Endometrial Signatures of Subfertility in Beef Heifers Reveal Dysregulation of MAPK Signaling and Ciliary Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management, Sample, and Heifer Classification
2.2. RNA Isolation, Library Preparation, and RNA Sequencing
2.3. RNA-Seq Data Analyses
2.4. Identification of Key Transcription Factors (TFs)
2.5. Creating Gene Co-Expression Networks
2.6. Pathway Analysis
3. Results
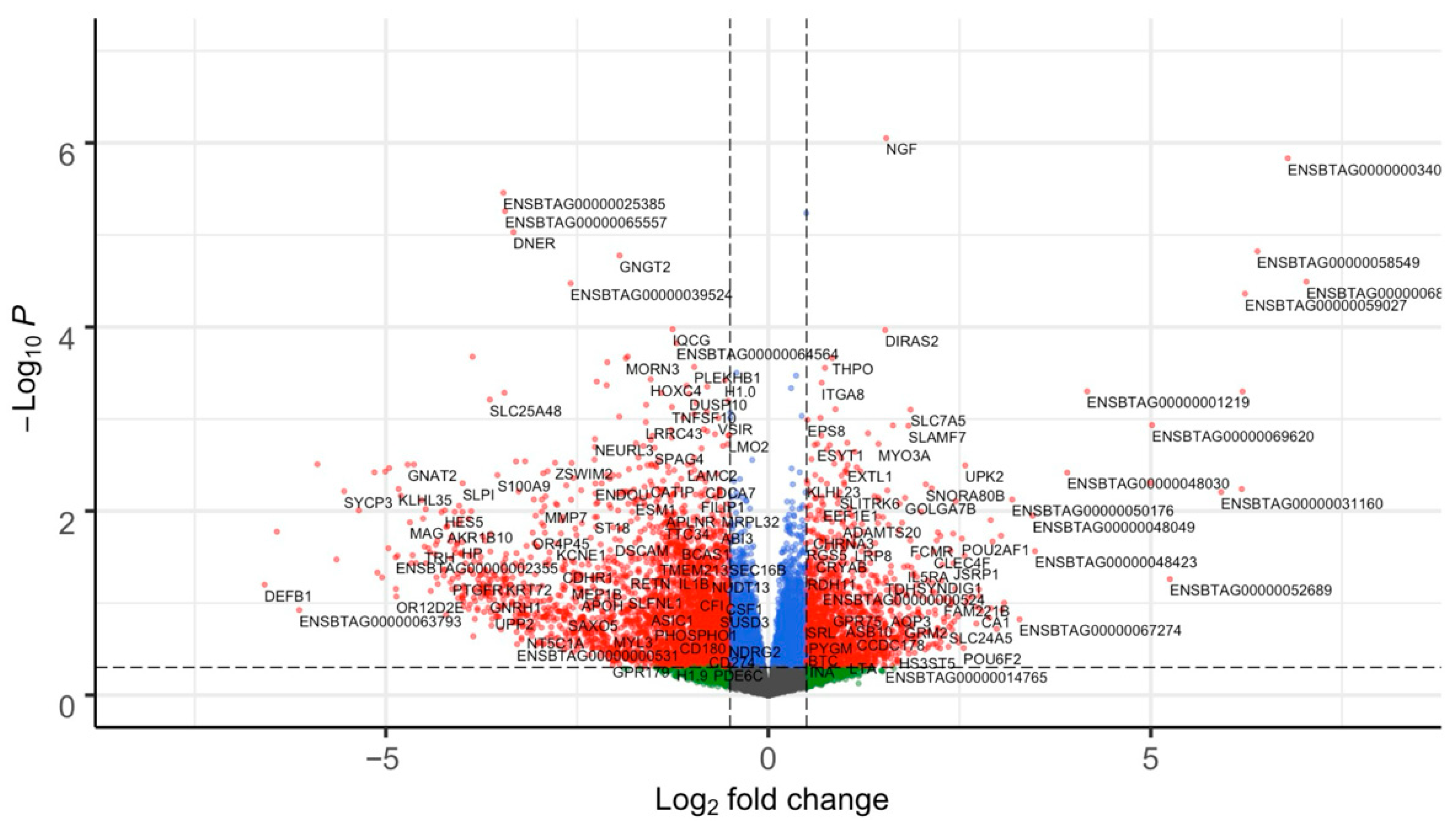
3.1. Altered Expression of Genes and Transcription Factors in Subfertile Heifers
3.2. Network Rewiring and Gain of Connectivity in the Subfertile Group
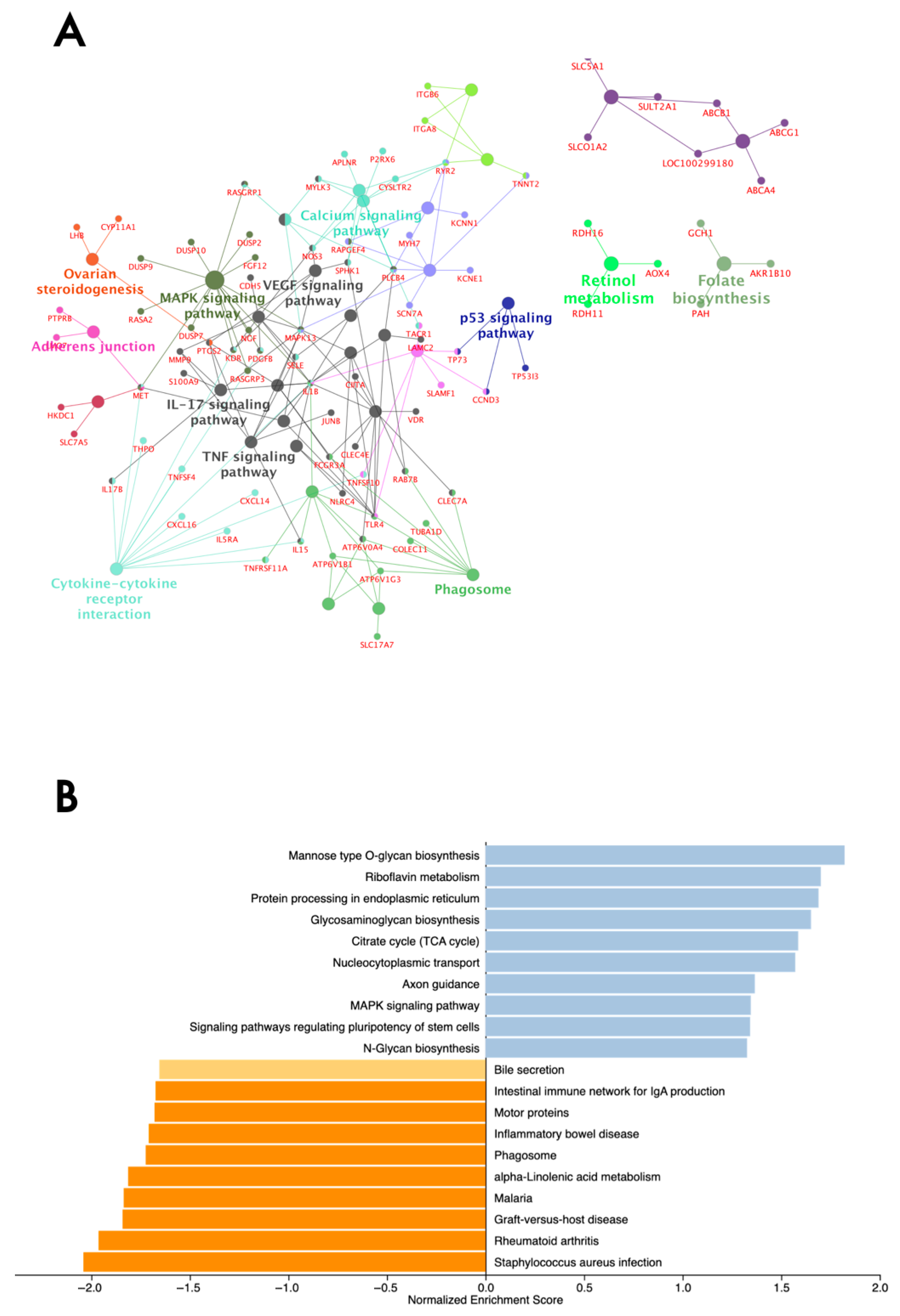
3.3. Genes Underlying MAPK, RAP1 Signaling, and Immune Response Pathways Are Over-Represented
4. Discussion
4.1. MAPK and Immune Signaling Pathways Are Over-Represented by Differentially Expressed Genes Between Subfertile and Fertile Classified Heifers
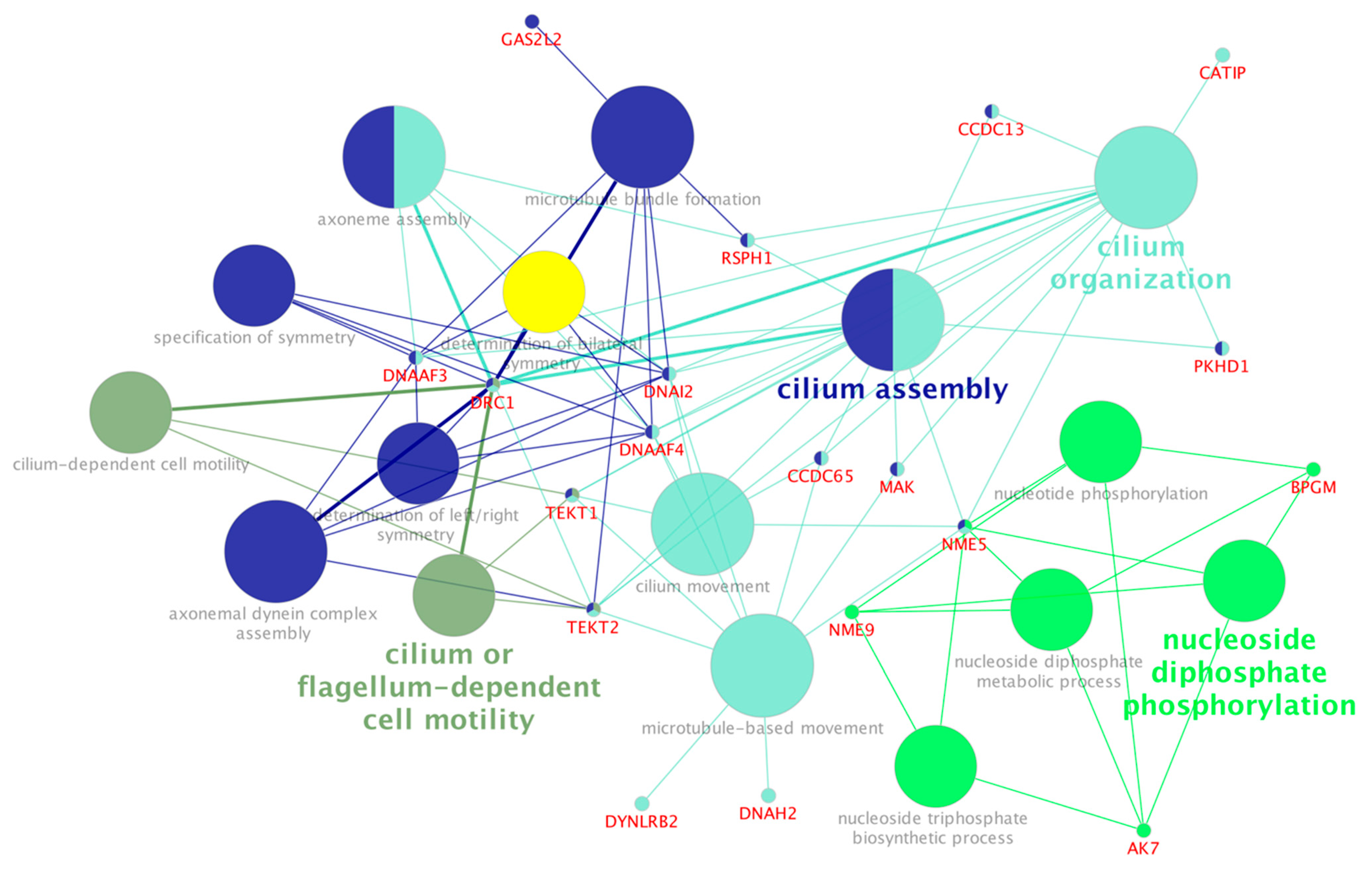
4.2. Hub Genes in the Subfertile Group Were Over-Represented for Microtubule-Based Movement, Cilium Movement and Assembly, and Axoneme Assembly
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial insemination |
| BP | Biological processes |
| CIDR | Controlled internal releasing device |
| DEGs | Differentially expressed genes |
| DK | Differential Connectivity |
| FC | Fold Change |
| GnRH | Gonadotropin-releasing hormone |
| GSEA | Gene Set Enrichment Analysis |
| IQ | Integrity quality |
| KEGG | Kyoto encyclopedia of genes and genomes |
| log2FC | log 2-fold change |
| IACUC | Institutional Animal Care and Use Committee |
| K | Connectivity |
| MAPK | Mitogen-activated protein kinase |
| NES | Normalized Enrichment Score |
| P4 | Progesterone |
| PCA | Principal component analysis |
| PCD | Primary ciliary dyskinesia |
| PCIT | Partial correlation and information theory |
| QC | Quality control |
| Rap1 | Ras-associated protein 1 |
| RIF | Regulatory impact factor |
| RNA-Seq | RNA sequencing |
| RTS | Reproductive tract score |
| SD | Standard deviation |
| TF | Transcription Factors |
References
