Increased Prevalence of Psychiatric Disorders in Children with RASopathies: Comparing NF1, Noonan Syndrome Spectrum Disorder, and the General Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognition
2.3. Psychiatric Disorders
2.4. Statistical Analysis
Psychiatric Diagnoses Analysis
3. Results
3.1. Rates of Psychiatric Disorders in NF1
3.2. Rates of Psychiatric Disorders in NSSD
3.3. Influence of Cognition on the Prevalence of Psychiatric Disorders
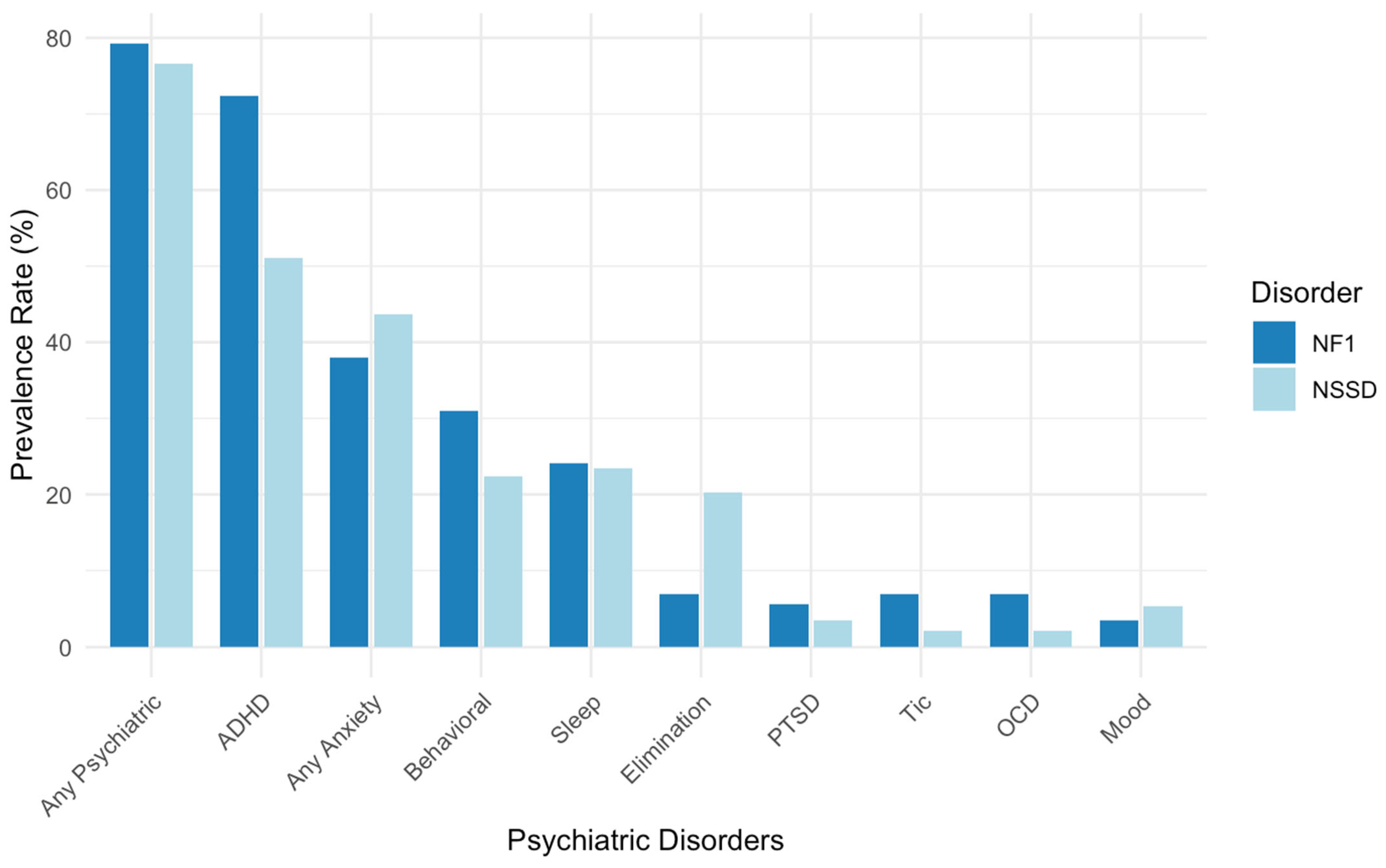
3.4. Comparison Between NF1 and NSSD Psychiatric Diagnoses
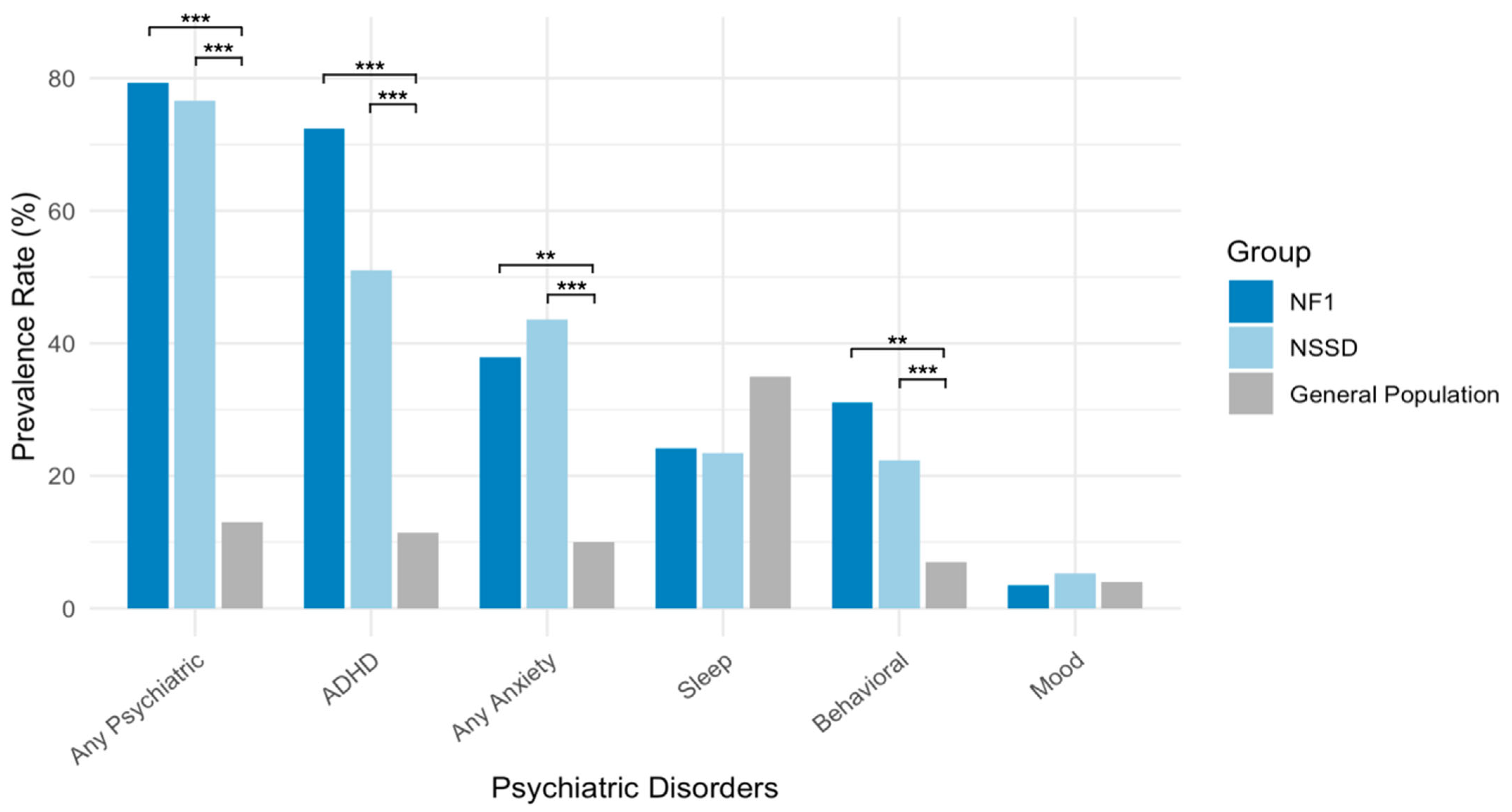
3.5. Comparing Psychiatric Prevalence in NF1 and NSSD Relative to the General Children’s Population
4. Discussion
4.1. ADHD
4.2. Anxiety Disorders
4.3. Sleep Disorders
4.4. Behavioral Disorders, Elimination Disorders, OCD, Tic Disorders, and PTSD
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Liu, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hebron, K.E.; Hernandez, E.R.; Yohe, M.E. The RASopathies: From Pathogenetics to Therapeutics. Dis. Model. Mech. 2022, 15, dmm049107. [Google Scholar] [CrossRef] [PubMed]
- Zenker, M. Clinical Overview on RASopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis Type 1. Nat. Rev. Dis. Primers 2017, 3, 17004. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, M.; Aoki, Y.; Gelb, B.D. The Molecular Genetics of RASopathies: An Update on Novel Disease Genes and New Disorders. Am. J. Med. Genet. C Semin. Med. Genet. 