Vitamin K Epoxide Reductase Complex Subunit 1 (VKORC1) Gene Polymorphisms Predict Arterial Stiffness and Serum MGP Levels in Chronic Kidney Disease Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Follow-Up Characteristics
2.2. Determination of Haemodynamic Parameters
2.3. Measurement of Arterial Calcification by Electron Beam Computed Tomography (EBCT)
2.4. Determination of Biochemical Markers
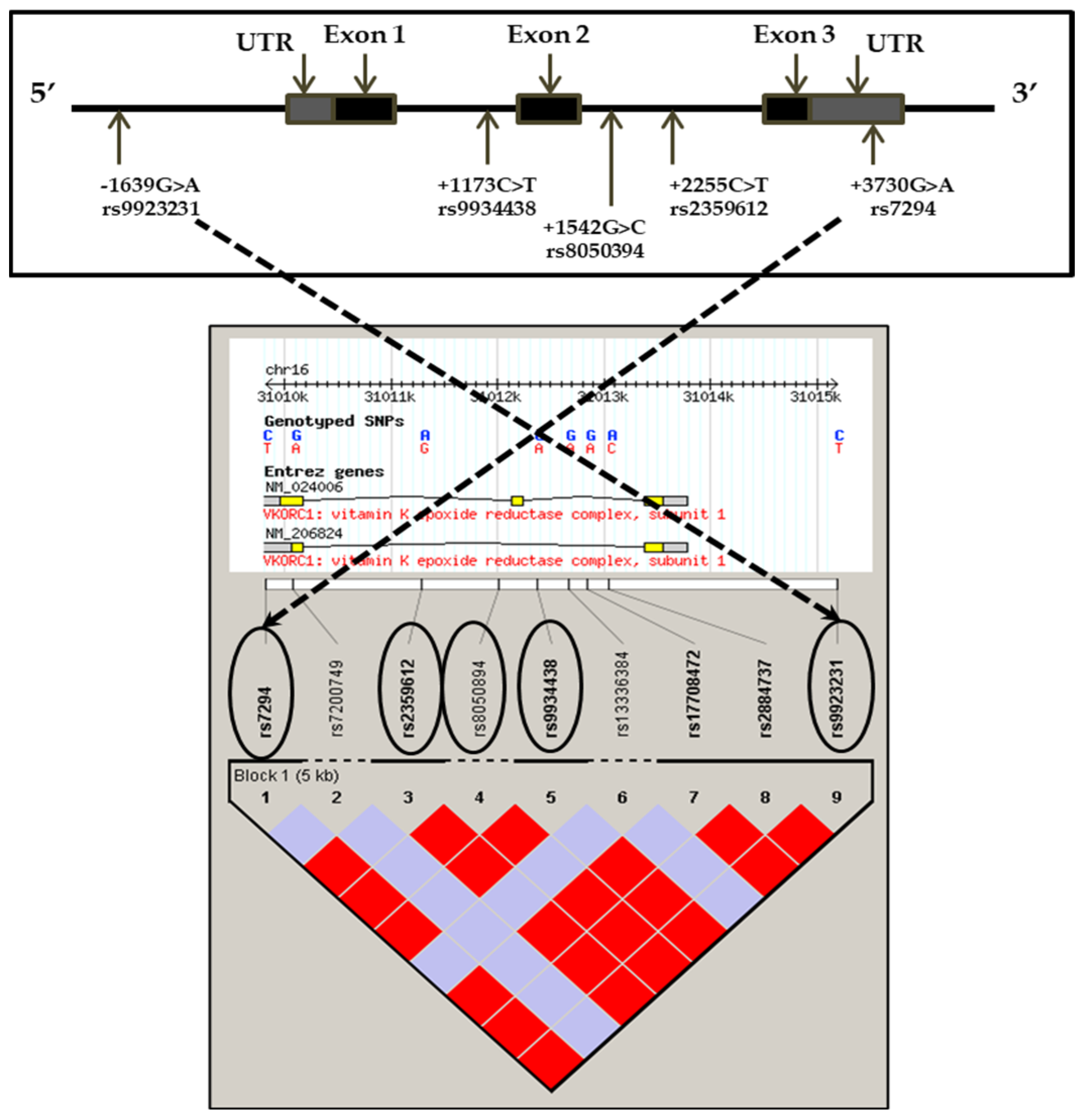
2.5. Determination of Genotypes by Taqman System
2.6. Data Analysis
3. Results
3.1. Clinical Characteristics
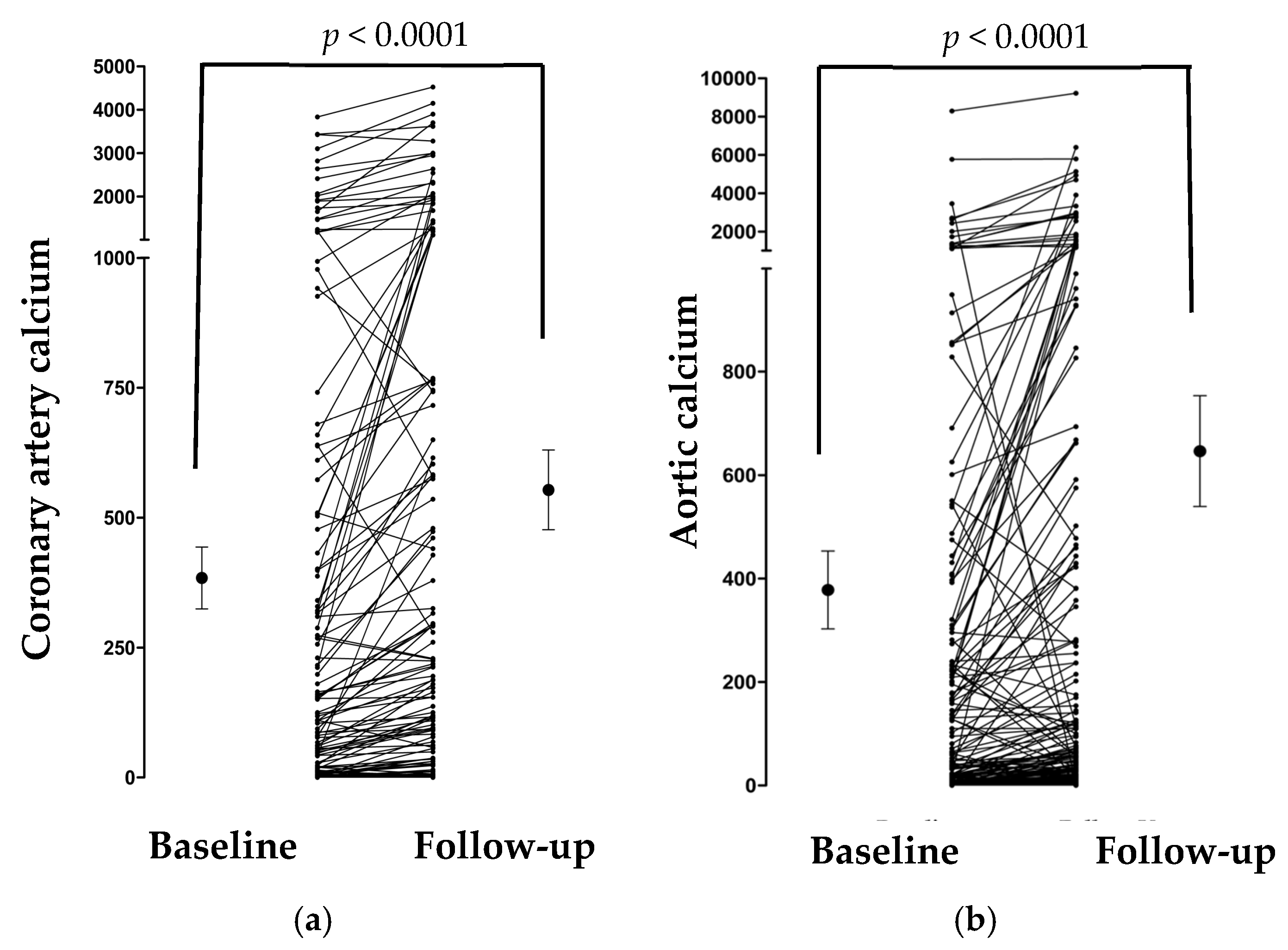
3.2. Changes in Calcification Markers over Time
3.3. Genotypes and Haplotypes
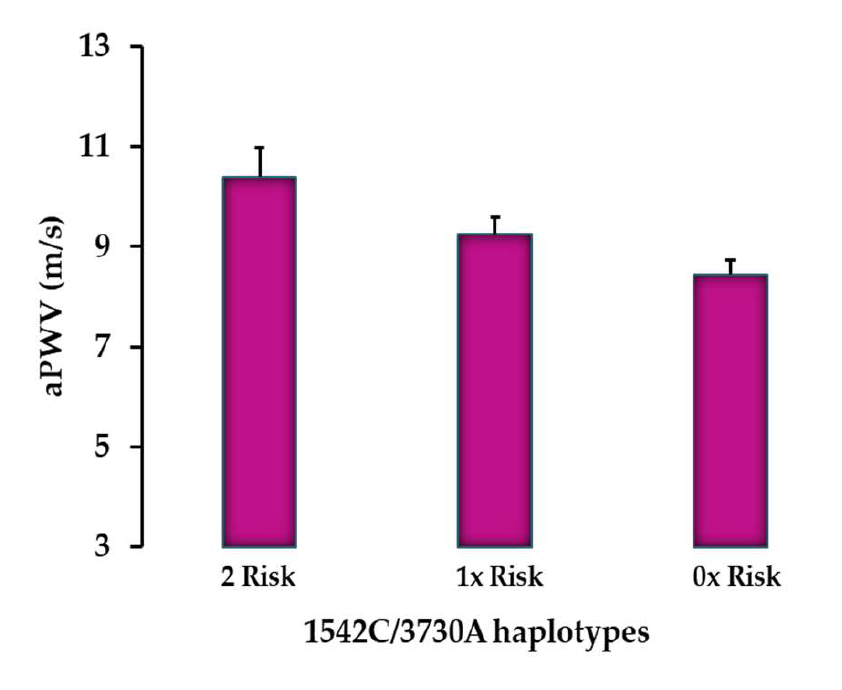
3.4. Differences in aPWV and Calcification Markers for Each SNP
3.5. Associations Between aPWV and Each SNP
3.6. Associations Between Age, Haemodynamic, and Calcification Markers
4. Discussion
4.1. Significant Association Between VKORC1 Gene Polymorphisms and Arterial Stiffness
4.2. No Association Between VKORC1 SNPs and Calcification
4.3. Association Between VKORC1 Gene Polymorphisms and Serum t-uncMGP
4.4. Serum MGP as a Biomarker of Calcification and Arterial Stiffness
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.-C.; Tsai, W.-C.; Chen, J.-Y.; Huang, J.-J. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am. J. Kidney Dis. 2005, 45, 494–501. [Google Scholar] [CrossRef]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; Safar, M.E.; London, G.M. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999, 99, 2434–2439. [Google Scholar] [CrossRef]
- Shoji, T.; Emoto, M.; Shinohara, K.; Kakiya, R.; Tsujimoto, Y.; Kishimoto, H.; Ishimura, E.; Tabata, T.; Nishizawa, Y. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J. Am. Soc. Nephrol. 2001, 12, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, M.; Tatara, Y.; Ito, N.; Takeya, Y.; Onishi, M.; Yaekawa, M.; Kato, N.; Kamide, K.; Rakugi, H. The combination of chronic kidney disease and increased arterial stiffness is a predictor for stroke and cardiovascular disease in hypertensive patients. Hypertens. Res. 2011, 34, 1209–1215. [Google Scholar] [CrossRef]