- Pohler, K.G.; Reese, S.T.; Franco, G.A.; Oliveira Filho, R.V.; Paiva, R.; Fernandez, L.; Melo, G.d.; Vasconcelos, J.L.M.; Cooke, R.; Poole, R.K. New approaches to diagnose and target reproductive failure in cattle. Anim. Reprod. 2020, 17, e20200057. [Google Scholar] [CrossRef]
- Altmae, S.; Martinez-Conejero, J.A.; Salumets, A.; Simon, C.; Horcajadas, J.A.; Stavreus-Evers, A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol. Hum. Reprod. 2010, 16, 178–187. [Google Scholar] [CrossRef]
- Spencer, T.E.; Forde, N.; Lonergan, P. The role of progesterone and conceptus-derived factors in uterine biology during early pregnancy in ruminants. J. Dairy Sci. 2016, 99, 5941–5950. [Google Scholar] [CrossRef] [PubMed]
- Tinning, H.; Edge, J.C.; DeBem, T.H.C.; Deligianni, F.; Giovanardi, G.; Pensabene, V.; Meirelles, F.V.; Forde, N. Review: Endometrial function in pregnancy establishment in cattle. Animal 2023, 17, 100751. [Google Scholar] [CrossRef] [PubMed]
- Simintiras, C.A.; Forde, N. Understanding the uterine environment in early pregnancy in cattle: How have the omics en-hanced our knowledge? Anim. Reprod. 2017, 14, 538–546. [Google Scholar] [CrossRef]
- Forde, N.; Lonergan, P. Transcriptomic Analysis of the Bovine Endometrium: What is Required to Establish Uterine Receptivity to Implantation in Cattle? J. Reprod. Dev. 2012, 58, 189–195. [Google Scholar] [CrossRef]
- Adhikari, B.; Lee, C.N.; Khadka, V.S.; Deng, Y.; Fukumoto, G.; Thorne, M.; Caires, K.; Odani, J.; Mishra, B. RNA-Sequencing based analysis of bovine endometrium during the maternal recognition of pregnancy. BMC Genom. 2022, 23, 494. [Google Scholar] [CrossRef]
- Davoodi, S.; Cooke, R.F.; Fernandes, A.C.C.; Cappellozza, B.I.; Vasconcelos, J.L.M.; Cerri, R.L.A. Expression of estrus modifies the gene expression profile in reproductive tissues on Day 19 of gestation in beef cows. Theriogenology 2016, 85, 645–655. [Google Scholar] [CrossRef]
- Mansouri-Attia, N.; Aubert, J.; Reinaud, P.; Giraud-Delville, C.; Taghouti, G.; Galio, L.; Everts, R.E.; Degrelle, S.; Richard, C.; Hue, I.; et al. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol. Genom. 2009, 39, 14–27. [Google Scholar] [CrossRef]
