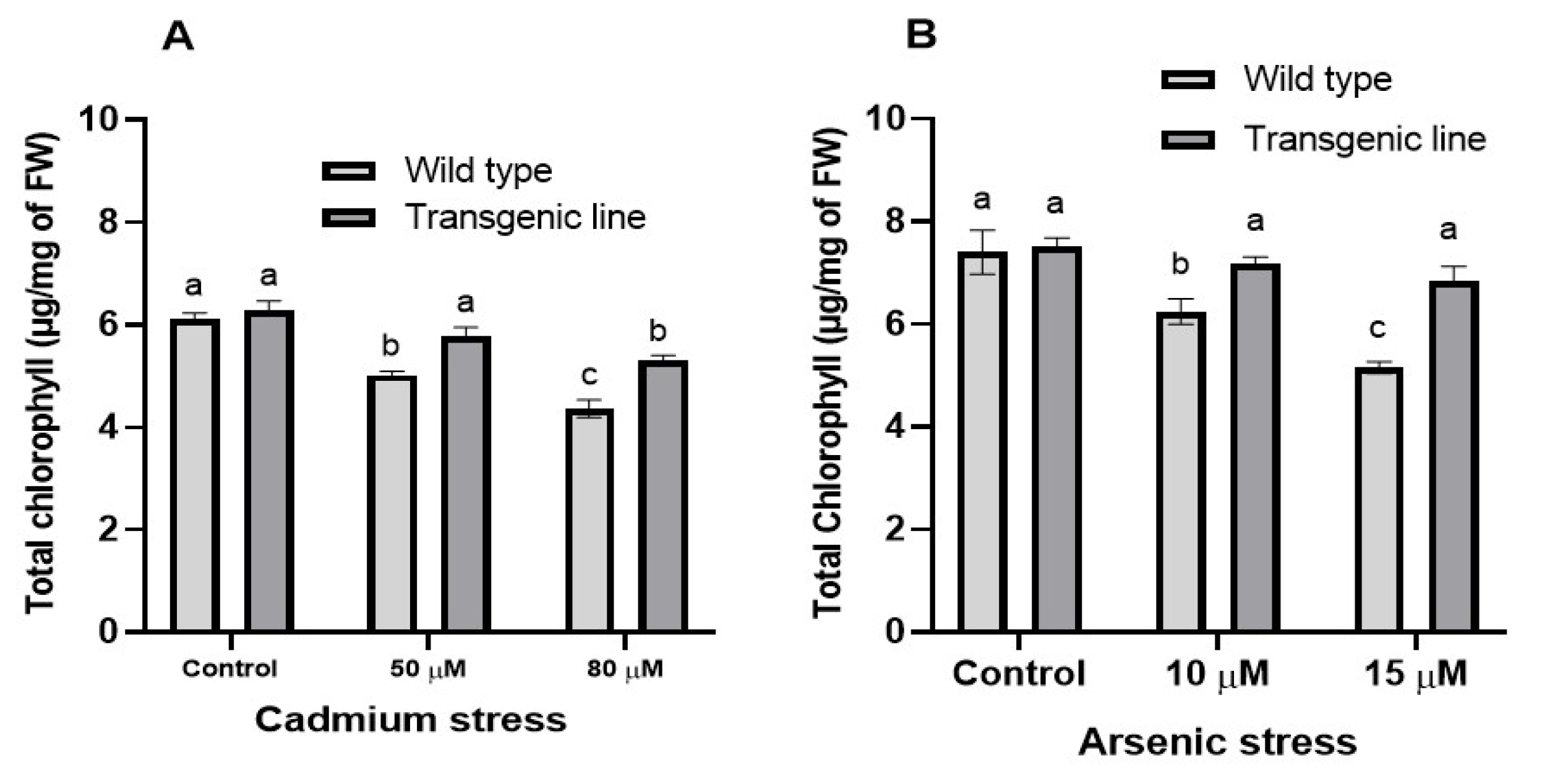
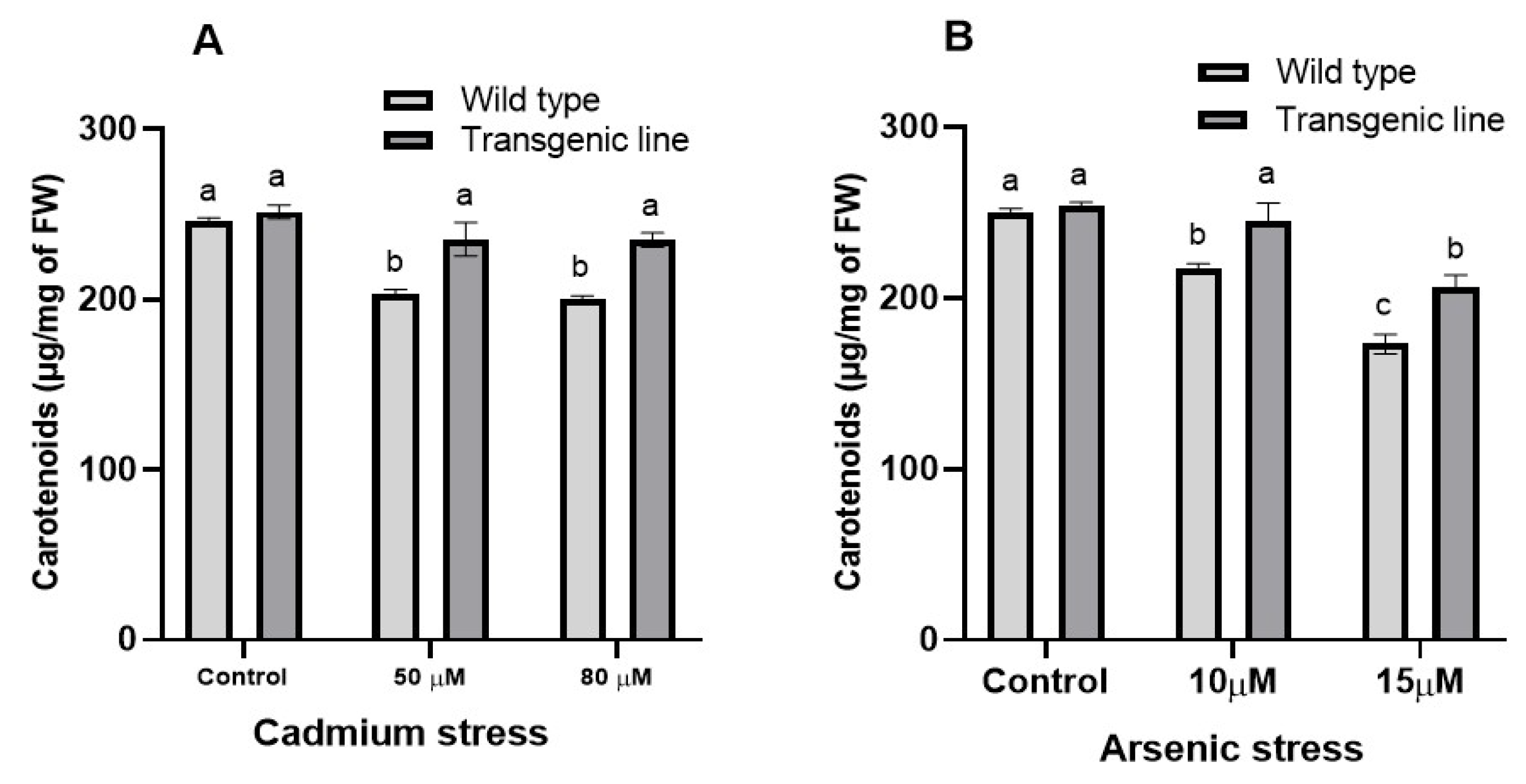
3.5. Photosynthetic Pigments and Carotenoid Content Decrease When Exposed to Cd and as Stress
3.6. Effects on Total Protein Content Under Cd and as Stress
4. Discussion
5. Conclusions and Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Klíma, M.; Roy, A.; Prášil, I.T. Biological networks underlying abiotic stress tolerance in temperate crops—A proteomic perspective. Int. J. Mol. Sci. 2015, 16, 20913–20942. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Singh Sidhu, G.P.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ali, H.M.; Ahmad, P. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 2019, 230, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Ronzan, M.; Piacentini, D.; Fattorini, L.; Federica, D.R.; Caboni, E.; Eiche, E.; Ziegler, J.; Hause, B.; Riemann, M.; Betti, C.; et al. Auxin-jasmonate crosstalk in Oryza sativa L. root system formation after cadmium and/or arsenic exposure. Environ. Exp. Bot. 2019, 165, 59–69. [Google Scholar] [CrossRef]
- Keyster, M.; Niekerk, L.-A.; Basson, G.; Carelse, M.; Bakare, O.; Ludidi, N.; Klein, A.; Mekuto, L.; Gokul, A. Decoding heavy metal stress signalling in plants: Towards improved food security and safety. Plants 2020, 9, 1781. [Google Scholar] [CrossRef]
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy metal stress in rice: Uptake, transport, signaling, and tolerance mechanisms. Physiolia Plant. 2021, 173, 430–448. [Google Scholar] [CrossRef]
- Amir, R.; Munir, F.; Kubra, G.; Iqbal, T.; Khan, M. Plant signaling molecules and cadmium stress tolerance. In Cadmium Tolerance in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–399. [Google Scholar]
- Xu, W.; Shi, W.; Yan, F.; Zhang, B.; Liang, J. Mechanisms of cadmium detoxification in cattail (Typha angustifolia L.). Aquat. Bot. 2011, 94, 37–43. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Gong, P.; Shen, Q.; Zhang, M.; Qiao, R.; Jiang, J.; Su, L.; Zhao, S.; Fu, S.; Ma, Y.; Ge, L.; et al. Plant and animal positive-sense single-stranded RNA viruses encode small proteins important for viral infection in their negative-sense strand. Mol. Plant 2023, 16, 1794–1810. [Google Scholar] [CrossRef]
