1. Introduction
RNA processing and modification encompasses key steps such as alternative splicing [
1], epigenetic modification [
2], and poly(A) tail formation [
3], which have been shown to play an important regulatory role in the replication process of various viral infections and exhibit diverse functional characteristics [
4,
5]. As the main causative agent of chronic hepatitis B and hepatocellular carcinoma (HCC), HBV undergoes series post-transcriptional processing modifications, while the precise and complex mechanisms remain unclear [
6].
The discovery of the hepatitis B virus (HBV) in 1965 led to the recognition of HBV infection as a global public health issue [
7]. Different hepatocellular carcinoma cell lines are used for developing therapeutics to resist infection of HBV subsequently [
8,
9]. Numerous studies have already clarified that the HBV genome is a relaxed circular DNA (rcDNA) molecule approximately 3.2 kilobases (kb) long and encodes four overlapping open reading frames (ORFs): C, P, S, and X [
10]. After infection, the rcDNA of HBV genome is then converted to covalently closed circular DNA (cccDNA), which can be transcribed into RNAs of various lengths, primarily 3.5 kb, 2.4 kb, 2.1 kb, and 0.7 kb [
11]. Finally, 3.5 kb RNAs are translated into C and P proteins, 2.4 kb RNAs are translated into L-HB, 2.1 kb RNAs are translated into two surface antigens (M-HB and S-HB), and the 0.7 kb RNA are translated into HBx [
12] (
Figure 1). As a crucial step of HBV replication in cells, the transcription of the HBV genome within cells and the series of regulatory mechanisms during transcription have rarely been studied, and the role of post-transcriptional processing and modification in HBV replication remains unclear as well.
Currently, studies on post-transcriptional processing modifications of the Hepatitis B virus (HBV) have identified over 20 HBV splicing variants, and they speculate that it may help regulate multiple viral and cellular processes in the course of HBV-related liver disease [
14,
15,
16]. In addition, epitranscriptomic analysis have shown that multiple types of epigenetic modifications play an important role in HBV replication. For example, m
6A modification plays a multifaceted role in HBV replication by regulating HBV RNA stability, nucleocapsid assembly, nuclear export of viral RNA, and HBx expression [
17,
18]; m
5C modification has been validated that it is not only essential for Aly/REF export factor recognition to promote viral mRNA export and HBx translation, but also necessary for inhibiting RIG-I binding and suppressing interferon-β production [
19]. Pseudouridine (Ψ) modification is another RNA epimodification type widely used in mRNA therapy. Research indicates that a single instance of pseudouridylation may also potentially alter key interactions between viral and host mechanisms in SARS-CoV-2 [
20,
21]. The formation and length of poly(A) tail show direct impact to RNA stability, for the reason why the process of 3′ adenylation of RNA is one of the most important parts of post-transcriptional modification [
22]. Series studies revealed that 3′ adenylation of primary RNA plays an essential role during the maturation and translation of mRNA, while the formation of poly(A) tail of HBV RNA have not been intensively studied yet.
In this study, to investigate the post-transcriptional processing modifications of HBV in various hepatocellular carcinoma cell lines, we employed Nanopore direct RNA sequencing (DRS) technology [
23,
24], which provides a new solution that can directly focus on individual RNA molecules without PCR amplification and overcome read length limitations since its appearance to sequence HBV transcriptome and then gain insight to HBV transcriptomic and epitranscriptomic features as well [
25,
26,
27]. Our study provides arguments to the post-transcriptional processing modifications of HBV in different hepatocellular carcinoma cell lines and their differences, and further explores the key roles of these regulatory mechanisms throughout the viral life cycle, which is of significant importance for understanding viral pathogenic mechanisms, developing novel antiviral drugs, and optimizing treatment strategies.
