A Nationwide Analysis of the Phenotype/Genotype Landscape of Hemophagocytic Lymphohistiocytosis: UNC13D Associates with Poor Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.2.1. Specialized Hematologic Tests
- NK Cytolytic Function Assay: NK cell-mediated lysis of K562-labeled cells was assessed using a CR51-release assay [25].
- Soluble IL2R: The quantitative determination of soluble interleukin-2 receptor (sIL2R) in serum was performed using a sandwich enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions. Serum samples were analyzed alongside calibrated standards to establish a standard curve, and sIL2R concentrations were calculated by interpolation. The assay demonstrated an analytical level of sIL2R in U/mL unit.
- Flow cytometry:
- o
- Degranulation assays quantifying CD107a surface expression were performed in a small subset of patients to direct molecular analyses for FHL [26].
- o
- Samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) in a small subset of patients to correlate with molecular findings [27].
2.2.2. Genetic Diagnosis
Sequencing Results and Bioinformatics Pipeline
Copy Number Variants Detection
2.3. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Clinical Presentation
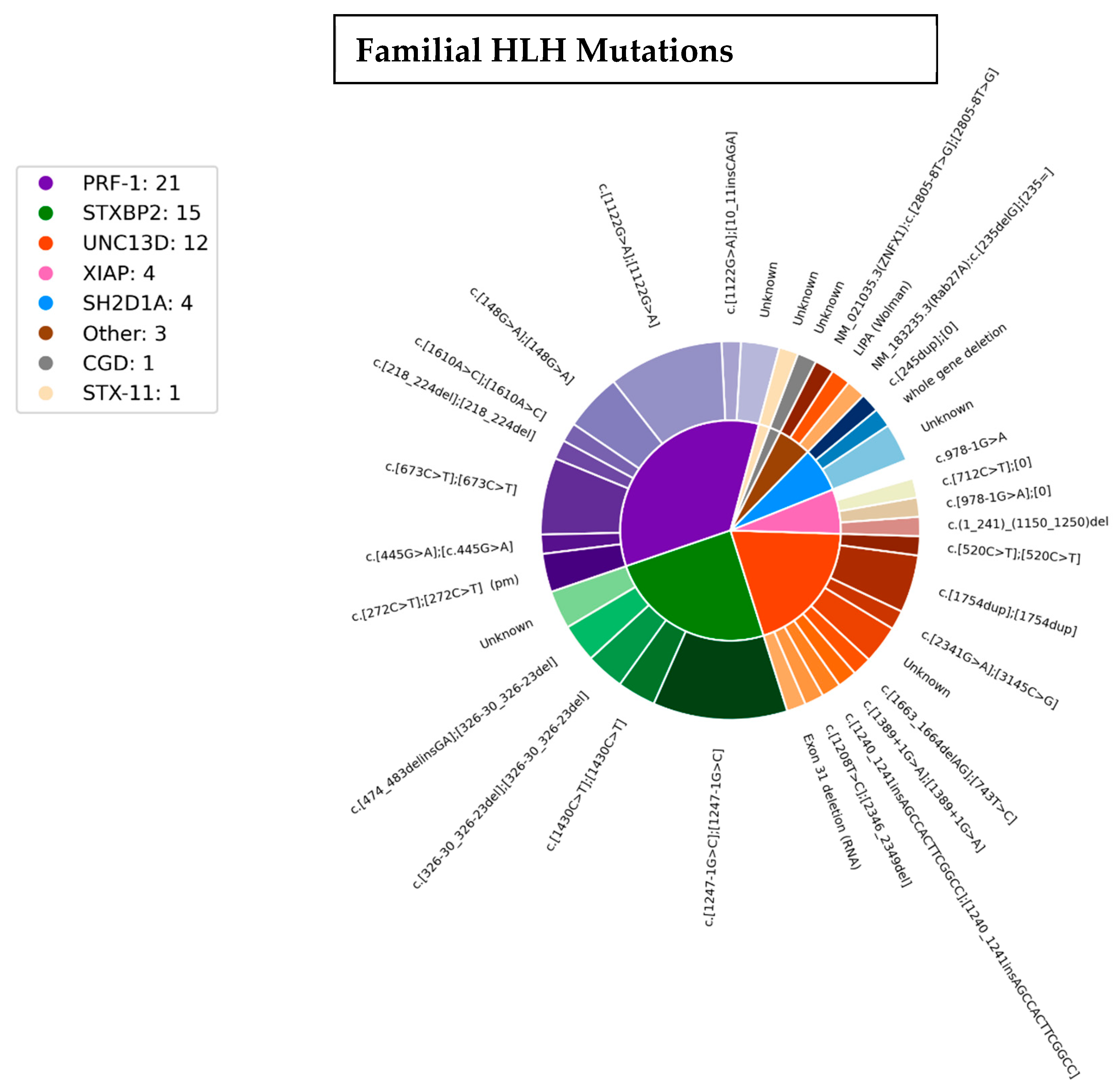
3.3. Genetics
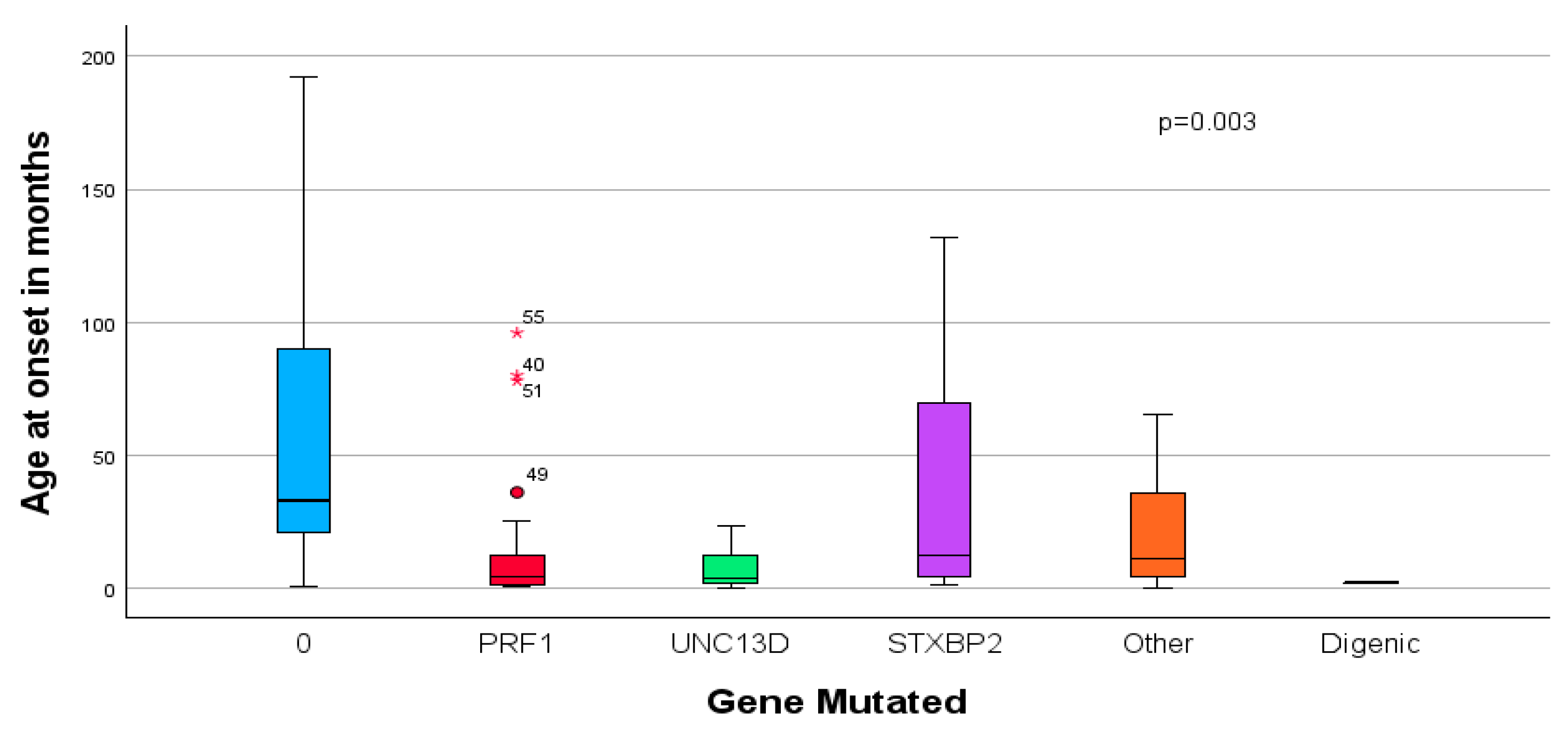
3.4. Outcome Prediction
3.5. Logistic Regression
4. Discussion
Outcome and Genetic Diagnoses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janka, G.E.; Lehmberg, K. Hemophagocytic syndromes–An update. Blood Rev. 2014, 28, 135–142. [Google Scholar] [CrossRef]
- Al-Samakri, H.; Berlinar, N. Hemophagocytic Lymphohistiocytosis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 27–49. [Google Scholar] [CrossRef]