- Rivetta, A.; Negrini, N.; Cocucci, M. Involvement of Ca2+-calmodulin in Cd2+ toxicity during the early phases of radish (Raphanus sativus L.) seed germination. Plant Cell Environ. 1997, 20, 600–608. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Z.; Yan, Z.; Yi, X.; Zhang, J.; Zhang, Y.; Zheng, X.; Zhu, Y. Deriving water quality criteria for trivalent and pentavalent arsenic. Sci. Total Environ. 2017, 587–588, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K. Public health. Worldwide occurrences of arsenic in ground water. Science 2002, 296, 2143–2145. [Google Scholar] [CrossRef] [PubMed]
- Dure, L., 3rd. A repeating 11-mer amino acid motif and plant desiccation. Plant J. 1993, 3, 363–369. [Google Scholar] [CrossRef]
- Bray, E.A. Molecular Responses to Water Deficit. Plant Physiol. 1993, 103, 1035–1040. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: A commonalty in the response of plants to dehydration and low temperature. Physiol. Plant. 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.X.; Wei, W.; Han, L.; Guan, Z.Q.; Wang, Z.; Chai, T.Y. BjDHNs confer heavy-metal tolerance in plants. Mol. Biotechnol. 2008, 38, 91–98. [Google Scholar] [CrossRef]
- Falavigna, V.d.S.; Malabarba, J.; Silveira, C.P.; Buffon, V.; Mariath, J.E.d.A.; Pasquali, G.; Margis-Pinheiro, M.; Revers, L.F. Characterization of the nucellus-specific dehydrin MdoDHN11 demonstrates its involvement in the tolerance to water deficit. Plant Cell Rep. 2019, 38, 1099–1107. [Google Scholar] [CrossRef]
- Peirats-Llobet, M.; Yi, C.; Liew, L.C.; Berkowitz, O.; Narsai, R.; Lewsey, M.G.; Whelan, J. Spatially resolved transcriptomic analysis of the germinating barley grain. Nucleic Acids Res. 2023, 51, 7798–7819. [Google Scholar] [CrossRef]
- Hara, M.; Fujinaga, M.; Kuboi, T. Metal binding by citrus dehydrin with histidine-rich domains. J. Exp. Bot. 2005, 56, 2695–2703. [Google Scholar] [CrossRef]
- Battaglia, M.; Olvera-Carrillo, Y.; Garciarrubio, A.; Campos, F.; Covarrubias, A.A. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008, 148, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Tunnacliffe, A.; Wise, M.J. The continuing conundrum of the LEA proteins. Die Naturwiss. 2007, 94, 791–812. [Google Scholar] [CrossRef] [PubMed]
- Koag, M.C.; Fenton, R.D.; Wilkens, S.; Close, T.J. The binding of maize DHN1 to lipid vesicles. Gain of structure and lipid specificity. Plant Physiol. 2003, 131, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Yu, F.; Cong, L.; Wang, L.; Burkard, G.; Chai, T. Cloning and expression analysis of SKn-type dehydrin gene from bean in response to heavy metals. Mol. Biotechnol. 2006, 32, 205–218. [Google Scholar] [CrossRef]