2022, 190, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.A.; Allanson, J.E.; Dahlgren, J.; Gelb, B.D.; Hall, B.; Pierpont, M.E.; Roberts, A.E.; Robinson, W.; Takemoto, C.M.; Noonan, J.A. Noonan Syndrome: Clinical Features, Diagnosis, and Management Guidelines. Pediatrics 2010, 126, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Brooks, A.; Burns, A.; Burkitt-Wright, E.; Kerr, B.; Huson, S.; Emsley, R.; Green, J. Autism Spectrum Disorder and Other Neurobehavioural Comorbidities in Rare Disorders of the Ras/MAPK Pathway. Dev. Med. Child. Neurol. 2017, 59, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Kenborg, L.; Andersen, E.W.; Duun-Henriksen, A.K.; Jepsen, J.R.M.; Doser, K.; Dalton, S.O.; Bidstrup, P.E.; Krøyer, A.; Frederiksen, L.E.; Johansen, C.; et al. Psychiatric Disorders in Individuals with Neurofibromatosis 1 in Denmark: A Nationwide Register-Based Cohort Study. Am. J. Med. Genet. A 2021, 185, 3706–3716. [Google Scholar] [CrossRef] [PubMed]
- Naylor, P.E.; Bruno, J.L.; Shrestha, S.B.; Friedman, M.; Jo, B.; Reiss, A.L.; Green, T. Neuropsychiatric Phenotypes in Children with Noonan Syndrome. Dev. Med. Child Neurol. 2023, 65, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Perrino, F.; Licchelli, S.; Serra, G.; Piccini, G.; Caciolo, C.; Pasqualetti, P.; Cirillo, F.; Leoni, C.; Digilio, M.C.; Zampino, G.; et al. Psychopathological Features in Noonan Syndrome. Eur. J. Paediatr. Neurol. 