- Jordan, M.B.; Allen, C.E.; Greenberg, J.; Henry, M.; Hermiston, M.L.; Kumar, M.; Hines, M.; Eckstein, O.; Ladisch, S.; Nichols, K.E.; et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendation from the North American Consortium for Histiocytes (NACHO). Pediatr. Blood Cancer 2019, 66, e27929. [Google Scholar] [CrossRef]
- Tesi, B.; Bryceson, Y.T. HLH: Genomic illuminates pathophysiological diversity. Blood 2018, 132, 5–7. [Google Scholar] [CrossRef]
- Barbosa, M.D.; Nguyen, Q.A.; Tchernev, V.T.; Ashley, J.A.; Detter, J.C.; Blaydes, S.M.; Brandt, S.J.; Chotai, D.; Hodgman, C.; Solari, R.C.; et al. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature 1996, 382, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.; Zieger, B.; Schwarz, K.; Yoshimi, A.; Speckmann, C.; Knoepfle, E.M.; Kontny, U.; Müller, C.; Nurden, A.; Rohr, J.; et al. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood 2006, 108, 81–87. [Google Scholar] [CrossRef]
- Ménasché, G.; Pastural, E.; Feldmann, J.; Certain, S.; Ersoy, F.; Dupuis, S.; Wulffraat, N.; Bianchi, D.; Fischer, A.; Le Deist, F.; et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 2000, 25, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.E.; Harkin, D.P.; Levitz, S.; Krainer, M.; Kolquist, K.A.; Genovese, C.; Bernard, A.; Ferguson, M.; Zuo, L.; Snyder, E.; et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA 1998, 95, 13765–13770. [Google Scholar] [CrossRef]
- Rigaud, S.; Fondanèche, M.C.; Lambert, N.; Pasquier, B.; Mateo, V.; Soulas, P.; Galicier, L.; Le Deist, F.; Rieux-Laucat, F.; Revy, P.; et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 2006, 444, 110–114. [Google Scholar] [CrossRef]
- Vavassori, S.; Chou, J.; Faletti, L.A.; Haunerdinger, V.; Opitz, L.; Joset, P.; Fraser, C.J.; Prader, S.; Gao, X.; Schuch, L.A.; et al. Multisystem inflammation and susceptibility to viral infections in human ZNFX1 deficiency. J. Allergy Clin. Immunol. 2021, 148, 381–393. [Google Scholar] [CrossRef] [PubMed]