2. Materials and Methods
2.1. Cell Culture and Transfection
The cell lines used in this study were obtained from commercial sources and maintained in our laboratory. PLC/PRF/5 cells were purchased from BeNa Culture Collection (BNCC, Beijing, China), and Huh7 cells were acquired from Procell Life Science & Technology Co., Ltd. (Wuhan, China). Both cell lines were cultured in DMEM medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with penicillin and streptomycin (Gibco), along with 10% fetal bovine serum (Gibco), under conditions of 37 °C and 5% CO2.
For transfection, cells were first seeded in T75 cell culture flasks. When ~90% confluent, they were digested with 0.25% trypsin-EDTA solution (Gibco) and re-plated in 6 cm dishes (1.5 × 106 cells/dish). Upon reaching ~80% confluence, cells were subjected to transfection. The HBV 1.3-mer WT replicon plasmid was purchased from Miaoling Biologicals (Wuhan, China) and transfected with Lipofectamine 2000 reagent (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s instructions.
2.2. Extraction of HBV Virion RNA
We used a kit (Takara, Takara Bio Inc., Kusatsu, Shiga, Japan) to extract RNA. We separated the total RNA from the cell sediment. We used the buffer provided in the kit (RL + DTT) to break the cells down, and then followed the instructions of the manufacturer. Finally, we washed the total RNA off using 100 μL of water without a nuclear acid. We measured the total RNA concentration in the sample using the Qubit RNA HS Assay kit (Invitrogen, Carlsbad, CA, USA).
2.3. HBV Viral Load Detection
The total sample volume was brought to 200 µL, after which the cellular RNA was extracted using the extraction reagent supplied with the Nucleic Acid Test Kit (Sansure Biotech Inc., Changsha, Hunan, China). The viral load of HBV was then detected by qPCR using the HBV Nucleic Acid Test Kit.
2.4. Nanopore Direct RNA Sequencing (DRS-Seq)
For nanopore sequencing of HBV-transfected cells, libraries were prepared using 1000 ng total RNA or 300 ng poly(A) tail RNA according to the manufacturer’s instructions (Oxford Nanopore DRS protocol, SQK-RNA004, Oxford Nanopore Technologies, Oxford, UK). Libraries from all samples were quantified using the Qubit fluorometer DNA HS assay (Invitrogen) and loaded onto a FLO-MIN106D flow cell (R10.4) and then run for 72 h for sequencing on the MinION device (Oxford Nanopore Technologies, Oxford, UK).
2.5. In Vitro Transcription
HBV fragments were PCR amplified from the HBV 1.3-mer WT replicon plasmid using designed specific primers (
Supplementary Materials,
Table S1), PrimeSTAR Max DNA Polymerase (Takara, Kusatsu, Shiga, Japan). The purification process was then initiated using the Promega Wizard SV Gel and PCR Purification System (Promega Corporation, Madison, WI, USA), followed by in vitro transcription using the Vazyme T7 High Yield Transcription Kit (Vazyme Biotech Co., Ltd., Nanjing, China). Subsequently, the transcription purification stage was executed employing the Invitrogen MEGAclear™ Transcription Purification RNA purification was then performed using the Invitrogen MEGAclear™ Transcription Purification Kit (Invitrogen).
2.6. DRS Data Preprocessing
Using Dorado (v0.8.1) software, raw electrical signal Pod5 files generated by nanopore direct sequencing were converted into BAM files and their quality assessed. Sequencing reads were directly mapped to the HBV genome using minimap2 (v2.17) with preset splicing parameters. Subsequent filtering, visualization, and sorting operations were performed using Samtools (v1.15.1).
2.7. The Splicing Events Analysis of HBV Transcriptome
Classified BAM files were used as input for splice site analysis using megadepth software (v1.2.0) (
http://github.com/ChristopherWilks/megadepth, accessed on 9 August 2025). Potential SD and SA site reads were filtered by determining the start and end positions of exons from the BAM file analysis. If the location of potential sites was unknown, only sites associated with typical GT-AG splice site pairs were annotated. Gene Structure Display Server (GSDS, V2.0) was used for genomics data visualization. Gene structure diagrams of the candidate genes were generated using the GSDS 2.0 online tool (
http://gsds.gao-lab.org, accessed on 15 October 2025) [
28]. The genomic DNA sequences and their corresponding coding sequences (CDS) were submitted to the server to generate the visualizations.