- Tamás, L.; Huttová, J.; Mistrík, I.; Simonovicová, M.; Siroká, B. Aluminium-induced drought and oxidative stress in barley roots. J. Plant Physiol. 2006, 163, 781–784. [Google Scholar] [CrossRef]
- Khan, N.Z.; Ali, A.; Ali, W.; Aasim, M.; Khan, T.; Khan, Z.; Munir, I. Heterologous expression of bacterial dehydrin gene in Arabidopsis thaliana promotes abiotic stress tolerance. Physiol. Mol. Biol. Plants 2023, 29, 1239–1246. [Google Scholar] [CrossRef]
- Shah, K.; Dubey, R.S. Cadmium elevates level of protein, amino acids and alters activity of proteolytic enzymes in germinating rice seeds. Acta Physiol. Plant. 1998, 20, 189–196. [Google Scholar] [CrossRef]
- Kumar, S.; Khare, R.; Trivedi, P.K. Arsenic-responsive high-affinity rice sulphate transporter, OsSultr1;1, provides abiotic stress tolerance under limiting sulphur condition. J. Hazard. Mater. 2019, 373, 753–762. [Google Scholar] [CrossRef]
- Aronsson, H.; Jarvis, R.P. Rapid isolation of Arabidopsis chloroplasts and their use for in vitro protein import assays. In Chloroplast Research in Arabidopsis: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2011; pp. 281–305. [Google Scholar]
- Huang, W.; Ratkowsky, D.A.; Hui, C.; Wang, P.; Su, J.; Shi, P. Leaf fresh weight versus dry weight: Which is better for describing the scaling relationship between leaf biomass and leaf area for broad-leaved plants? Forests 2019, 10, 256. [Google Scholar] [CrossRef]
- Bouchabke, O.; Chang, F.; Simon, M.; Voisin, R.; Pelletier, G.; Durand-Tardif, M. Natural variation in Arabidopsis thaliana as a tool for highlighting differential drought responses. PLoS ONE 2008, 3, e1705. [Google Scholar] [CrossRef]
- Gopalasatheeskumar, K.; Ariharasiva, G.K.; Sengottuvel, T.; Sanish, V.D.; Srividhya, V. Quantification of total phenolic and flavonoid content in leaves of Cucumis melo var. agrestis using UV-spectrophotometer. Asian J. Res. Chem. 2019, 12, 335–337. [Google Scholar] [CrossRef]