- van Montfrans, J.M.; Hoepelman, A.I.M.; Otto, S.; van Gijn, M.; van de Corput, L.; de Weger, R.A.; Monaco-Shawver, L.; Banerjee, P.P.; Sanders, E.A.M.; van der Zijde, C.M.J.; et al. CD27 deficiency is associated with combined immunodeficiency and persistent symptomatic EBV viremia. J. Allergy Clin. Immunol. 2012, 129, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Linka, R.M.; Risse, S.L.; Bienemann, K.; Werner, M.; Linka, Y.; Krux, F.; Synaeve, C.; Deenen, R.; Ginzel, S.; Dvorsky, R.; et al. Loss-of-function mutations within the IL-2 inducible kinase ITK in patients with EBV-associated lymphoproliferative diseases. Leukemia 2012, 26, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.; Ramme, K.G.; Rudd, E.; Zheng, C.; Wali, Y.; al-Lamki, Z.; Gürgey, A.; Yalman, N.; Nordenskjöld, M.; Henter, J.I. Characterization of PRF1, STX11 and UNC13D genotype-phenotype correlations in familial hemophagocytic lymphohistiocytosis. Br. J. Haematol. 2008, 143, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zur Stadt, U.; Beutel, K.; Kolberg, S.; Schneppenheim, R.; Kabisch, H.; Janka, G.; Hennies, H.C. Mutation spectrum in children with primary hemophagocytic lymphohistiocyto sis: Molecular and functional analyses of PRF1, UNC13D, STX11, and RABA27A. Hum. Mutat. 2006, 27, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Gadoury-Levesque, V.; Dong, L.; Su, R.; Chen, J.; Zhang, K.; Risma, K.A.; Marsh, R.A.; Sun, M. Frequency and spectrum of disease-causing variants in 1892 patients with suspected genetic HLH disorders. Blood Adv. 2020, 4, 2578–2594. [Google Scholar] [CrossRef]
- Chinn, I.K.; Eckstein, O.S.; Peckham-Gregory, E.C.; Goldberg, B.R.; Forbes, L.R.; Nicholas, S.K.; Mace, E.M.; Vogel, T.P.; Abhyankar, H.A.; Diaz, M.I.; et al. Genetic and mechanistic diversity in pediatric hemophagocytic lymphohistiocytosis. Blood 2018, 132, 89–100. [Google Scholar] [CrossRef]