2.8. Poly (A) Tail Length Analysis of HBV Transcriptome
Poly(A) tail length detection was performed using Oxford Nanopore Dorado (v0.8.1). The tail interrupt length (default 10 nt) was set to exclude the interference of non-adenylate sequences in the tail interrupt region. The basecalling model was selected in ultra-high precision mode (sup) to enhance the signal segmentation accuracy. The final Poly(A) tail lengths were extracted from the tl tags of the BAM files and summarized statistically by a custom script.
2.9. RNA Modification Data Analysis of HBV Transcriptome
The analysis of RNA modification was conducted utilizing the Dorado basecaller (v0.8.1), which incorporates an integrated modification base detection function. Direct RNA sequencing data were analyzed using a dorado basecaller—modified-bases sup,m6A_DRACH, m5C, pseU/path/to/pod5_files > calls. bam, which simultaneously performs ultra-high-precision basecalls (sup model) and m6A_DRACH (context optimization for DRACH-themed motifs), 5-methylcytosine (m6A), and 5-methylcytosine (m5C). The base probabilities of modifications were extracted from BAM MM/ML markers using modkit (v0.4.3). These were then filtered using a ≥20 probability threshold, and the m6A site was further restricted to typical DRACH motifs validated by bedtools nuc (D = A/G/U, R = A/G, H = A/C/U). Finally, the predictions were background corrected against in vitro transcriptional controls.
2.10. Statistical Analysis
In GraphPad Prism 8.0.2, the F-test is first performed to assess homogeneity of variance. If p < 0.05, the t-test with Welch’s correction is used; otherwise, the conventional t-test is applied. A p-value < 0.05 was considered statistically significant, while a p-value < 0.01 was deemed highly significant.
4. Discussion
Given the widespread global transmission of HBV infection, in-depth research into its life cycle and its effects on host cells is of particular importance [
36]. While traditional molecular biology techniques have provided us with basic information about HBV, research into its post-transcriptional processing modifications remains relatively limited. This study investigated post-transcriptional processing modifications of HBV using two distinct hepatocellular carcinoma cell lines: PLC/PRF/5 cells (derived from the primary hepatocellular carcinoma tissue of a 24-year-old African male) harbor an integrally intact HBV genome, while Huh7 cells (derived from the well-differentiated hepatocellular carcinoma tissue of a 57-year-old Japanese male) exhibit high transfection efficiency and transcriptional activity, making them an ideal model for HBV plasmid transfection. Using Nanopore direct RNA sequencing technology, we systematically analyzed alternative splicing, epigenetic modifications, and poly(A) tail characteristics of HBV transcripts, elucidating post-transcriptional processing modifications of HBV in different hepatocellular carcinoma cell lines [
37,
38].
RNA is a highly structured macromolecule with various post-transcriptional modifications, including 3′ polyadenylation, alternative splicing, and chemical modifications [
33]. As with the eukaryotic transcriptome, selective splicing plays a crucial role in the replication cycle of numerous viral families, contributing to the production of infectious proteins through expression. For instance, HIV-1 primary transcripts undergo extensive and complex selective splicing to produce regulatory viral proteins [
39]. Similarly, human papillomavirus (HPV) requires constitutive and selective splicing to generate 20 different mRNAs that encode proteins essential for completing its life cycle [
40]. In this study, a total of 34 HBV splice variants were identified (20 of which have been reported), 16 of which were shared by the two-cell lineage and 9 of which were unique to PLC/PRF/5 and Huh7, respectively. As in other studies, we found that D2 (461 nt) and D7 (2450 nt) were the major splice donor sites and that A3 (490 nt) and A7 (1386 nt) were the major acceptor sites. The major HBV splice variant was 2447/489 (with shear editing between 2447 and 489 nt), accounting for the majority of spliced transcripts in this study [
31]. The nine unique isoforms for each of the two cell lines may suggest that host factors regulate splice site selection. For example, the enrichment of particular isoforms in cells could be linked to the expression of cell-specific splicing factors (e.g., hnRNPs or SR proteins), which control splicing efficiency by binding to exon/intron silencers (ESS/ISS) or enhancers (ESE/ISE) [
41].