2018, 22, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence-(WASI-II); NCS Pearson: San Antonio, TX, USA, 2011. [Google Scholar]
- Hodge, M.A.; Sutherland, R.; Jeng, K.; Bale, G.; Batta, P.; Cambridge, A.; Detheridge, J.; Drevensek, S.; Edwards, L.; Everett, M.; et al. Agreement between Telehealth and Face-to-Face Assessment of Intellectual Ability in Children with Specific Learning Disorder. J. Telemed. Telecare 2019, 25, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Kobak, K.; Kearney, C.; Milham, M.; Andreotti, C.; Escalera, J.; Alexander, L.; Gill, M.K.; Birmaher, B.; Sylvester, R.; et al. Development of Three Web-Based Computerized Versions of the Kiddie Schedule for Affective Disorders and Schizophrenia Child Psychiatric Diagnostic Interview: Preliminary Validity Data. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Maternal Child Health Bureau (MCHB). National Survey Children’s Health; Data Resource Center Child Adolescent Health Supported U.S. Department Health Human Services, Health Resources Services Administration (HRSA): Rockville, MD, USA, 2021–2022. [Google Scholar]
- NSCH Interactive Data Query (2022—Present)—Data Resource Center for Child and Adolescent Health. Available online: https://nschdata.org/browse/survey?s=2&y=51&r=1& (accessed on 20 May 2025).
