Expression Profile and Clinical Relevance of ADAR Family Genes in Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Patient Material
2.3. Database
2.4. Analysis of ADAR, ADARB1, and ADARB2 Expression
- RNA Isolation
- Reverse transcription reaction
- Primers design and Real-time PCR
2.5. Pathological and Clinical Analysis
2.6. Analysis of VIM Promoter Methylation Using the UALCAN Web Portal
2.7. Gene Group Enrichment Analysis
2.8. Statistical Analysis
3. Results
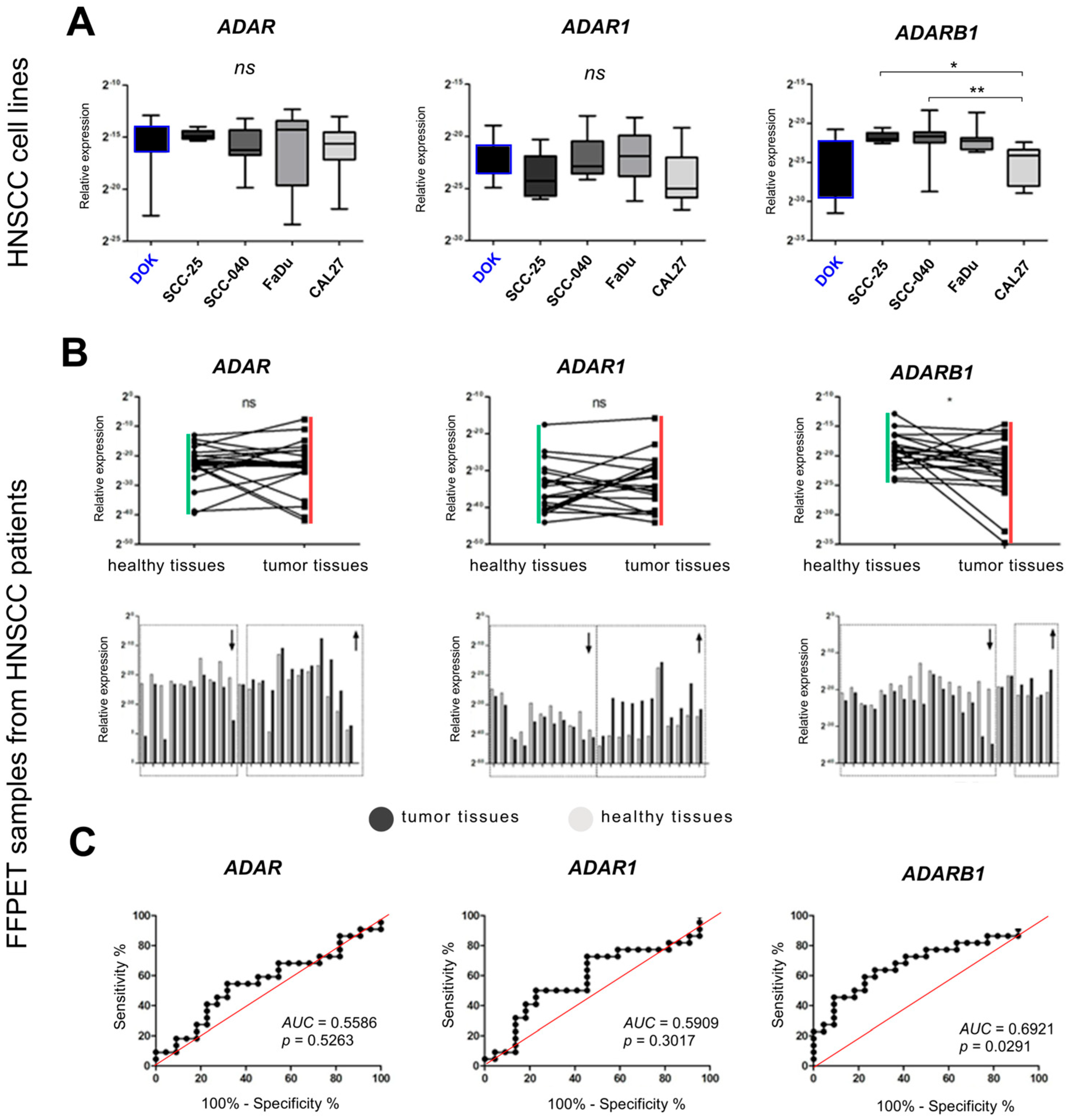
3.1. Expression of ADAR Family Genes in HNSCC Cell Lines