- Dunne, L.; Diskin, M.; Sreenan, J. Embryo and foetal loss in beef heifers between day 14 of gestation and full term. Anim. Reprod. Sci. 2000, 58, 39–44. [Google Scholar] [CrossRef]
- Mazzoni, G.; Pedersen, H.S.; Rabaglino, M.B.; Hyttel, P.; Callesen, H.; Kadarmideen, H.N. Characterization of the endometrial transcriptome in early diestrus influencing pregnancy status in dairy cattle after transfer of in vitro-produced embryos. Physiol. Genom. 2020, 52, 269–279. [Google Scholar] [CrossRef]
- Forde, N.; Beltman, M.E.; Duffy, G.B.; Duffy, P.; Mehta, J.P.; O’Gaora, P.; Roche, J.F.; Lonergan, P.; Crowe, M.A. Changes in the Endometrial Transcriptome During the Bovine Estrous Cycle: Effect of Low Circulating Progesterone and Consequences for Conceptus Elongation. Biol. Reprod. 2011, 84, 266–278. [Google Scholar] [CrossRef]
- Killeen, A.P.; Morris, D.G.; Kenny, D.A.; Mullen, M.P.; Diskin, M.G.; Waters, S.M. Global gene expression in endometrium of high and low fertility heifers during the mid-luteal phase of the estrous cycle. BMC Genom. 2014, 15, 234. [Google Scholar] [CrossRef]
- Ponsuksili, S.; Tesfaye, D.; Schellander, K.; Hoelker, M.; Hadlich, F.; Schwerin, M.; Wimmers, K. Differential Expression of miRNAs and Their Target mRNAs in Endometria Prior to Maternal Recognition of Pregnancy Associates with Endometrial Receptivity for In Vivo- and In Vitro-Produced Bovine Embryos1. Biol. Reprod. 2014, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Minten, M.A.; Bilby, T.R.; Bruno, R.G.S.; Allen, C.C.; Madsen, C.A.; Wang, Z.; Sawyer, J.E.; Tibary, A.; Neibergs, H.L.; Geary, T.W.; et al. Effects of fertility on gene expression and function of the bovine endometrium. PLoS ONE 2013, 8, e69444. [Google Scholar] [CrossRef] [PubMed]
- Chankeaw, W.; Lignier, S.; Richard, C.; Ntallaris, T.; Raliou, M.; Guo, Y.; Plassard, D.; Bevilacqua, C.; Sandra, O.; Andersson, G.; et al. Analysis of the transcriptome of bovine endometrial cells isolated by laser micro-dissection (1): Specific signatures of stromal, glandular and luminal epithelial cells. BMC Genom. 2021, 22, 451. [Google Scholar] [CrossRef] [PubMed]