- Hou, Y.; Yu, L.; Liu, D.; Wilson-Lemoine, E.; Wu, X.; Moreira, J.P.; Mujica, B.F.; Mukhopadhyay, E.S.; Novotney, A.N.; Payne, J.M. Systematic Review and Meta-Analysis: Attention-Deficit/Hyperactivity Disorder Symptoms in Children with Neurofibromatosis Type 1. J. Am. Acad. Child Adolesc. Psychiatry 2025, 64, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, E.I.; Tworog-Dube, E.; Roberts, A.E. Attention Skills and Executive Functioning in Children with Noonan Syndrome and Their Unaffected Siblings. Dev. Med. Child Neurol. 2015, 57, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Halevy, A.; Aharon, S.; Shuper, A. Attention Deficit Hyperactivity Disorder in Neurofibromatosis Type 1: Evaluation with a Continuous Performance Test. J. Clin. Neurol. 2018, 14, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Torres Nupan, M.M.; Velez Van Meerbeke, A.; López Cabra, C.A.; Herrera Gomez, P.M. Cognitive and Behavioral Disorders in Children with Neurofibromatosis Type 1. Front. Pediatr. 2017, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, A.; Howie, E.; Trump, D.; Huson, S.M. Behaviour in Children with Neurofibromatosis Type 1: Cognition, Executive Function, Attention, Emotion, and Social Competence. Dev. Med. Child Neurol. 2013, 55, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.M.; Hyman, S.L.; Shores, E.A.; North, K.N. Assessment of Executive Function and Attention in Children with Neurofibromatosis Type 1: Relationships between Cognitive Measures and Real-World Behavior. Child Neuropsychol. 2011, 17, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, E.I. Neuropsychological Functioning in Individuals with Noonan Syndrome: A Systematic Literature Review with Educational and Treatment Recommendations. J. Pediatr. Neuropsychol. 2016, 2, 14–33. [Google Scholar] [CrossRef]
- Ayano, G.; Demelash, S.; Gizachew, Y.; Tsegay, L.; Alati, R. The Global Prevalence of Attention Deficit Hyperactivity Disorder in Children and Adolescents: An Umbrella Review of Meta-Analyses. J. Affect. Disord. 2023, 339, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Serur, Y.; Fuhrmann, N.; Russo, O.; Green, T. Irritability in Children with RASopathies, Insights into Emotional Dysregulation and Social Impairment. Eur. Child Adolesc. Psychiatry 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Naylor, P.E.; Siqueiros-Sanchez, M.; Wintermark, M.; Raman, M.M.; Jo, B.; Reiss, A.L.; Green, T. Novel Effects of Ras-MAPK Pathogenic Variants on the Developing Human Brain and Their Link to Gene Expression and Inhibition Abilities. Transl. Psychiatry 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Green, T.; Naylor, P.E.; Davies, W. Attention Deficit Hyperactivity Disorder (ADHD) in Phenotypically Similar Neurogenetic Conditions: Turner Syndrome and the RASopathies. J. Neurodev. Disord. 