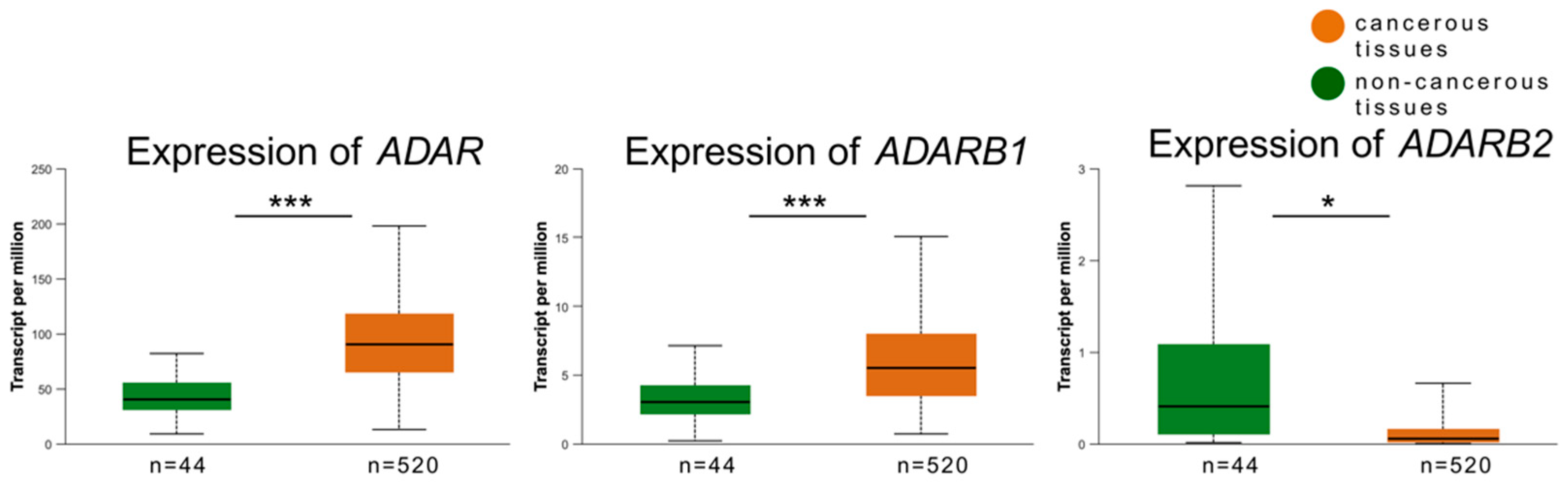
3.2. Expression of ADAR, ADARB1, and ADARB2 in Tumor vs. Non-Cancerous Tissues
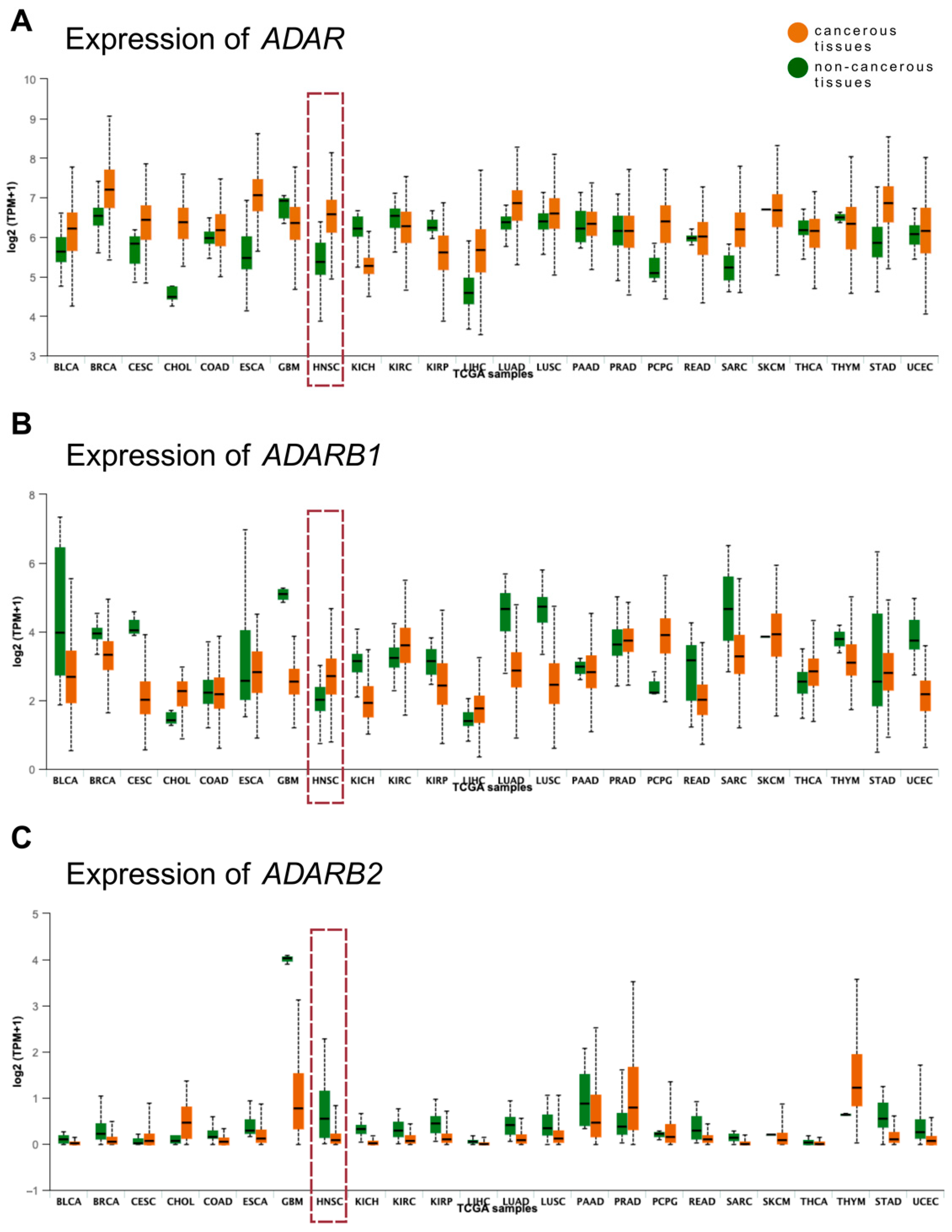
3.3. Validation of Expression Patterns Using TCGA Data
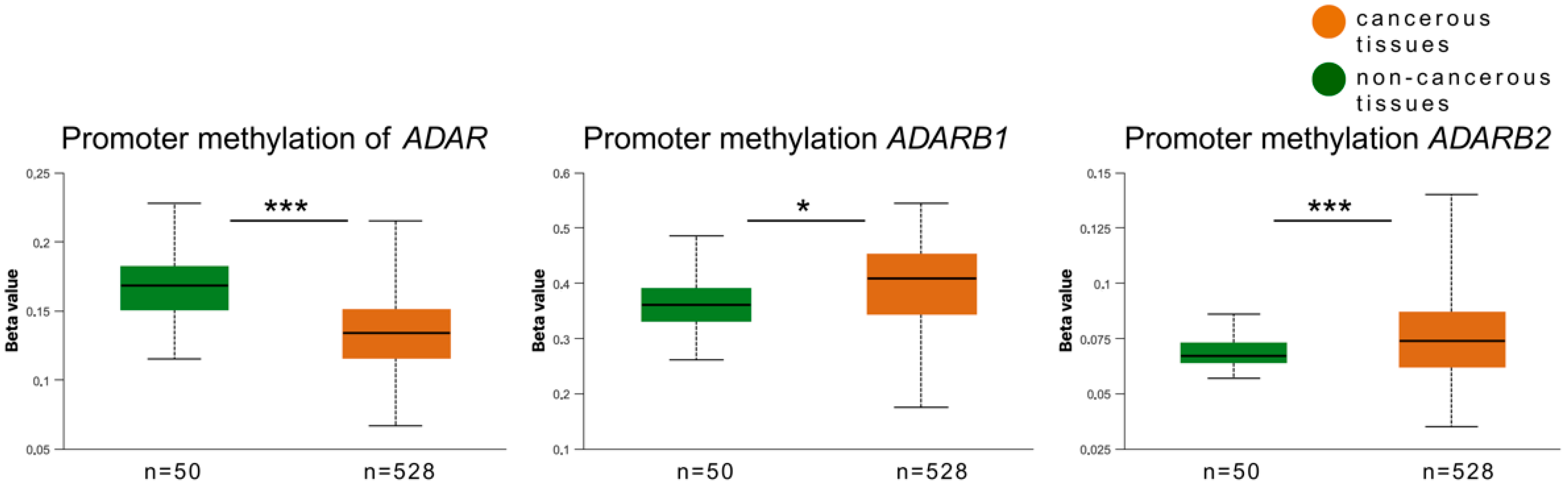