Polyadenylation is a conserved regulatory process in eukaryotic gene expression: triggered by RNA polymerase II termination, it initiates 3′-end splicing and catalyzes the formation of a poly(A) tail via polyadenylate polymerase (PAP). By coordinating mRNA maturation, nuclear export, stability, and translation initiation, it plays a central role in cellular homeostasis and disease [
42]. Previous study found that the length of the poly(A) tail of bovine coronavirus mRNA changes during infection, decreasing from 45 nucleotides (nt) immediately after viral entry to 65 nt at 6–9 h post-infection and increasing to 120–144 nt at 30 h post-infection. This suggests that longer poly(A) tails may favor translation in coronaviruses [
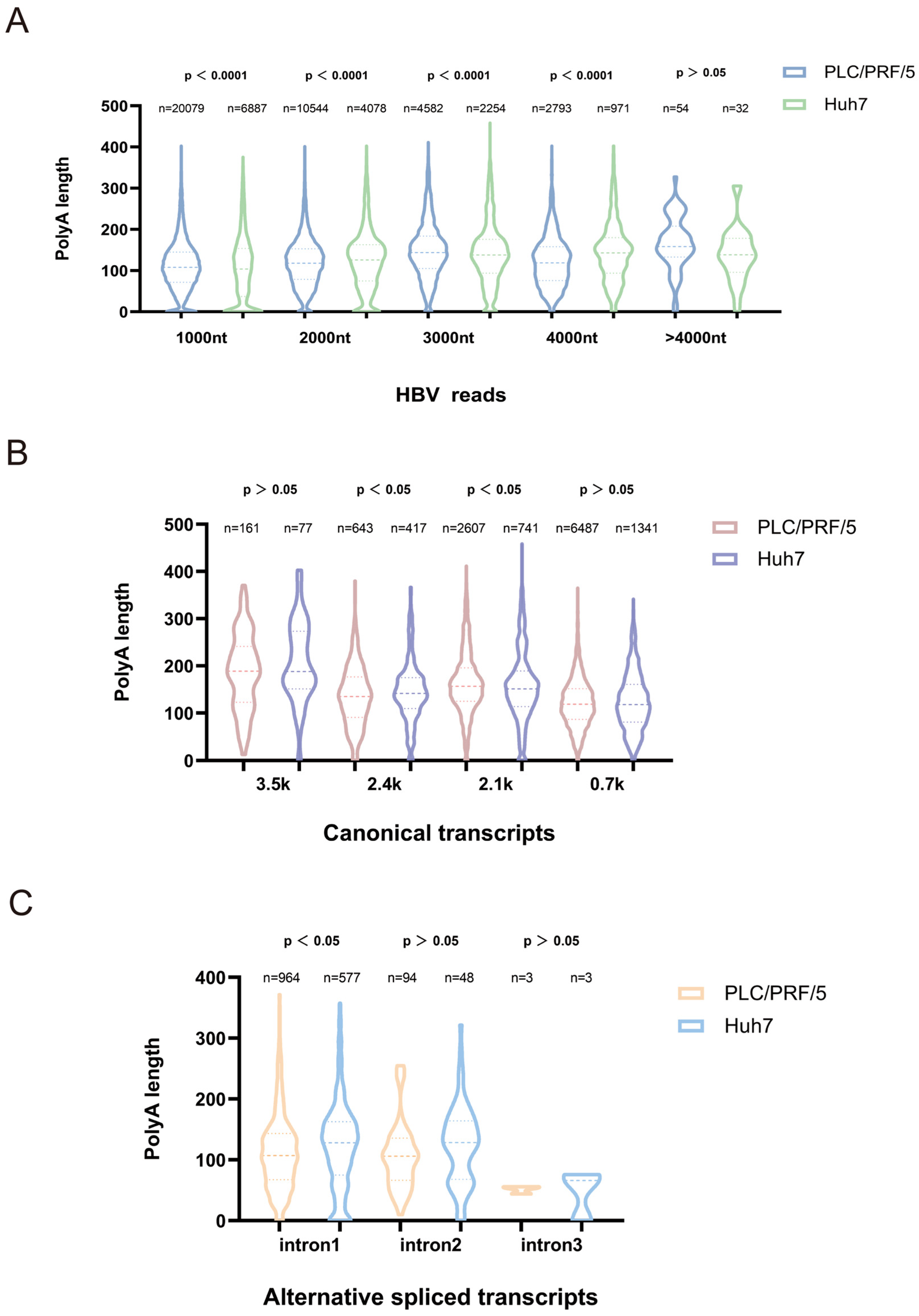
43]. In our study, we found that poly(A) tail lengths differed significantly between classical and long HBV transcripts in PLC/PRF/5 and Huh7 cells, indicating the stability of long HBV transcripts differs between the two cell types. Additionally, the average poly(A) tail length of most variable shear transcript species in Huh7 cells was slightly longer than in PLC/PRF/5 cells. This finding may provide new insights into the heterogeneity of viral antigen expression in different hepatocellular carcinoma models.
Since the late 1950s, more than 300 chemical modifications (epitranscriptomes) have been identified, regulating cellular/viral processes and more than 100 human diseases. There are trans-viral studies showing the universality of the biological functions of such conserved clusters: in HIV-1, for example, the m
6A cluster at the 3′ end was shown to be an essential element for viral replication, and its absence leads to replication defects at the level of the single-molecule epitranscriptome [
37]. The m
5C modification in the Epstein–Barr virus-encoded protein 1 (EBER1) is crucial for viral lytic replication and negatively impacts RNA stability [
19]. In our study, 30 (PLC/PRF/5) and 28 (Huh7) HBV modification sites were detected, including conserved m
6A (20 shared, 95% overlap), m