- Binelli, M.; Scolari, S.C.; Pugliesi, G.; Van Hoeck, V.; Gonella-Diaza, A.M.; Andrade, S.C.S.; Gasparin, G.R.; Coutinho, L.L. The Transcriptome Signature of the Receptive Bovine Uterus Determined at Early Gestation. PLoS ONE 2015, 10, e0122874. [Google Scholar] [CrossRef]
- Silva, F.A.C.C.; Martins, T.; Sponchiado, M.; Rocha, C.C.; Pohler, K.G.; Peñagaricano, F.; Binelli, M. Hormonal profile prior to luteolysis modulates the uterine luminal transcriptome in the subsequent cycle in beef cross-bred cows. Biol. Reprod. 2023, 108, 922–935. [Google Scholar] [CrossRef]
- Martins, T.; Sponchiado, M.; Silva, F.A.C.C.; Estrada-Cortés, E.; Hansen, P.J.; Peñagaricano, F.; Binelli, M. Progesterone-dependent and progesterone-independent modulation of luminal epithelial transcription to support pregnancy in cattle. Physiol. Genom. 2022, 54, 71–85. [Google Scholar] [CrossRef]
- Rocha, C.C.; Silva, F.A.C.C.; Cavani, L.; Cordeiro, A.L.L.; Maldonado, M.B.C.; Bennett, A.; Waheed, A.; Campbell, M.; Pohler, K.G.; Peñagaricano, F.; et al. Endometrial Gene Expression Predicts Pregnancy Outcome in Brahman Cows. Mol. Reprod. Dev. 2025, 92, e70047. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Banerjee, P.; Rodning, S.P.; Dyce, P.W. Machine learning-based co-expression network analysis unravels potential fertility-related genes in beef cows. Animals 2022, 12, 2715. [Google Scholar] [CrossRef]
- Holm, D.E.; Thompson, P.N.; Irons, P.C. The value of reproductive tract scoring as a predictor of fertility and production outcomes in beef heifers1. J. Anim. Sci. 2009, 87, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Read, C.C.; Kriese-Anderson, L.A.; Rodning, S.P.; Brandebourg, T.D.; Biase, F.H.; Marks, M.L.; Elmore, J.B.; Stanford, M.K.; Dyce, P.W. Plasma metabolomic profiles differ at the time of artificial insemination based on pregnancy outcome, in Bos taurus beef heifers. Sci. Rep. 2018, 8, 13196. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.; FASTQC. A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 21 September 2025).