- Cetica, V.; Sieni, E.; Pende, D.; Danesino, C.; De Fusco, C.; Locatelli, F.; Micalizzi, C.; Putti, M.C.; Biondi, A.; Fagioli, F.; et al. Genetic predisposition to hemophagocytic lymphohistiocytosis: Report on 500 patients from the Italian registry. J. Allergy Clin. Immunol. 2016, 137, 188–196.e4. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Miyashita, T.; Herbst, H.; Niedobitek, G.; Asada, M.; Tsuchida, M.; Hanada, R.; Kinoshita, A.; Sakurai, M.; Kobayashi, N.; et al. Epstein-Barr virus-infected T lymphocytes in Epstein-Barr virus-associated hemophagocytic syndrome. J. Clin. Investig. 1993, 92, 1444–1450. [Google Scholar] [CrossRef]
- Janka, G.E.; Lehmberg, K. Hemophagocytic lymphohistiocytosis: Pathogenesis and treatment. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 605–611. [Google Scholar] [CrossRef]
- Henter, J.I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Henter, J.I.; Samuelsson-Horne, A.; Aricò, M.; Egeler, R.M.; Elinder, G.; Filipovich, A.H.; Gadner, H.; Imashuku, S.; Komp, D.; Ladisch, S.; et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 2002, 100, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, E.; Horne, A.; Aricó, M.; Astigarraga, I.; Egeler, R.M.; Filipovich, A.H.; Ishii, E.; Janka, G.; Ladisch, S.; Lehmberg, K.; et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: Long-term results of the cooperative HLH-2004 study. Blood 2017, 130, 2728–2738. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Jordan, M.B.; Allen, C.; Cesaro, S.; Rizzari, C.; Rao, A.; Degar, B.; Garrington, T.P.; Sevilla, J.; Putti, M.C.; et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020, 382, 1811–1822. [Google Scholar] [CrossRef]
- Keenan, C.; Nichols, K.E.; Albeituni, S. Use of the JAK Inhibitor Ruxolitinib in the Treatment of Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2021, 12, 614704. [Google Scholar] [CrossRef]