- Lacolley, P.; Challande, P.; Osborne-Pellegrin, M.; Regnault, V. Genetics and pathophysiology of arterial stiffness. Cardiovasc. Res. 2009, 81, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.M.; Fitzpatrick, L.A.; Inoue, D.; Qiao, J.-H.; Fishbein, M.C.; Detrano, R.C.; Shah, P.K.; Rajavashisth, T.B. Molecular, Endocrine, and Genetic Mechanisms of Arterial Calcification. Endocr. Rev. 2004, 25, 629–672. [Google Scholar] [CrossRef]
- Islam, S.; Jayaram, D.T.; Biswas Stuehr, D.J. Functional maturation of cytochromes P450 3A4 and 2D6 relies on GAPDH- and Hsp90-Dependent heme allocation. J. Biol. Chem. 2024, 300, 105633. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Cranenburg, E.C.; Vermeer, C. Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost. 2008, 100, 593–603. [Google Scholar] [CrossRef]
- Besir, B.; Kapadia, S.R. The role of vitamin K2 in cardiovascular health. Interv. Cardiol. 2024, 16, 679–686. [Google Scholar]
- Mishima, E.; Wahida, A.; Seibt, T.; Conrad, M. Diverse biological functions of vitamin K: From coagulation to ferroptosis. Nat. Metab. 2023, 924–932. [Google Scholar]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arter. Thromb. Vasc. Biol. 1998, 18, 1400–1407. [Google Scholar] [CrossRef]
- Schurgers, L.; Spronk, H.; Skepper, J.; Hackeng, T.; Shanahan, C.; Vermeer, C.; Weissberg, P.; Proudfoot, D. Post-translational modifications regulate matrix Gla protein function: Importance for inhibition of vascular smooth muscle cell calcification. J. Thromb. Haemost. 2007, 5, 2503–2511. [Google Scholar] [CrossRef]
- Kohn, M.H.; Price, R.E.; Pelz, H.J. A cardiovascular phenotype in warfarin-resistant Vkorc1 mutant rats. Artery Res. 2008, 2, 138–147. [Google Scholar] [CrossRef]
- Teichert, M.; Visser, L.E.; van Schaik, R.H.; Hofman, A.; Uitterlinden, A.G.; De Smet, P.A.G.M.; Witteman, J.C.M.; Stricker, B.H.C. Vitamin K epoxide reductase complex subunit 1 (VKORC1) polymorphism and aortic calcification: The Rotterdam Study. Arter. Thromb. Vasc. Biol. 2008, 28, 771–776. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Andrea, G.; D’Ambrosio, R.L.; Di Perna, P.; Chetta, M.; Santacroce, R.; Brancaccio, V.; Grandone, E.; Margaglione, M. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood 2005, 105, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ge, W.; Yu, F.; Zhu, H. Impact of VKORC1 gene polymorphism on interindividual and interethnic warfarin dosage requirement—A systematic review and meta-analysis. Thromb. Res. 2010, 125, e159–e166. [Google Scholar] [CrossRef] [PubMed]