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Banerjee, P.; Diniz, W.J.S.; Hollingsworth, R.; Rodning, S.P.; Dyce, P.W. mRNA signatures in peripheral white blood cells predict reproductive potential in beef heifers at weaning. Genes 2023, 14, 498. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and visualize the results of multivariate data analyses. In R Package Version 1.5; C-RAN: Vienna, Austria, 2017; Volume 1, pp. 337–354. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-ready volcano plots with enhanced colouring and labeling. 2024. Available online: https://bioconductor.org/packages/release/bioc/vignettes/EnhancedVolcano/inst/doc/EnhancedVolcano.html (accessed on 21 September 2025).
- Reverter, A.; Hudson, N.J.; Nagaraj, S.H.; Pérez-Enciso, M.; Dalrymple, B.P. Regulatory impact factors: Unraveling the transcriptional regulation of complex traits from expression data. Bioinformatics 2010, 26, 896–904. [Google Scholar] [CrossRef]
- Shen, W.-K.; Chen, S.-Y.; Gan, Z.-Q.; Zhang, Y.-Z.; Yue, T.; Chen, M.-M.; Xue, Y.; Hu, H.; Guo, A.-Y. AnimalTFDB 4.0: A comprehensive animal transcription factor database updated with variation and expression annotations. Nucleic Acids Res. 2023, 51, D39–D45. [Google Scholar] [CrossRef]
- Reverter, A.; Chan, E.K.F. Combining partial correlation and an information theory approach to the reversed engineering of gene co-expression networks. Bioinformatics 2008, 24, 2491–2497. [Google Scholar] [CrossRef]
- Fuller, T.F.; Ghazalpour, A.; Aten, J.E.; Drake, T.A.; Lusis, A.J.; Horvath, S. Weighted gene coexpression network analysis strategies applied to mouse weight. Mamm. Genome 2007, 18, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Diniz, W.J.S.; Crouse, M.S.; Cushman, R.A.; McLean, K.J.; Caton, J.S.; Dahlen, C.R.; Reynolds, L.P.; Ward, A.K. Cerebrum, liver, and muscle regulatory networks uncover maternal nutrition effects in developmental programming of beef cattle during early pregnancy. Sci. Rep. 2021, 11, 2771. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Ziemann, M. Pathway Analysis: DAVID Versus GSEA. Available online: https://genomespot.blogspot.com/2016/02/pathway-analysis-david-versus-gsea.html (accessed on 21 September 2025).