3.4. Promoter Methylation Analysis of ADAR Family Genes
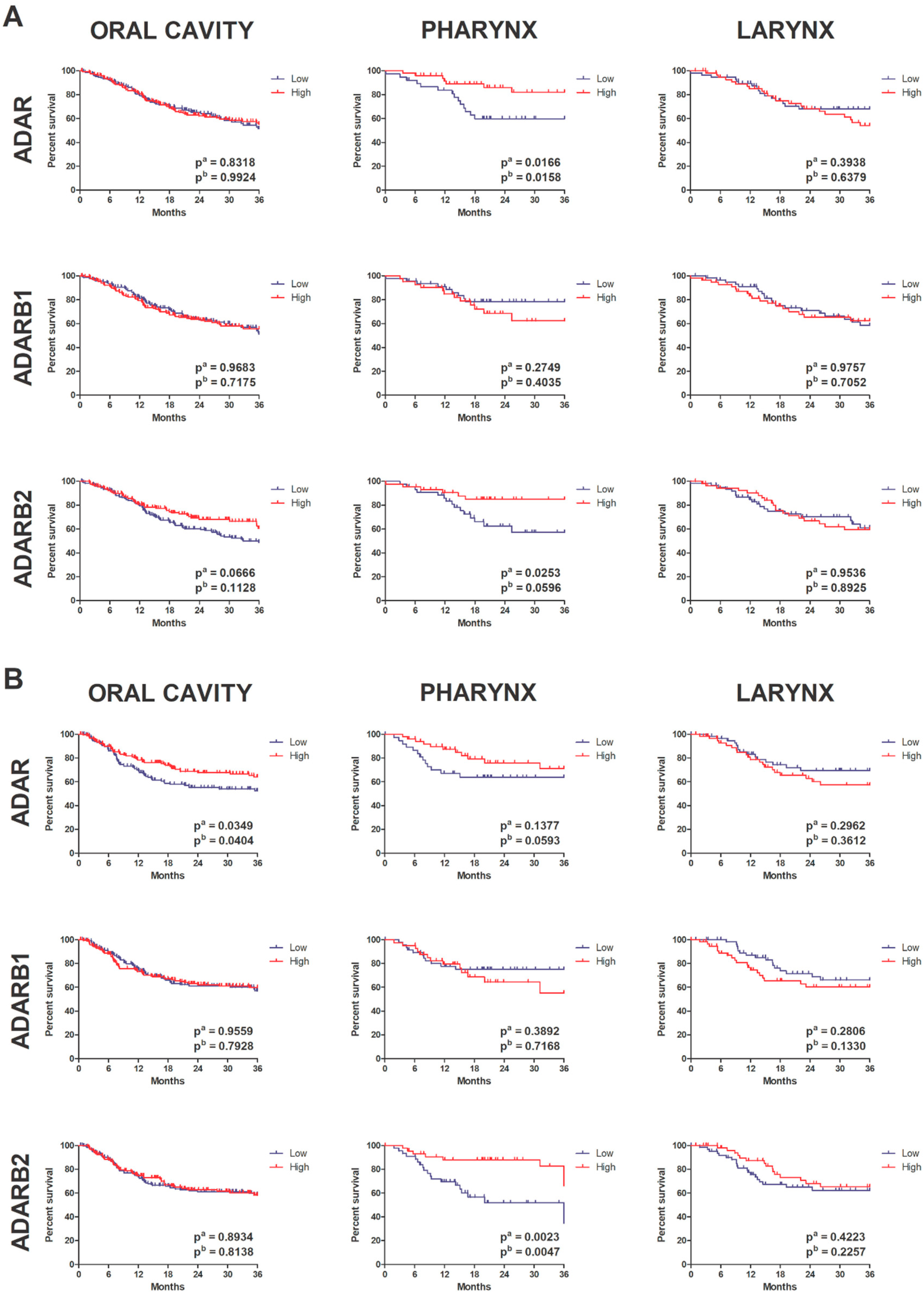
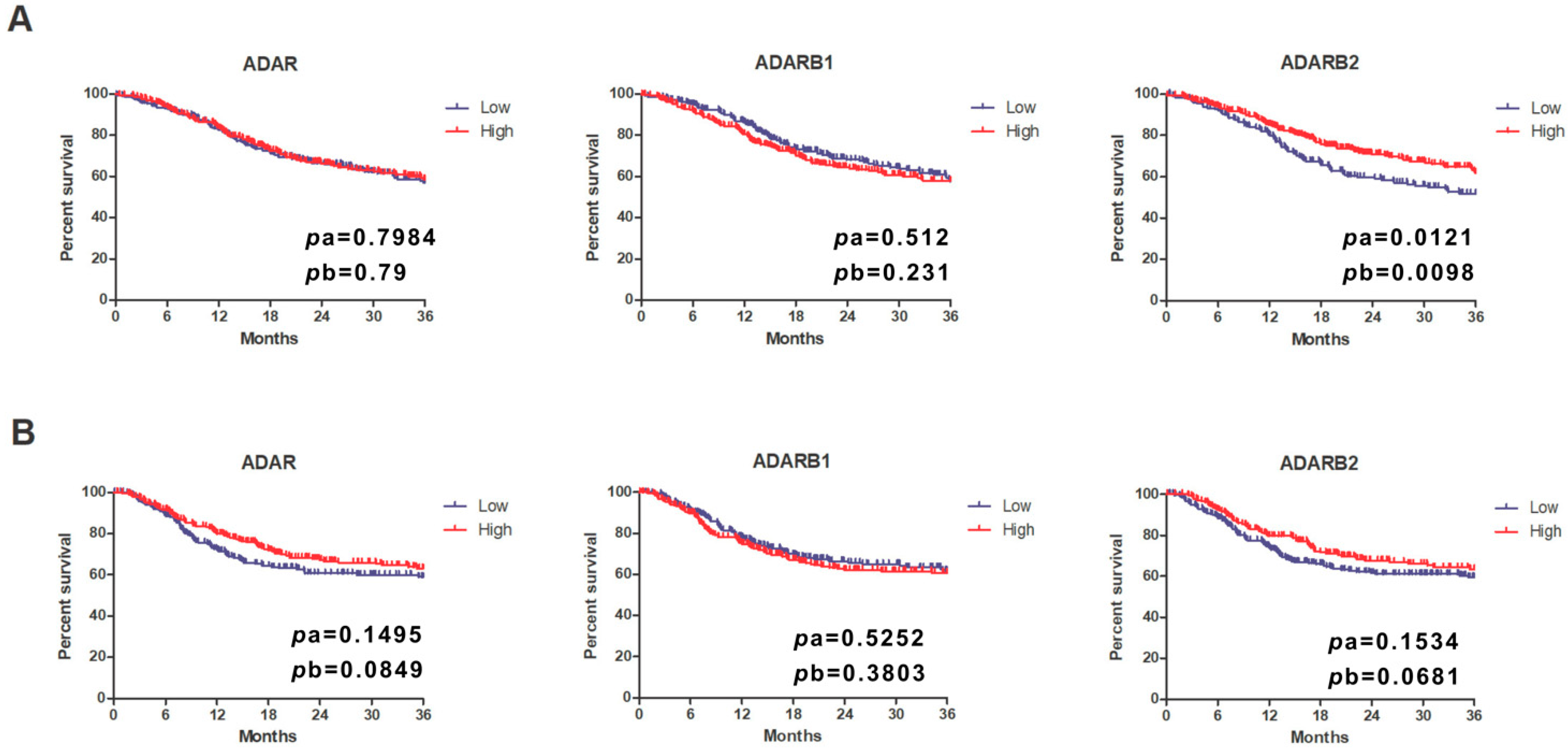
3.5. Prognostic Significance of ADAR Family Expression
3.6. Association with Clinicopathological Parameters
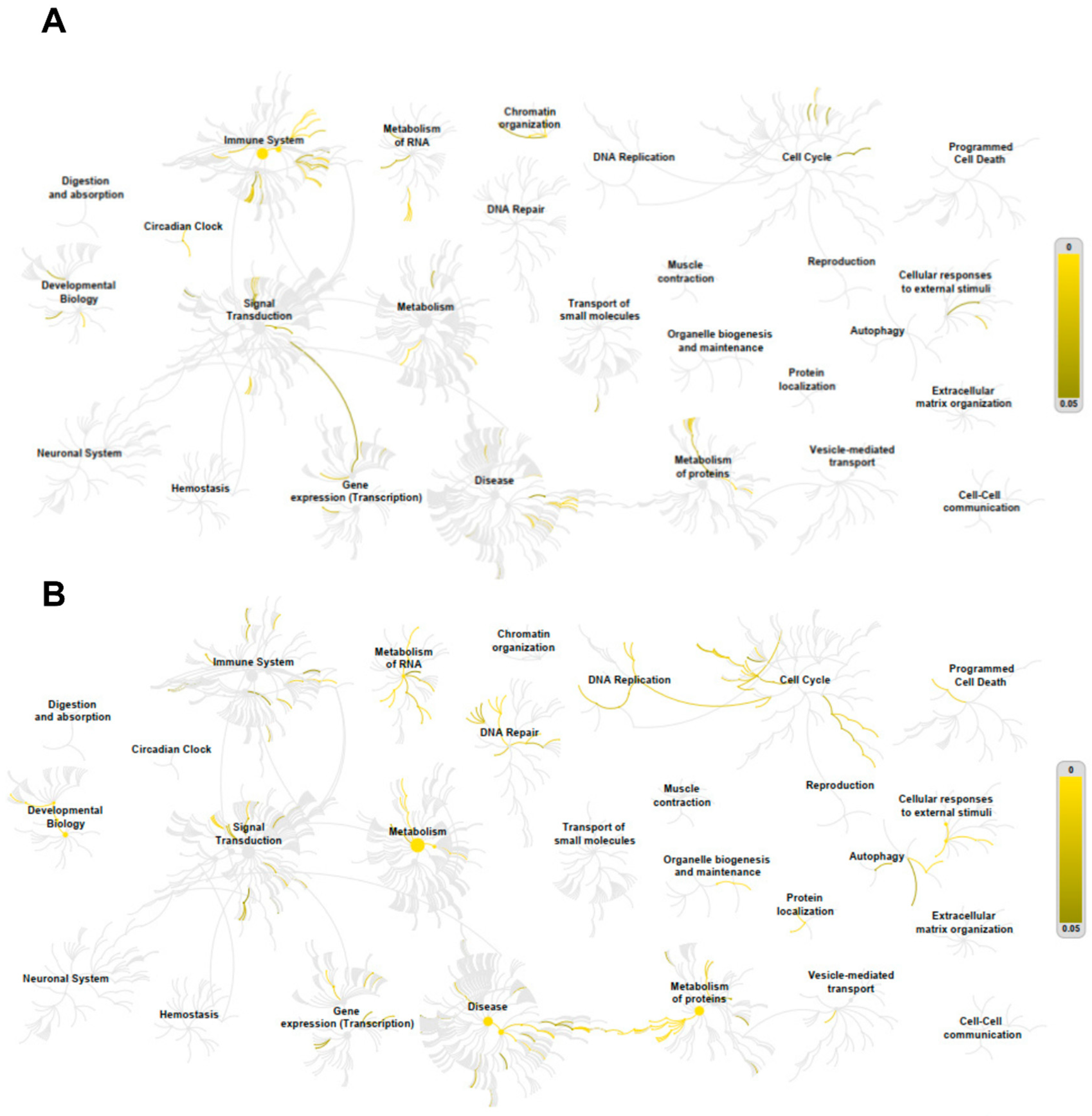