- Rieder, M.J.; Reiner, A.P.; Gage, B.F.; Nickerson, D.A.; Eby, C.S.; McLeod, H.L.; Blough, D.K.; Thummel, K.E.; Veenstra, D.L.; Rettie, A.E. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005, 352, 2285–2293. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Ding, J. VKORC1-1639G/A and 1173 C/T Genetic Polymorphisms influence individual differences in warfarin maintenance dose. Genet. Test. Mol. Biomark. 2015, 19, 488–493. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Zhang, Y.; Yang, Y.; Sun, L.; Hu, S.; Chen, J.; Zhang, C.; Zheng, Y.; Zhen, Y.; et al. VKORC1 haplotypes are associated with arterial vascular diseases (stroke, coronary heart disease, and aortic dissection). Circulation 2006, 113, 1615–1621. [Google Scholar] [CrossRef]
- Wang, D.; Chen, H.; Momary, K.M.; Cavallari, L.H.; Johnson, J.A.; Sadée, W. Regulatory polymorphism 4n vitamin K epoxide reductase complex subunit 1 (VKORC1) affects gene expression and warfarin dose requirement. Blood 2008, 112, 1013–1021. [Google Scholar] [CrossRef]
- Caplin, B.; Nitsch, D.; Gill, H.; Hoefield, R.; Blackwelln, S.; Mackenzie, D.; Cooper, J.A.; Middleton, R.J.; Talmud, P.J.; Veitch, P.; et al. Circulating methylarginine levels and the decline in renal function in patients with chronic kidney disease are modulated by DDAH1 polymorphisms. Kidney Int. 2010, 77, 459–467. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Fuchs, S.A.; Jansen, I.M.; Spratt, J.C.; Murray, G.D.; Cockcroft, J.R.; Webb, D.J. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J. Hypertens. 1998, 16, 2079–2084. [Google Scholar] [CrossRef]
- O’Rourke, R.A.; Brundage, B.H.; Froelicher, V.F.; Greenland, P.; Grundy, S.M.; Hachamovitch, R.; Pohost, G.M.; Shaw, L.J.; Weintraub, W.S.; Winters, J.W.L.; et al. American College of Cardiology/American Heart Association Expert Consensus Document on Electron-Beam Computed Tomography for the Diagnosis and Prognosis of Coronary Artery Disease. Circulation 2000, 102, 126–140. [Google Scholar] [CrossRef]