5C (6 shared), and Ψ (2 shared) clusters—likely evolutionarily selected core regulatory elements. Such conserved clusters may serve as core functional elements for adaptive selection of HBV during long-term evolution, driving key processes in viral transcriptional regulation. Meanwhile, cell-specific modification sites (e.g., m
6A-512 nt and m
5C-254 nt unique to PLC/PRF/5) reflect HBV’s dynamic adaptation to host epigenetic heterogeneity, enabling plasticity in response to liver injury stages, immune pressure, and replication demands.
This study reveals the epigenomic characteristics of hepatitis B virus (HBV), though the findings represent preliminary research. The identified modification sites and splicing variants require further validation. The functional roles of these modifications and splicing variants in influencing HBV biological properties, viral replication, pathogenicity, and carcinogenicity should be elucidated in future studies.
Collectively, our nanopore direct RNA sequencing study achieved four key outcomes: single-molecule analysis enabled precise identification of low-frequency HBV splicing variants; direct detection of natural RNA modifications avoided chemical biases of traditional methylation sequencing; poly(A) tail profiling overcame short-read sequencing limitations; and integrated multidimensional analysis revealed a synergistic regulatory network involving splicing, modifications, and poly(A) tails. Additionally, by comparing differences in post-transcriptional processing modifications of HBV between PLC/PRF/5 and Huh7 cells, we can infer that PLC/PRF/5 cells may better mimic HBV chronic infection immune evasion by restricting viral replication (host-mediated transcriptional silencing) while maintaining HBsAg secretion. In contrast, the long poly(A) tail in Huh7 cells supports efficient replication, making it potentially more suitable for studying HBV transcription and replication. These findings may provide clues for selecting cellular models relevant to HBV mechanism research and lay the groundwork for predicting antiviral targets.