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Liaqat Ali Khan, N.; Nafee, T.; Shao, T.; Hart, A.R.; Elliott, S.; Ola, B.; Heath, P.R.; Fazeli, A. Dysregulation in Multiple Transcriptomic Endometrial Pathways Is Associated with Recurrent Implantation Failure and Recurrent Early Pregnancy Loss. Int. J. Mol. Sci. 2022, 23, 16051. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, S.; Song, M. Fundamental gene network rewiring at the second order within and across mammalian systems. Bioinformatics 2021, 37, 3293–3301. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2016, 6, a023002. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Zhang, N.; Xian, Y.; Tang, Y.; Ye, J.; Reza, F.; He, G.; Wen, X.; Jiang, X. The multiple roles of interferon regulatory factor family in health and disease. Signal Transduct. Target. Ther. 2024, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lin, S.; Hsiao, K.; Tsai, S. Hypoxia-inhibited dual-specificity phosphatase-2 expression in endometriotic cells regulates cyclooxygenase-2 expression. J. Pathol. 2011, 225, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Rohan, P.J.; Davis, P.; Moskaluk, C.A.; Kearns, M.; Krutzsch, H.; Siebenlist, U.; Kelly, K. PAC-1: A Mitogen-Induced Nuclear Protein Tyrosine Phosphatase. Science 1993, 259, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jiao, Y.; Postlethwaite, A.; Stuart, J.M.; Wang, Y.; Sun, D.; Gu, W. Dual-specificity phosphatases 2: Surprising positive effect at the molecular level and a potential biomarker of diseases. Genes Immun. 2013, 14, 1–6. [Google Scholar] [CrossRef]
- Xiong, J.; Ran, L.; Zhu, Y.; Wang, Y.; Wang, S.; Wang, Y.; Lan, Q.; Han, W.; Liu, Y.; Huang, Y.; et al. DUSP2-mediated inhibition of tubular epithelial cell pyroptosis confers nephroprotection in acute kidney injury. Theranostics 2022, 12, 5069–5085. [Google Scholar] [CrossRef]
- Banerjee, P.; Diniz, W.J.S.; Rodning, S.P.; Dyce, P.W. miRNA expression profiles of peripheral white blood cells from beef heifers with varying reproductive potential. Front. Genet. 2023, 14, 1174145. [Google Scholar] [CrossRef]
- Chen, H.-F.; Chuang, H.-C.; Tan, T.-H. Regulation of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein Stability. Int. J. Mol. Sci. 2019, 20, 2668. [Google Scholar] [CrossRef]
- Radi, Z.A.; Marusak, R.A.; Morris, D.L. Species Comparison of the Role of p38 MAP Kinase in the Female Reproductive System. J. Toxicol. Pathol. 2009, 22, 109–124. [Google Scholar] [CrossRef]
- Dai, M.; Xu, Y.; Gong, G.; Zhang, Y. Roles of immune microenvironment in the female reproductive maintenance and regulation: Novel insights into the crosstalk of immune cells. Front. Immunol. 2023, 14, 1109122. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, M.; Su, Q.; Li, H.; Wang, F. Impaired fertility and perinatal outcomes in adenomyosis: Insights from a novel murine model and uterine gene profile alterations during implantations. Am. J. Obstet. Gynecol. 2025, 233, 180.E1–180.E18. [Google Scholar] [CrossRef]
- Dickson, M.J.; Bishop, J.V.; Hansen, T.R.; Sheldon, I.M.; Bromfield, J.J. The endometrial transcriptomic response to pregnancy is altered in cows after uterine infection. PLoS ONE 2022, 17, e0265062. [Google Scholar] [CrossRef]
- Bliss, S.P.; Miller, A.; Navratil, A.M.; Xie, J.; McDonough, S.P.; Fisher, P.J.; Landreth, G.E.; Roberson, M.S. ERK Signaling in the Pituitary Is Required for Female But Not Male Fertility. Mol. Endocrinol. 2009, 23, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, H.; Yang, X.; Song, W. BarH-like homeobox 1 induces the progression of cell malignant phenotype in endometrial carcinoma through the regulation of ERK/MEK signaling pathway. Reprod. Biol. 2021, 21, 100502. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in Ovarian Granulosa Cells Are Essential for Female Fertility. Science 2009, 324, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, A.; Bisinotto, R.S.; Peñagaricano, F. Genes and pathways associated with pregnancy loss in dairy cattle. Sci. Rep. 2021, 11, 13329. [Google Scholar] [CrossRef]
- Kusama, K.; Yoshie, M.; Tamura, K.; Daikoku, T.; Takarada, T.; Tachikawa, E. Possible roles of the cAMP-mediators EPAC and RAP1 in decidualization of rat uterus. Reproduction 2014, 147, 897–906. [Google Scholar] [CrossRef]
- Chrzanowska-Wodnicka, M.; White, G.C.; Quilliam, L.A.; Whitehead, K.J. Small GTPase Rap1 Is Essential for Mouse Development and Formation of Functional Vasculature. PLoS ONE 2015, 10, e0145689. [Google Scholar] [CrossRef]