- Braun, J.; Oldendorf, M.; Moshage, W.; Heidler, R.; Zeitler, E.; Luft, F.C. Electron beam computed tomography in the evaluation of cardiac calcifications in chronic dialysis patients. Am. J. Kidney Dis. 1996, 27, 394–401. [Google Scholar] [CrossRef]
- Janowitz, W.R.; Agatston, A.S.; Kaplan, G.; Viamonte, M., Jr. Differences in prevalence and extent of coronary artery calcium detected by ultrafast computed tomography in asymptomatic men and women. Am. J. Cardiol. 1993, 72, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowith, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr. Detrano R: Quantification of coronary calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Matthew, J.; Budoff, M.J.; Achenbach, S.; Blumenthal, R.S.; Carr, J.J.; Goldin, J.G.; Greenland, P.; Guerci, A.D.; Lima, J.A.C.; Rader, D.J.; et al. Assessment of Coronary Artery Disease by Cardiac Computed Tomography. A Scientific Statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006, 114, 1761–1791. [Google Scholar]
- Cranenburg, E.C.; Vermeer, C.; Koos, R.; Boumans, M.-L.; Hackeng, T.M.; Bouwman, F.G.; Kwaijtaal, M.; Brandenburg, V.M.; Ketteler, M.; Schurgers, L.J. The circulating inactive form of matrix Gla Protein (ucMGP) as a biomarker for cardiovascular calcification. J. Vasc. Res. 2008, 45, 427–436. [Google Scholar] [CrossRef]
- Shaw, L.J.; Raggi, P.; Schisterman, E.; Berman, D.S.; Callister, T.Q. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2008, 228, 826–833. [Google Scholar] [CrossRef]
- Limdi, N.A.; Wadelius, M.; Cavallari, L.; Eriksson, N.; Crawford, D.C.; Lee, M.-T.M.; Chen, C.-H.; Motsinger-Reif, A.; Sagreiya, H.; Liu, N.; et al. Warfarin pharmacogenetics: A single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood 2010, 115, 3827–3834. [Google Scholar] [CrossRef]
- Yin, T.; Miyata, T. Warfarin dose and the pharmacogenomics of CYP2C9 and VKO. Thromb. Res. 2007, 120, 1–10. [Google Scholar] [CrossRef]
- Wadelius, M.; Chen, L.Y.; Downes, K.; Ghori, J.; Hunt, S.; Eriksson, N.; Wallerman, O.; Melhus, H.; Wadelius, C.; Bentley, D.; et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005, 5, 262–270. [Google Scholar] [CrossRef]