- Cox, J.H.; deSouza, M. Evaluation of Natural Killer Cell Activity. Mol. Biotechnol. 2000, 15, 147–154. [Google Scholar] [CrossRef]
- Marcenaro, S.; Gallo, F.; Martini, S.; Santoro, A.; Griffiths, G.M.; Aricò, M.; Moretta, L.; Pende, D. Analysis of natural killer-cell function in familial Hemophagocytic lymphohistiocytosis (FHL): Defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood 2006, 108, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Kogawa, K.; Lee, S.M.; Villanueva, J.; Marmer, D.; Sumegi, J.; Filipovich, A.H. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood 2002, 99, 61–66. [Google Scholar] [CrossRef]
- Shefer Averbuch, N.; Steinberg-Shemer, O.; Dgany, O.; Krasnov, T.; Noy-Lotan, S.; Yacobovich, J.; Kuperman, A.A.; Kattamis, A.; Ben Barak, A.; Roth-Jelinek, B.; et al. Targeted next generation sequencing for the diagnosis of patients with rare congenital anemias. Eur. J. Haematol. 2018, 101, 297–304. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Committee ALQA. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Rehm, H.L.; Bale, S.J.; Bayrak-Toydemir, P.; Berg, J.S.; Brown, K.K.; Deignan, J.L.; Friez, M.J.; Funke, B.H.; Hegde, M.R.; Lyon, E. Working Group of the American College of Medical G, Genomics Laboratory Quality Assurance C. ACMG clinical laboratory standards for next-generation sequencing. Genet. Med. 2013, 15, 733–747. [Google Scholar] [CrossRef]
- Scionti, F.; Di Martino, M.T.; Pensabene, L.; Bruni, V.; Concolino, D. The Cytoscan HD Array in the Diagnosis of Neurodevelopmental Disorders. High-throughput 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Population of Israel on the Eve of 2024. Available online: https://www.cbs.gov.il/he/mediarelease/DocLib/2023/424/11_23_424b.pdf (accessed on 22 May 2025).