- Ohlsson, R.; Falck, P.; Hellström, M.; Lindahl, P.; Boström, H.; Franklin, G.; Ährlund-Richter, L.; Pollard, J.; Soriano, P.; Betsholtz, C. PDGFB Regulates the Development of the Labyrinthine Layer of the Mouse Fetal Placenta. Dev. Biol. 1999, 212, 124–136. [Google Scholar] [CrossRef]
- Basavaraja, R.; Drum, J.N.; Sapuleni, J.; Bibi, L.; Friedlander, G.; Kumar, S.; Sartori, R.; Meidan, R. Downregulated luteolytic pathways in the transcriptome of early pregnancy bovine corpus luteum are mimicked by interferon-tau in vitro. BMC Genom. 2021, 22, 452. [Google Scholar] [CrossRef]
- Biswas Shivhare, S.; Lu, Q.; Sun, D.; Hou, H.; Bulmer, J.N.; Innes, B.A.; Hapangama, D.K.; Lash, G.E. Platelet-derived growth factor BB is reduced in endometrial endothelial cells of women with abnormal uterine bleeding–endometrial disorder. Reprod. Biomed. Online 2022, 45, 531–543. [Google Scholar] [CrossRef]
- Nagy, Z.; Kovács, I.; Török, M.; Tóth, D.; Vereb, G.; Buzás, K.; Juhász, I.; Blumberg, P.M.; Bíró, T.; Czifra, G. Function of RasGRP3 in the formation and progression of human breast cancer. Mol. Cancer 2014, 13, 96. [Google Scholar] [CrossRef]
- Cremades-Jimeno, L.; López-Ramos, M.; Fernández-Santamaría, R.; De Pedro, M.Á.; Mahillo, I.; Rosales-Ariza, C.; Olaguibel, J.M.; Pozo, V.d.; Caballero, M.L.; Luna-Porta, J.A.; et al. Molecular Study from the Signaling Pathways of Four Potential asthma triggers: AKT1, MAPK13, STAT1, and TLR4. Int. J. Mol. Sci. 2025, 26, 6240. [Google Scholar] [CrossRef]
- Kahnamouyi, S.; Nouri, M.; Farzadi, L.; Darabi, M.; Hosseini, V.; Mehdizadeh, A. The role of mitogen-activated protein kinase–extracellular receptor kinase pathway in female fertility outcomes: A focus on pituitary gonadotropins regulation. Ther. Adv. Endocrinol. Metab. 2018, 9, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Hirohashi, Y.; Kuroda, T.; Takaya, A.; Kubo, T.; Kanaseki, T.; Tsukahara, T.; Hasegawa, T.; Saito, T.; Sato, N.; et al. MAPK13 is preferentially expressed in gynecological cancer stem cells and has a role in the tumor-initiation. Biochem. Biophys. Res. Commun. 2016, 472, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Rudolf Vegas, A.; Podico, G.; Canisso, I.F.; Bollwein, H.; Almiñana, C.; Bauersachs, S. Spatiotemporal endometrial transcriptome analysis revealed the luminal epithelium as key player during initial maternal recognition of pregnancy in the mare. Sci. Rep. 2021, 11, 22293. [Google Scholar] [CrossRef] [PubMed]
- Fair, T. The contribution of the maternal immune system to the establishment of pregnancy in cattle. Front. Immunol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Hansen, P. The immunology of early pregnancy in farm animals. Reprod. Domest. Anim. 2011, 46, 18–30. [Google Scholar] [CrossRef]
- De los Santos, J.A.; Andrade, J.P.N.; Cangiano, L.R.; Iriarte, A.; Peñagaricano, F.; Parrish, J.J. Transcriptomic analysis reveals gene expression changes in peripheral white blood cells of cows after embryo transfer: Implications for pregnancy tolerance. Reprod. Domest. Anim. 2023, 58, 946–954. [Google Scholar] [CrossRef]
- Correia-Álvarez, E.; Gómez, E.; Martín, D.; Carrocera, S.; Pérez, S.; Otero, J.; Peynot, N.; Giraud-Delville, C.; Caamaño, J.N.; Sandra, O.; et al. Expression and localization of interleukin 1 beta and interleukin 1 receptor (type I) in the bovine endometrium and embryo. J. Reprod. Immunol. 2015, 110, 1–13. [Google Scholar] [CrossRef]
- Peralta, M.B.; Cainelli, S.; Stassi, A.F.; Angeli, E.; Rey, F.; Ortega, H.H.; Salvetti, N.R.; Velázquez, M.M.L. Endometrial expression of members of the IL-1 family: Their involvement in delayed conception of dairy cows. Theriogenology 2023, 195, 168–175. [Google Scholar] [CrossRef]
- Velázquez, M.M.L.; Peralta, M.B.; Angeli, E.; Stassi, A.F.; Gareis, N.C.; Durante, L.; Cainelli, S.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Immune status during postpartum, peri-implantation and early pregnancy in cattle: An updated view. Anim. Reprod. Sci. 2019, 206, 1–10. [Google Scholar] [CrossRef]
- Yoshinaga, K.; PrabhuDas, M.; Davies, C.; White, K.; Caron, K.; Golos, T.; Fazleabas, A.; Paria, B.; Mor, G.; Paul, S.; et al. Interdisciplinary Collaborative Team for Blastocyst Implantation Research: Inception and perspectives. Am. J. Reprod. Immunol. 2014, 71, 1–11. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of Gelatinases MMP-2 and MMP-9 in Healthy and Complicated Pregnancy and Their Future Potential as Preeclampsia Biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef]
- Bisgrove, B.W.; Yost, H.J. The roles of cilia in developmental disorders and disease. Development 2006, 133, 4131–4143. [Google Scholar] [CrossRef]