- Yuan, H.-Y.; Chen, J.-J.; Lee, M.T.M.; Wung, J.-C.; Chen, Y.-F.; Charng, M.-J.; Lu, M.-J.; Hung, C.-R.; Wei, C.-Y.; Chen, C.-H.; et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum. Mol. Genet. 2005, 14, 1745–1751. [Google Scholar] [CrossRef]
- Crawford, D.C.; Ritchie, M.D.; Rieder, M.J. Identifying the genotype behind the phenotype: A role model found in VKORC1 and its association with warfarin dosing. Pharmacogenomics 2007, 8, 487–496. [Google Scholar] [CrossRef]
- Hikami, K.; Kawasaki, A.; Ito, I.; Koga, M.; Ito, S.; Hayashi, T.; Matsumoto, I.; Tsutsumi, A.; Kusaoi, M.; Takasaki, Y.; et al. Association of a functional polymorphism in the 3′-untranslated region of SPI1 with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 755–763. [Google Scholar] [CrossRef]
- Liang, C.L.; Hsi, E.; Chen, K.C.; Pan, Y.R.; Wang, Y.S.; Juo, S.H. A functional polymorphism at 3′UTR of the PAX6 gene may confer risk for extreme myopia in Chinese. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3500–3505. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: A preliminary report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, S.B.; Rathcke, C.N.; Zerahn, B.; Vestergaard, H. Increased levels of the calcification marker matrix Gla Protein and the inflammatory markers YKL-40 and CRP in patients with type 2 diabetes and ischemic heart disease. Cardiovasc. Diabetol. 2010, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Gullestad, L.; Dahl, C.P.; Aukrust, P.; Aakhus, S.; Solberg, O.G.; Vermeer, C.; Schurgers, L.J. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J. Int. Med. 2010, 268, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Chan, W.S.; Jolson, D.M.; Williamson, M.K. The elastic lamellae of devitalized arteries calcify when incubated in serum: Evidence for a serum calcification factor. Arter. Thromb. Vasc. Biol. 2006, 26, 1079–1085. [Google Scholar] [CrossRef] [PubMed]


| Variables | Mean ± SD |
|---|---|
| Age (years) | 57.8 ± 15.6 |
| Gender (m/f) | 221/81 |
| Height (m) | 1.7 ± 0.1 |
| Weight (kg) | 81.1 ± 18.8 |
| BMI (kg/m2) | 27.6 ± 5.5 |
| Current smokers (n) | 40 |
| Hypertension (n) | 242 |
| Diabetes (n) | 61 |
| Myocardial infarction (n) | 23 |
| CVD medications used (n) | |
| ACE inhibitor | 139 |