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Serur, Y.; Sofrin Frumer, D.; Daon, K.; Sobol-Havia, D.; Weinberger, R.; Shulman, C.; Gothelf, D. Psychiatric Disorders and Autism in Young Children with 22q11.2 Deletion Syndrome Compared to Children with Idiopathic Autism. Eur. Psychiatry 2019, 55, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.; Senior, J.; Murtagh, L. ASD, ADHD, Mental Health Conditions and Psychopharmacology in Neurogenetic Syndromes: Parent Survey: Psychopathology in Neurogenetic Syndromes. J. Intellect. Disabil. Res. 2015, 59, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, E.I.; Hudock, R.L.; Foy, A.M.; Semrud-Clikeman, M.; Pierpont, M.E.; Berry, S.A.; Shanley, R.; Rubin, N.; Sommer, K.; Moertel, C.L. Social Skills in Children with RASopathies: A Comparison of Noonan Syndrome and Neurofibromatosis Type 1. J. Neurodev. Disord. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Foy, A.M.H.; Hudock, R.L.; Shanley, R.; Pierpont, E.I. Social Behavior in RASopathies and Idiopathic Autism. J. Neurodev. Disord. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Wiener, L.; Battles, H.; Bedoya, S.Z.; Baldwin, A.; Widemann, B.C.; Pao, M. Identifying Symptoms of Distress in Youth Living with Neurofibromatosis Type 1 (NF1). J. Genet. Couns. 2018, 27, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, P.; Cumbo, F.; Serra, G.; Trasolini, M.; Frattini, C.; Scibelli, F.; Licchelli, S.; Cirillo, F.; Caciolo, C.; Casini, M.P.; et al. Manic and Depressive Symptoms in Children Diagnosed with Noonan Syndrome. Brain Sci. 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Licis, A.K.; Vallorani, A.; Gao, F.; Chen, C.; Lenox, J.; Yamada, K.A.; Duntley, S.P.; Gutmann, D.H. Prevalence of Sleep Disturbances in Children with Neurofibromatosis Type 1. J. Child Neurol. 2013, 28, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, M.; Messina, G.; Esposito, M.; Santoro, C.; Iacono, D.; Spruyt, K. Polysomnographic Study in Pediatric Neurofibromatosis Type 1. Front. Neurol. 2023, 14, 1213430. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Chaudhury, S.; Saldanha, D. Noonan Syndrome with Somnambulism: A Rare Case Report. Ind. Psychiatry J. 2020, 29, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Prioli, A.; Sacchetto, L.; Gasperi, E.; Bisceglia, A.; Ghobert, A.L.; Piacentini, G. Sleep Disordered Breathing in Noonan Syndrome: A Rare Case Report. J. Pediatr. Neonat. Individ. Med. 2018, 7, 1–6. [Google Scholar]
- Sutton, E.L. Psychiatric Disorders and Sleep Issues. Med. Clin. N. Am. 2014, 98, 1123–1143. [Google Scholar] [CrossRef] [PubMed]
- Sarsour, K.; Morin, C.M.; Foley, K.; Kalsekar, A.; Walsh, J.K. Association of Insomnia Severity and Comorbid Medical and Psychiatric Disorders in a Health Plan-Based Sample: Insomnia Severity and Comorbidities. Sleep Med. 2010, 11, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, L.; Postorino, V.; Siracusano, M.; Riccioni, A.; Curatolo, P. The Relationship between Sleep Problems, Neurobiological Alterations, Core Symptoms of Autism Spectrum Disorder, and Psychiatric Comorbidities. J. Clin. Med. 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed]

| NF1 | NSSD | Overall | p Values (Chi-Square or t-Test or Mann–Whitney U Test) | |