- Lyons, R.A.; Saridogan, E.; Djahanbakhch, O. The reproductive significance of human Fallopian tube cilia. Hum. Reprod. Update 2006, 12, 363–372. [Google Scholar] [CrossRef]
- Narita, K.; Takeda, S. Cilia in the choroid plexus: Their roles in hydrocephalus and beyond. Front. Cell. Neurosci. 2015, 9, 39. [Google Scholar] [CrossRef]
- Hunter, M.I.; Thies, K.M.; Winuthayanon, W. Hormonal regulation of cilia in the female reproductive tract. Curr. Opin. Endocr. Metab. Res. 2024, 34, 100503. [Google Scholar] [CrossRef]
- Long, X.; Chen, L.; Xiao, X.; Min, X.; Wu, Y.; Yang, Z.; Wen, X. Structure, function, and research progress of primary cilia in reproductive physiology and reproductive diseases. Front. Cell Dev. Biol. 2024, 12, 1418928. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, J.; Yan, F.; Chen, Y.; Wu, Y.; Luo, J.; Duan, L.; Zou, J.; Guo, J.; Pang, J.; et al. Ciliary IFT-B Transportation Plays an Important Role in Human Endometrial Receptivity Establishment and is Disrupted in Recurrent Implantation Failure Patients. Cell Prolif. 2025, 58, e13819. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Wei, Y.; Lin, Z.; Wu, H.; Yang, F.; Cen, X.; Lu, W.; Qin, H.; Wang, R.; Wang, J. A new perspective on endometriosis: Integrating eQTL mendelian randomization with transcriptomics and single-cell data analyses. Funct. Integr. Genom. 2025, 25, 75. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Chopra, J.; Dossett, C.; Shepherd, E.; Bercusson, A.; Carroll, M.; Walker, W.; Lucas, J.S.; Cheong, Y. The impact of primary ciliary dyskinesia on female and male fertility: A narrative review. Hum. Reprod. Update 2023, 29, 347–367. [Google Scholar] [CrossRef]
- Jiang, G.; Zou, L.; Long, L.; He, Y.; Lv, X.; Han, Y.; Yao, T.; Zhang, Y.; Jiang, M.; Peng, Z.; et al. Homozygous mutation in DNAAF4 causes primary ciliary dyskinesia in a Chinese family. Front. Genet. 2022, 13, 1087818. [Google Scholar] [CrossRef]
- Patir, A.; Fraser, A.M.; Barnett, M.W.; McTeir, L.; Rainger, J.; Davey, M.G.; Freeman, T.C. The transcriptional signature associated with human motile cilia. Sci. Rep. 2020, 10, 10814. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Carvalho, V.; Dias, C.; Barbosa, T.; Oliveira, J.; Alves, Â.; Oliveira, E.; Sá, R.; Sousa, M. Characterization of a DRC1 null variant associated with primary ciliary dyskinesia and female infertility. J. Assist. Reprod. Genet. 2023, 40, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Brewer, A.; Cormican, P.; Lim, J.J.; Chapwanya, A.; O’Farrelly, C.; Meade, K.G. Qualitative and quantitative differences in endometrial inflammatory gene expression precede the development of bovine uterine disease. Sci. Rep. 2020, 10, 18275. [Google Scholar] [CrossRef] [PubMed]
- Kumro, F.G.; O’Neil, E.V.; Ciernia, L.A.; Moraes, J.G.N.; Spencer, T.E.; Lucy, M.C. Scanning electron microscopy of the surface epithelium of the bovine endometrium. J. Dairy Sci. 2020, 103, 12083–12090. [Google Scholar] [CrossRef]
- Pereira, G.; Guo, Y.; Silva, E.; Bevilacqua, C.; Charpigny, G.; Lopes-da-Costa, L.; Humblot, P. Progesterone differentially affects the transcriptomic profiles of cow endometrial cell types. BMC Genom. 2022, 23, 82. [Google Scholar] [CrossRef]
- Walker, L.A.; Sovic, M.G.; Chiang, C.-L.; Hu, E.; Denninger, J.K.; Chen, X.; Kirby, E.D.; Byrd, J.C.; Muthusamy, N.; Bundschuh, R.; et al. CLEAR: Coverage-based limiting-cell experiment analysis for RNA-seq. J. Transl. Med. 2020, 18, 63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kertz, N.C.; Banerjee, P.; Dyce, P.W.; Rodning, S.P.; Diniz, W.J.S. Endometrial Signatures of Subfertility in Beef Heifers Reveal Dysregulation of MAPK Signaling and Ciliary Function. Genes 2025, 16, 1323. https://doi.org/10.3390/genes16111323
Kertz NC, Banerjee P, Dyce PW, Rodning SP, Diniz WJS. Endometrial Signatures of Subfertility in Beef Heifers Reveal Dysregulation of MAPK Signaling and Ciliary Function. Genes. 2025; 16(11):1323. https://doi.org/10.3390/genes16111323
Chicago/Turabian StyleKertz, Nicholas C., Priyanka Banerjee, Paul W. Dyce, Soren P. Rodning, and Wellison J. S. Diniz. 2025. "Endometrial Signatures of Subfertility in Beef Heifers Reveal Dysregulation of MAPK Signaling and Ciliary Function" Genes 16, no. 11: 1323. https://doi.org/10.3390/genes16111323
APA StyleKertz, N. C., Banerjee, P., Dyce, P. W., Rodning, S. P., & Diniz, W. J. S. (2025). Endometrial Signatures of Subfertility in Beef Heifers Reveal Dysregulation of MAPK Signaling and Ciliary Function. Genes, 16(11), 1323. https://doi.org/10.3390/genes16111323