| Aspirin | 114 |
| α-Blocker | 55 |
| α2-Blocker | 113 |
| β-Blocker | 92 |
| Ca2+ blockers | 102 |
| Diuretics | 156 |
| Nitrate | 16 |
| Statins | 160 |
| Warfarin | 12 |
| Variables | Mean ± SD |
|---|---|
| Arterial Calcification Scores a | |
| Total coronary calcification score (baseline, n = 240) | 46.6 (0.0–344.3) |
| Total coronary calcification score (follow-up, n = 162) | 98.6 (1.5–587.7) |
| Aortic calcification score (baseline, n = 241) | 52.7 (6.2–397.1) |
| Aortic calcification score (follow-up, n = 162) | 95.6 (25.4–661.6) |
| Haemodynamic and Arterial Stiffness Measures (n = 302) | |
| Peripheral systolic BP, mmHg | 133 ± 19 |
| Peripheral diastolic BP, mmHg | 79 ± 11 |
| Mean arterial pressure, mmHg | 97 ± 12 |
| Heart rate, bpm | 69 ± 13 |
| Aortic pulse wave velocity, m/s (baseline, n = 199) a | 8.5 (6.8–10.9) |
| Aortic pulse wave velocity, m/s (follow-up, n = 88) a | 7.7 (5.4–10.6) |
| Biochemical Markers of Vascular Calcification (n = 296) | |
| Total cholesterol, mmol/L | 4.7 ± 1.1 |
| HDL cholesterol, mmol/L | 1.5 ± 0.5 |
| LDL cholesterol, mmol/L | 2.4 ± 1.0 |
| Triglyceride, mmol/L | 2.5 ± 6.9 |
| Glucose, mmol/L | 5.7 ± 2.8 |
| C-reactive protein, mg/L | 3.3 ± 4.9 |
| Phosphate, nM | 1.3 ± 0.8 |
| Total uncarboxylated matrix Gla protein, nM a | 2670.5 (2004.0–3359.2) |
| Estimated glomerular filtration rate, mL/min a | 39.2 (25.8–50.9) |
| Age Groups (n) | Men (n) Mean ± SEM | Women (n) Mean ± SEM |
|---|---|---|
| <40 years (31) | 4.5 ± 3.0 (23) | 0.13 ± 0.1 (8) |
| 41–50 years (34) | 89.3 ± 59.3 (21) | 77.5 ± 51.4 (13) |
| 51–60 years (47) | 425 ± 194 (33) | 54.6 ± 18.7 (14) |
| 61–70 years (53) | 762 ± 196 (42) | 75.8 ± 26.3 (11) |
| >71 years (64) | 875 ± 138 (50) | 213 ± 130 (14) |
| Variables | Beta | t-Value | Significance (p) |
|---|---|---|---|
| −1639G>A polymorphism | |||
| Age | 0.416 | 6.615 | <0.001 |
| Gender | 0.031 | 0.490 | 0.624 |
| MAP | 0.151 | 2.3096 | 0.018 |
| BMI | 0.227 | 3.608 | <0.001 |
| G>A polymorphism | 0.132 | 2.115 | 0.036 |
| Adjusted R2 = 0.284; F value = 15.865; p < 0.001 | |||
| +1173C>T polymorphism | |||
| Age | 0.422 | 6.724 | <0.001 |
| Gender | 0.038 | 0.602 | 0.548 |
| MAP | 0.152 | 2.417 | 0.017 |
| BMI | 0.229 | 3.629 | <0.001 |