- Al Ahmari, A.; Alsmadi, O.; Sheereen, A.; Elamin, T.; Jabr, A.; El-Baik, L.; Alhissi, S.; Saud, B.A.; Al-Awwami, M.; Fawaz, I.A.; et al. Genetic and clinical characteristics of pediatric patients with familial hemophagocytic lymphohistiocytosis. Blood Res. 2021, 56, 86–101. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Zhang, Y.; Teng, W.; Wang, M.; Nie, D.; Zhou, X.; Wang, D.; Zhao, H.; Zhu, P.; et al. Genetic variant spectrum in 265 Chinese patients with hemophagocytic lymphohistiocytosis: Molecular analyses of PRF1, UNC13D, STX11, STXBP2, SH2D1A, and XIAP. Clin. Genet. 2018, 94, 200–212. [Google Scholar] [CrossRef]
- Verkamp, B.; Zoref-Lorenz, A.; Francisco, B.; Kieser, P.; Mack, J.; Blackledge, T.; Brik Simon, D.; Yacobovich, J.; Jordan, M.B. Early response markers predict survival after etoposide-based therapy of hemophagocytic lymphohistiocytosis. Blood Adv. 2023, 7, 7258–7269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bracaglia, C.; Prencipe, G.; Bemrich-Stolz, C.; Beukelman, T.; Dimmitt, R.A.; Chatham, W.W.; Zhang, K.; Li, H.; Walter, M.A.; et al. A Heterozygous RAB27A Mutation Associated with Delayed Cytolytic Granule Polarization and Hemophagocytic Lymphohistiocytosis. J. Immunol. 2016, 196, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
| Total | Percent (%) | |
|---|---|---|
| Age at onset ≤ 1 yr | 54/95 | 55 |
| Age at diagnosis (months) | ||
| ≤12 | 43/96 | 45 |
| >12 | 53/96 | 55 |
| Gender (M) | 54 | 55 |
| Ethnicity | ||
| Muslim | 50 | 51 |
| Jewish | 42 | 43 |
| Druze | 6 | 6 |
| Consanguinity | ||
| Muslim | 36 | 39.5 |
| Jewish | 0 | |
| Druze | 4 | 4.5 |
| Total | 40/91 | 44 |
| Presenting clinical manifestations | ||
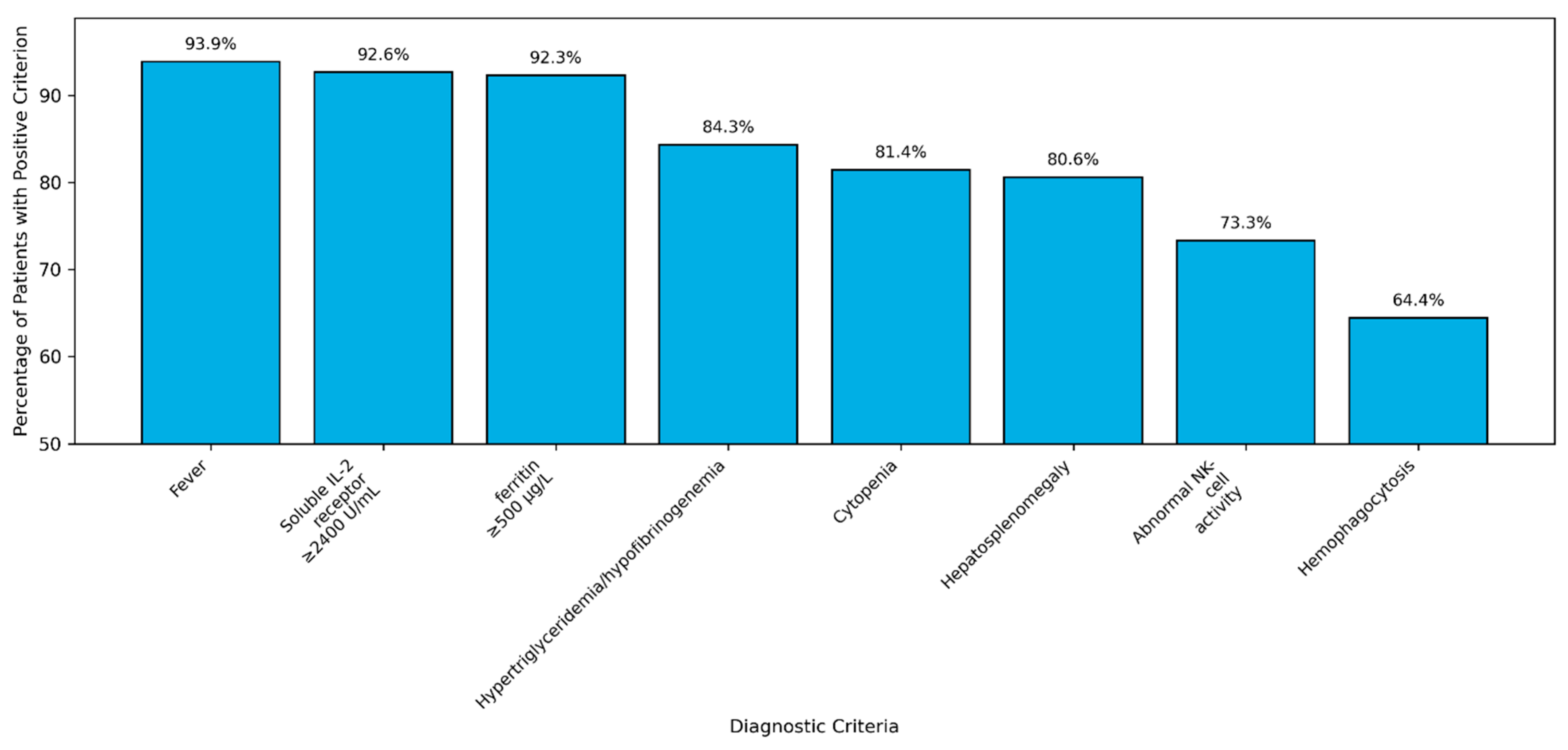
| Fever | 92 | 94 |
| Hepatosplenomegaly | 79 | 81 |
| Adenopathy | 24 | 24 |
| Rash | 25 | 25 |
| CNS involvement | 30 | 31 |
| Edema/anasarca | 30 | 31 |
| Presenting laboratory manifestations | ||
| Cytopenias | 79/95 | 81 |
| Triglycerides ≥ 265 mg/dL | 65/83 | 78 |
| Fibrinogen ≤ 150 mg/dL | 68/83 | 82 |