- Uysal, S.; Aumeeruddy-Elalfi, Z.; Zengin, G.; Aktumsek, A.; Mašković, P.Z.; Vujić, J.M.; Mahomoodally, M.F. In vitro antioxidant, cytotoxicity and chemical profile of different extracts from Acanthus hirsutus Boiss used in Anatolian folk medicine. Eur. J. Integr. Med. 2018, 17, 135–140. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Porra, R.J.; Scheer, H. Towards a more accurate future for chlorophyll a and b determinations: The inaccuracies of Daniel Arnon’s assay. Photosynth. Res. 2019, 140, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, M.; Lee, J.-H.; Lee, H.-J.; Park, C.-M. The unified ICE–CBF pathway provides a transcriptional feedback control of freezing tolerance during cold acclimation in Arabidopsis. Plant Mol. Biol. 2015, 89, 187–201. [Google Scholar] [CrossRef]
- Dai, J.; Shen, L.; Zhou, J.; Liu, X.; Chen, S. A YSK-Type Dehydrin from Nicotiana tabacum Enhanced Copper Tolerance in Escherichia coli. Int. J. Mol. Sci. 2022, 23, 15162. [Google Scholar] [CrossRef]
- Han, L.; Hu, L.; Lv, Y.; Li, Y.; Ma, Z.; Li, B.; Gao, P.; Liang, Y.; Zhao, X. Effects of Bacillus amyloliquefaciens QST713 on mineral nutrient utilization of alfalfa (Medicago sativa L.) under drought stress. Agronomy 2024, 14, 1793. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Huang, T.; Deng, S.; Sheng, J.; Zhang, D. In vivo protective effect of late embryogenesis abundant protein (ApSK3 dehydrin) on Agapanthus praecox to promote post-cryopreservation survival. Biocell 2022, 46, 2507. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Qin, J.; Xiao, G.; Zhu, S.; Hu, T. OsLEA1a overexpression enhances tolerance to diverse abiotic stresses by inhibiting cell membrane damage and enhancing ROS scavenging capacity in transgenic rice. Funct. Plant Biol. 2021, 48, 860–870. [Google Scholar] [CrossRef]
- Pasternak, T.; Palme, K.; Pérez-Pérez, J.M. Role of reactive oxygen species in the modulation of auxin flux and root development in Arabidopsis thaliana. Plant J. 2023, 114, 83–95. [Google Scholar] [CrossRef]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef]
- Murray, M.R.; Graether, S.P. Physiological, structural, and functional insights into the cryoprotection of membranes by the dehydrins. Front. Plant Sci. 2022, 13, 886525. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Reyes, J.L.; Wei, H.; Yang, Y.; Karlson, D.; Covarrubias, A.A.; Krebs, S.L.; Fessehaie, A.; Arora, R. RcDhn5, a cold acclimation-responsive dehydrin from Rhododendron catawbiense rescues enzyme activity from dehydration effects in vitro and enhances freezing tolerance in RcDhn5-overexpressing Arabidopsis plants. Physiol. Plant. 2008, 134, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Kumar, P.; Sharma, P.N.; Bisht, S.S. Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Sci. 2002, 162, 381–388. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Kovacs, D.; Kalmar, E.; Torok, Z.; Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 2008, 147, 381–390. [Google Scholar] [CrossRef]