| G>C polymorphism | 0.122 | 1.949 | 0.053 |
| Adjusted R2 = 0.282; F value = 15.676; p < 0.001 | |||
| +1542G>C polymorphism | |||
| Age | 0.416 | 6.582 | <0.001 |
| Gender | 0.032 | 0.513 | 0.609 |
| MAP | 0.131 | 2.081 | 0.039 |
| BMI | 0.247 | 3.869 | <0.001 |
| C>T polymorphism | 0.131 | 2.068 | 0.040 |
| Adjusted R2 = 0.281; F value = 15.482; p < 0.001 | |||
| +2255C>T polymorphism | |||
| Age | 0.414 | 6.542 | <0.001 |
| Gender | 0.035 | 0.549 | 0.584 |
| MAP | 0.151 | 2.405 | 0.017 |
| BMI | 0.230 | 3.645 | <0.001 |
| C>T polymorphism | −0.121 | −1.921 | 0.056 |
| Adjusted R2 = 0.281; F value = 15.646; p < 0.001 | |||
| +3730G>A polymorphism | |||
| Age | 0.417 | 6.671 | <0.001 |
| Gender | 0.016 | 0.253 | 0.801 |
| MAP | 0.154 | 2.459 | 0.015 |
| BMI | 0.212 | 3.367 | <0.001 |
| G>A polymorphism | 0.159 | 2.543 | 0.012 |
| Adjusted R2 = 0.292; F value = 16.345; p < 0.001. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.H.; Vermeer, C.; Cockcroft, J.R.; Wheeler, D.C.; O’Shaughnessy, K.M.; Yasmin. Vitamin K Epoxide Reductase Complex Subunit 1 (VKORC1) Gene Polymorphisms Predict Arterial Stiffness and Serum MGP Levels in Chronic Kidney Disease Patients. Genes 2025, 16, 1396. https://doi.org/10.3390/genes16121396
Chen DH, Vermeer C, Cockcroft JR, Wheeler DC, O’Shaughnessy KM, Yasmin. Vitamin K Epoxide Reductase Complex Subunit 1 (VKORC1) Gene Polymorphisms Predict Arterial Stiffness and Serum MGP Levels in Chronic Kidney Disease Patients. Genes. 2025; 16(12):1396. https://doi.org/10.3390/genes16121396
Chicago/Turabian StyleChen, David H., Cees Vermeer, John R. Cockcroft, David C. Wheeler, Kevin M. O’Shaughnessy, and Yasmin. 2025. "Vitamin K Epoxide Reductase Complex Subunit 1 (VKORC1) Gene Polymorphisms Predict Arterial Stiffness and Serum MGP Levels in Chronic Kidney Disease Patients" Genes 16, no. 12: 1396. https://doi.org/10.3390/genes16121396
APA StyleChen, D. H., Vermeer, C., Cockcroft, J. R., Wheeler, D. C., O’Shaughnessy, K. M., & Yasmin. (2025). Vitamin K Epoxide Reductase Complex Subunit 1 (VKORC1) Gene Polymorphisms Predict Arterial Stiffness and Serum MGP Levels in Chronic Kidney Disease Patients. Genes, 16(12), 1396. https://doi.org/10.3390/genes16121396