|---|---|---|---|---|
| N | 29 | 94 | 123 | NF1: NSSD |
| Age (Mean (SD)) | 8.97 (2.27) | 9.83 (2.99) | 9.62 (2.85) | 0.278 |
| Sex | ||||
| Female (%) | 14 (48.3%) | 48 (51.1%) | 62 (50.4%) | 0.960 |
| Male (%) | 15 (51.7%) | 46 (48.9%) | 61 (49.6%) | |
| FSIQ (Mean (SD)) * | 98.8 (12.2) | 97.1 (13.5) | 97.5 (13.2%) | 0.535 |
| Gene Variants (%) | ||||
| NF1 | 26 (89.65%) | |||
| NF1-Other | 3 (10.34%) | |||
| PTPN11 | 66 (70.2%) | |||
| SOS1 | 8 (6.5%) | |||
| NSSD-Other | 20 (21.27%) | |||
| Medication status (%) | ||||
| Any psychoactive medication | 12 (41.4%) | 18 (19.1%) | 30 (24.4%) | |
| Stimulants | 12 (41.4%) | 10 (10.6%) | 22 (17.9%) | |
| SSRI | 2 (6.9%) | 2 (2.1%) | 4 (3.3%) | |
| Atomoxetine | 0 (0%) | 3 (3.2%) | 3 (2.4%) | |
| Melatonin | 0 (0%) | 3 (3.2%) | 3 (2.4%) | |
| Clonidine | 1 (3.4%) | 1 (1.1%) | 2 (1.6%) | |
| Benzodiazepines | 0 (0%) | 1 (1.1%) | 1 (0.8%) | |
| Buspirone | 0 (0%) | 1 (1.1%) | 1 (0.8%) | |
| Gabapentin | 1 (3.4%) | 0 (0%) | 1 (0.8%) | |
| Guanfacine | 0 (0%) | 1 (1.1%) | 1 (0.8%) | |
| Remeron | 0 (0%) | 1 (1.1%) | 1 (0.8%) |
| Psychiatric Diagnosis | NF1 (n/N) | NSSD (n/N) | Children’s Population * | Fisher’s Test (NF1 vs. NSSD) | Binomial Test (NF1 vs. Pop) | Binomial Test (NSSD vs. Pop) |
|---|---|---|---|---|---|---|
| Any Psychiatric Disorder | 23/29 (79.3%) | 72/94 (76.6%) | 13% | p = 1.000 | p < 0.001 | p < 0.001 |
| ADHD | 21/29 (72.4%) | 48/94 (51.1%) | 11.4% | p = 0.544 | p < 0.001 | p < 0.001 |
| Anxiety Disorders | 11/29 (37.9%) | 41/94 (43.6%) | 10% | p = 1.000 | p = 0.001 | p < 0.001 |
| Mood Disorders | 1/29 (3.4%) | 5/94 (5.3%) | 4% | p = 1.000 | p = 1.000 | p = 1.000 |
| Behavioral Disorders | 9/29 (31.0%) | 21/94 (22.3%) | 7% | p = 1.000 | p = 0.002 | p < 0.001 |
| Sleep Disorders | 7/29 (24.1%) | 22/94 (23.4%) | 35% | p = 1.000 | p = 1.000 | p = 0.313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serur, Y.; Russo, O.; McGhee, C.A.; Green, T. Increased Prevalence of Psychiatric Disorders in Children with RASopathies: Comparing NF1, Noonan Syndrome Spectrum Disorder, and the General Population. Genes 2025, 16, 843. https://doi.org/10.3390/genes16070843
Serur Y, Russo O, McGhee CA, Green T. Increased Prevalence of Psychiatric Disorders in Children with RASopathies: Comparing NF1, Noonan Syndrome Spectrum Disorder, and the General Population. Genes. 2025; 16(7):843. https://doi.org/10.3390/genes16070843
Chicago/Turabian StyleSerur, Yaffa, Odeya Russo, Chloe Alexa McGhee, and Tamar Green. 2025. "Increased Prevalence of Psychiatric Disorders in Children with RASopathies: Comparing NF1, Noonan Syndrome Spectrum Disorder, and the General Population" Genes 16, no. 7: 843. https://doi.org/10.3390/genes16070843
APA StyleSerur, Y., Russo, O., McGhee, C. A., & Green, T. (2025). Increased Prevalence of Psychiatric Disorders in Children with RASopathies: Comparing NF1, Noonan Syndrome Spectrum Disorder, and the General Population. Genes, 16(7), 843. https://doi.org/10.3390/genes16070843