- Szopiński, M.; Sitko, K.; Rusinowski, S.; Zieleźnik-Rusinowska, P.; Corso, M.; Rostański, A.; Rojek-Jelonek, M.; Verbruggen, N.; Małkowski, E. Different strategies of Cd tolerance and accumulation in Arabidopsis halleri and Arabidopsis arenosa. Plant Cell Environ. 2020, 43, 3002–3019. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Moravčíková, D.; Žiarovská, J. The Effect of Cadmium on Plants in Terms of the Response of Gene Expression Level and Activity. Plants 2023, 12, 1848. [Google Scholar] [CrossRef]
- Gieroń, Ż.; Sitko, K.; Małkowski, E. The Different Faces of Arabidopsis arenosa—A Plant Species for a Special Purpose. Plants 2021, 10, 1342. [Google Scholar] [CrossRef]
- Pasternak, T.; Pérez-Pérez, J.M.; Ruperti, B.; Aleksandrova, T.; Palme, K. A new in vitro growth system for phenotypic characterization and seed propagation of Arabidopsis thaliana. J. Plant Growth Regul. 2024, 43, 652–658. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Usman, M.; Ali, W.; Khan, N.Z.; Aasim, M.; Staykov, N.; Ali, A.; Munir, I.; Gechev, T. Heterologous Expression of Bacterial Dehydrin Promotes Arabidopsis Tolerance to Cadmium and Arsenic Stress. Genes 2025, 16, 1413. https://doi.org/10.3390/genes16121413
Ali A, Usman M, Ali W, Khan NZ, Aasim M, Staykov N, Ali A, Munir I, Gechev T. Heterologous Expression of Bacterial Dehydrin Promotes Arabidopsis Tolerance to Cadmium and Arsenic Stress. Genes. 2025; 16(12):1413. https://doi.org/10.3390/genes16121413
Chicago/Turabian StyleAli, Asmat, Muhammad Usman, Waqar Ali, Nadir Zaman Khan, Muhammad Aasim, Nikola Staykov, Akhtar Ali, Iqbal Munir, and Tsanko Gechev. 2025. "Heterologous Expression of Bacterial Dehydrin Promotes Arabidopsis Tolerance to Cadmium and Arsenic Stress" Genes 16, no. 12: 1413. https://doi.org/10.3390/genes16121413
APA StyleAli, A., Usman, M., Ali, W., Khan, N. Z., Aasim, M., Staykov, N., Ali, A., Munir, I., & Gechev, T. (2025). Heterologous Expression of Bacterial Dehydrin Promotes Arabidopsis Tolerance to Cadmium and Arsenic Stress. Genes, 16(12), 1413. https://doi.org/10.3390/genes16121413