3.7. Correlation and Pathway Enrichment Analyses
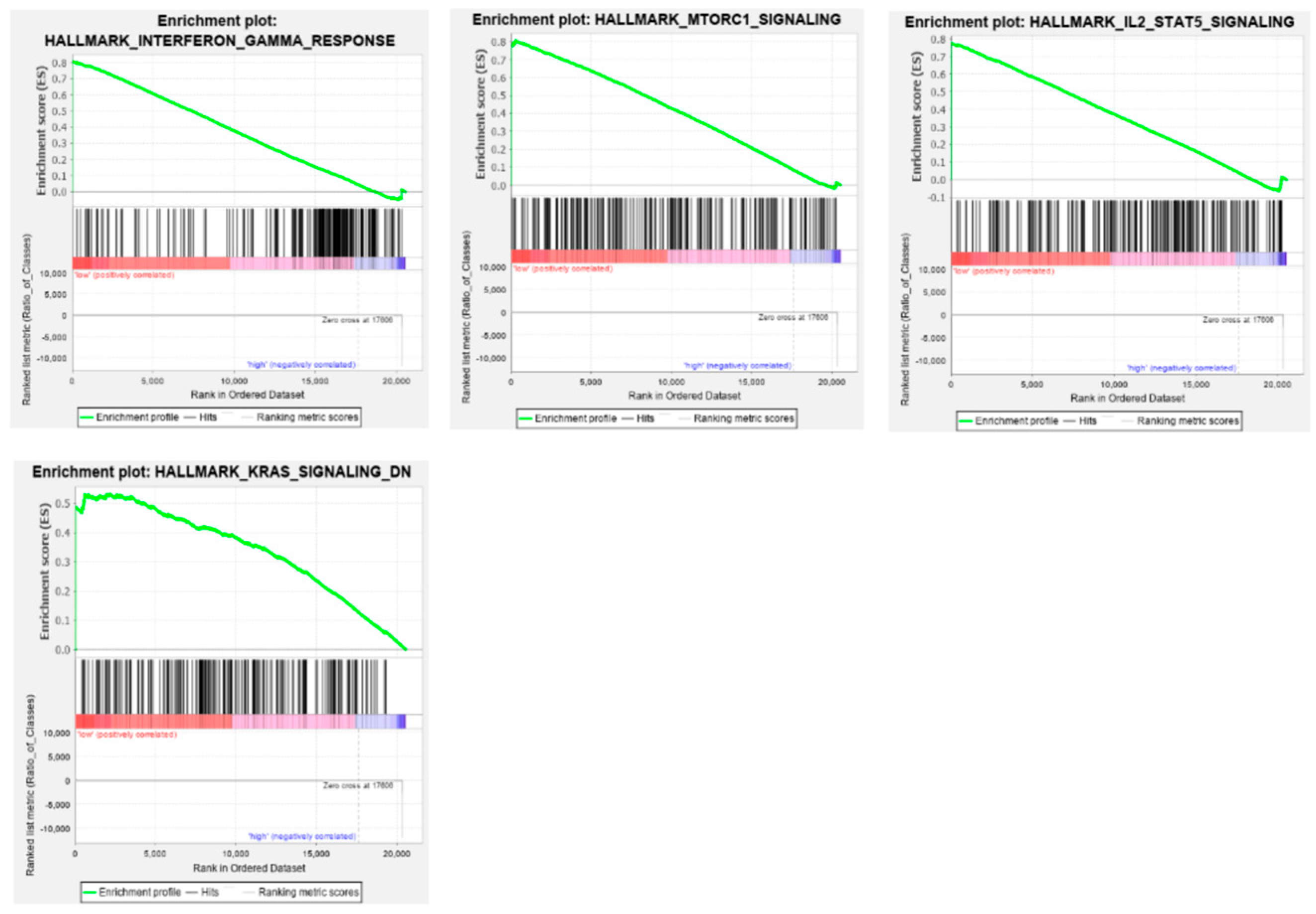
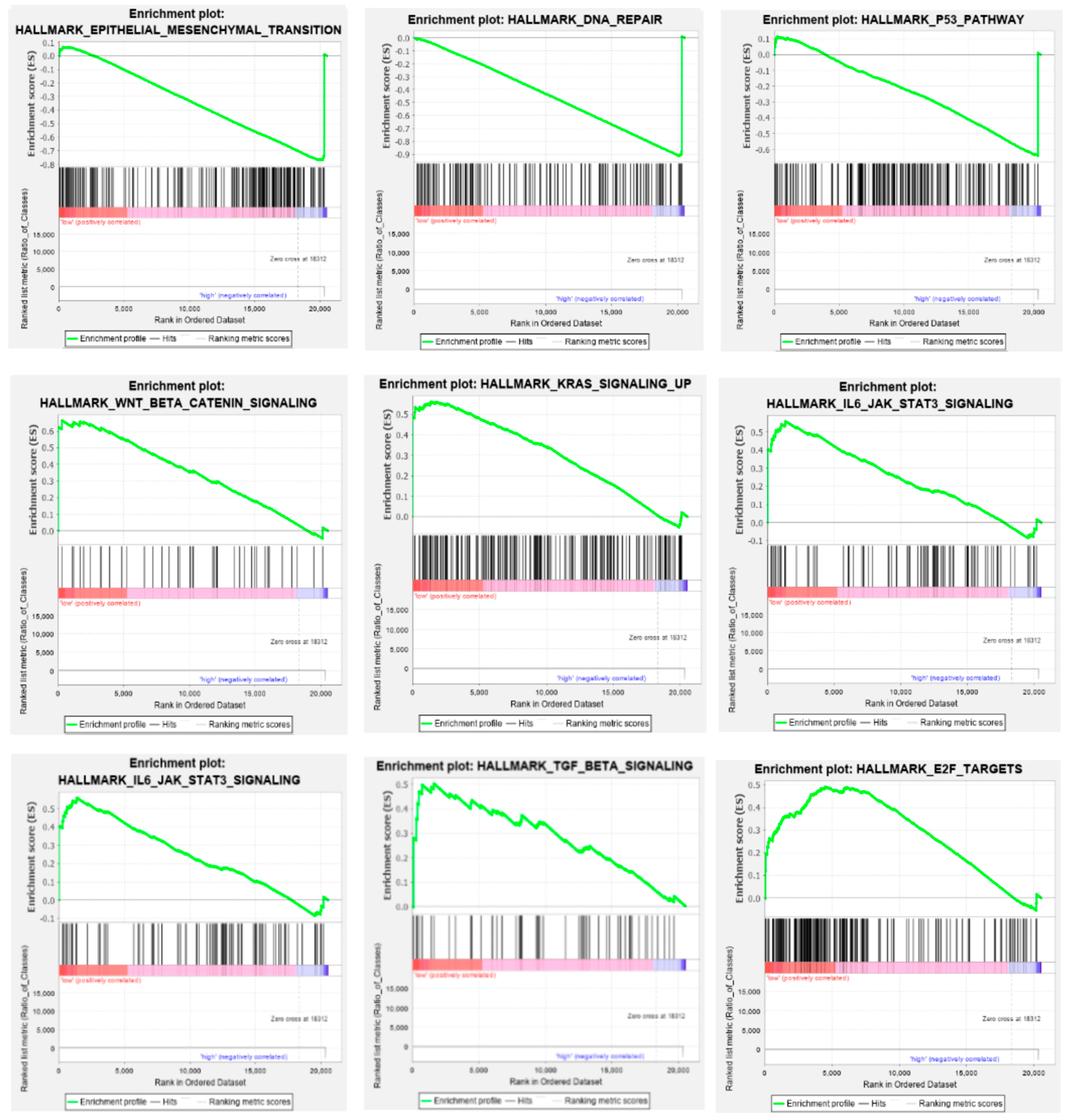
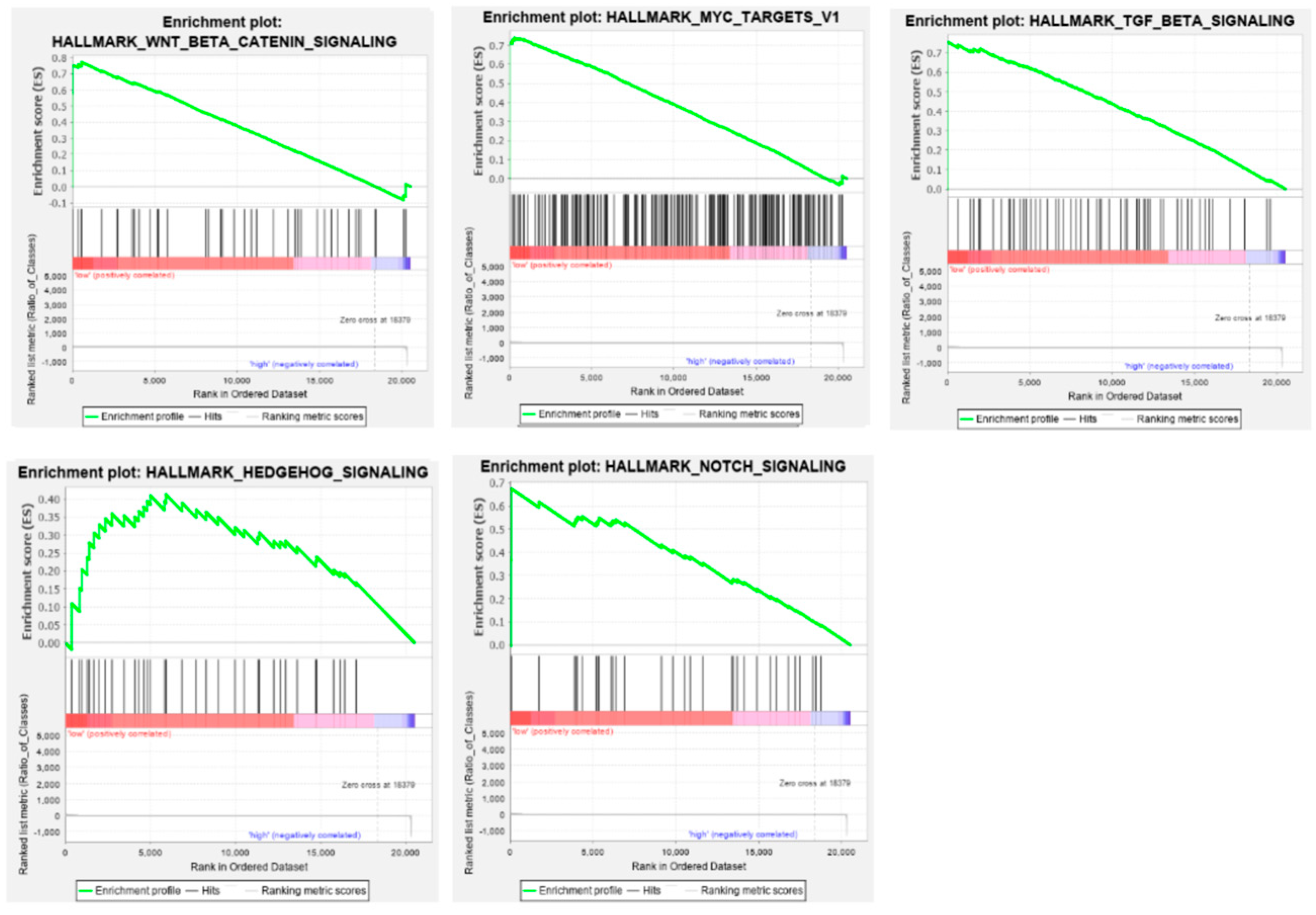
3.8. Gene Set Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Dyzmann-Sroka, A.; Malicki, J.; Jędrzejczak, A. Cancer incidence in the Greater Poland region as compared to Europe. Rep. Pract. Oncol. Radiother. 2020, 25, 632–636. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Dyzmann-Sroka, A.; Kubiak, A.; Łagoda-Bulczyńska, S.; Łyjak, M.; Śledzińska, R.; Taraszkiewicz, L.; Trojanowski, M.; Wojciechowska, U. Nowotwory Złośliwe w Wielkopolsce w 2022 r. Biuletyn nr 21. 2022. Available online: https://www.wco.pl/wrn/u/publications/0-wrn-2024-book-20250210-67aaf6bc324df046826814.pdf?v7.2 (accessed on 12 August 2025).