| Ferritin ≥ 500 μg/L | 85/92 | 92 |
| NK activity < 20% | 38/52 | 73 |
| Soluble IL2 receptor ≥ 2400 U/mL | 64/69 | 92 |
| Treatment | ||
| HLH-94 | 12 | 12 |
| HLH-2004 | 62 | 63 |
| Steroids/IVIG | 21 | 21 |
| Other/Second-line therapy | 22 | 22 |
| Bone marrow transplantation | 58 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brik Simon, D.; Greental Ness, Y.; Dgany, O.; Noy-Lotan, S.; Krasnov, T.; Berger, G.; Feuerstein, T.; Stein, J.; Kraus, A.; Yanir, A.; et al. A Nationwide Analysis of the Phenotype/Genotype Landscape of Hemophagocytic Lymphohistiocytosis: UNC13D Associates with Poor Prognosis. Genes 2025, 16, 1315. https://doi.org/10.3390/genes16111315
Brik Simon D, Greental Ness Y, Dgany O, Noy-Lotan S, Krasnov T, Berger G, Feuerstein T, Stein J, Kraus A, Yanir A, et al. A Nationwide Analysis of the Phenotype/Genotype Landscape of Hemophagocytic Lymphohistiocytosis: UNC13D Associates with Poor Prognosis. Genes. 2025; 16(11):1315. https://doi.org/10.3390/genes16111315
Chicago/Turabian StyleBrik Simon, Dafna, Yarden Greental Ness, Orly Dgany, Sharon Noy-Lotan, Tanya Krasnov, Galit Berger, Tamar Feuerstein, Jerry Stein, Aviva Kraus, Asaf Yanir, and et al. 2025. "A Nationwide Analysis of the Phenotype/Genotype Landscape of Hemophagocytic Lymphohistiocytosis: UNC13D Associates with Poor Prognosis" Genes 16, no. 11: 1315. https://doi.org/10.3390/genes16111315
APA StyleBrik Simon, D., Greental Ness, Y., Dgany, O., Noy-Lotan, S., Krasnov, T., Berger, G., Feuerstein, T., Stein, J., Kraus, A., Yanir, A., Barg, A., Jacoby, E., Mandel-Shorer, N., Harlev, D., Even-Or, E., Tamary, H., Gilad, O., Steinberg-Shemer, O., & Yacobovich, J. (2025). A Nationwide Analysis of the Phenotype/Genotype Landscape of Hemophagocytic Lymphohistiocytosis: UNC13D Associates with Poor Prognosis. Genes, 16(11), 1315. https://doi.org/10.3390/genes16111315