- Markowski, J.; Pietruszewska, W.; Mikaszewski, B.; Jurkiewicz, D.; Rogowski, M.; Maciejczyk, A.; Niemczyk, K.; Klatka, J.; Wierzbicka, M. A rapid parallel increase in the incidence and mortality of head and neck cancer among the Polish elderly over the last two decades and upward trends until 2035. Otolaryngol. Pol. 2024, 78, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pazdrowski, J.; Szewczyk, M.; Pazdrowski, P.; Seraszek-Jaros, A.; Niewinski, P.; Golusiński, W. Risk factors for local and nodal recurrence in patients with head and neck cutaneous squamous cell carcinoma in a high-reference oncological center in Poland. Rep. Pract. Oncol. Radiother. 2024, 29, 204–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F., Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar] [PubMed]
- La Vecchia, C.; Tavani, A.; Franceschi, S.; Levi, F.; Corrao, G.; Negri, E. Epidemiology and prevention of oral cancer. Oral Oncol. 1997, 33, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Petti, S. Lifestyle risk factors for oral cancer. Oral Oncol. 2009, 45, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Gallus, S.; Garavello, W.; Bosetti, C.; La Vecchia, C. Cancer risk associated with alcohol and tobacco use: Focus on upper aero-digestive tract and liver. Alcohol. Res. Health 2006, 29, 193–198. [Google Scholar] [PubMed] [PubMed Central]
- Rose Ragin, C.C.; Taioli, E. Second primary head and neck tumor risk in patients with cervical cancer--SEER data analysis. Head Neck 2008, 30, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282, Erratum in Nat. Rev. Cancer 2018, 18, 662. [Google Scholar] [CrossRef] [PubMed]
- Toledo, L.; Neelsen, K.J.; Lukas, J. Replication Catastrophe: When a Checkpoint Fails because of Exhaustion. Mol. Cell 2017, 66, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Teng, Y. Potential roles of FAT1 somatic mutation in progression of head and neck cancer. Oncoscience 2022, 9, 30–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirota, T.; Kunitoku, N.; Sasayama, T.; Marumoto, T.; Zhang, D.; Nitta, M.; Hatakeyama, K.; Saya, H. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell 2003, 114, 585–598. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomaviruses and cancer: From basic studies to clinical application. Nat. Rev. Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Alayón, L.F.; Salas, B.S.; Diaz-Saavedra, R.C.; Ortiz, A.R.; Martin, J.Z.; Jimenez, P.C.L.; Sáez-Bravo, M.L. Screening oropha-ryngeal dysphagia in patients with head and neck cancer in a radiation oncology department. Rep. Pract. Oncol. Radiother. 2024, 28, 756–763. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, N.; O’Connell, L.; Browne, D.; Khosravi, B.; Brennan, S.; Duane, F.; Armstrong, J.; Boychak, O.; McArdle, O. Treatment of oropharyngeal cancer during the COVID-19 lockdown—Outcomes for patients treated during the pandemic. Rep. Pract. Oncol. Radiother. 2024, 29, 606–613. [Google Scholar] [CrossRef]
- Maxwell, J.H.; Grandis, J.R.; Ferris, R.L. HPV-Associated Head and Neck Cancer: Unique Features of Epidemiology and Clinical Management. Annu. Rev. Med. 2016, 67, 91–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogawa, T.; Matsuura, K.; Shiga, K.; Tateda, M.; Katagiri, K.; Kato, K.; Saijo, S.; Kobayashi, T. Surgical treatment is recommended for advanced oral squamous cell carcinoma. Tohoku J. Exp. Med. 2011, 223, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.M.; Weber, R.S.; Lai, S.Y. Head and neck cancer: An evolving treatment paradigm. Cancer 2008, 113 (Suppl. S7), 1911–1932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffmann, T.K.; Schuler, P.J.; Laban, S.; Grässlin, R.; Beer, M.; Beer, A.J.; Friebe-Hoffmann, U.; Bullinger, L.; Möller, P.; Wiegel, T. Response Evaluation in Head and Neck Oncology: Definition and Prediction. ORL J. Otorhinolaryngol. Relat. Spec. 2017, 79, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Koiwai, K.; Hirasawa, D.; Sugimura, M.; Endo, Y.; Mizuhata, K.; Ina, H.; Fukazawa, A.; Kitoh, R.; Sakai, H.; Fujinaga, Y. Impact of upgraded radiotherapy system on outcomes in postoperative head and neck squamous cell carcinoma patients. Rep. Pract. Oncol. Radiother. 2022, 27, 954–962. [Google Scholar] [CrossRef]
- Viani, G.A.; Faustino, A.C.; Danelichen, A.F.B.; Maatsura, F.K.; Neves, L.V.F.; Fernandes, M.H.; Fernandes, J.P. Radiotherapy for locally advanced head and neck cancer in elderly patients: Results and prognostic factors a single cohort. Rep. Pract. Oncol. Radiother. 2021, 26, 12–19. [Google Scholar] [CrossRef]
- Mauro, G.P.; Da Roz, L.M.; de Carvalho Gico, V.; Weltman, E.; De Souza, E.C.; Baraldi, H.E.; Figueiredo, E.G.; Carlotti, C.G. Debulking surgery prior to stereotactic radiotherapy for head and neck paragangliomas. Rep. Pract. Oncol. Radiother. 2024, 29, 454–459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basu, S.; Chatterjee, S.; Chatterjee, K.; Samanta, S.; Saha, S.; Hossain, S.T.; Mondal, P.; Biswas, S. Correlation of degree of acute radiation dermatitis (RD) with skin dose distribution in head and neck squamous cell carcinoma patients treated with definitive concurrent chemoradiation. Rep. Pract. Oncol. Radiother. 2024, 29, 579–587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sindhu, S.K.; Bauman, J.E. Current Concepts in Chemotherapy for Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 145–154. [Google Scholar] [CrossRef]
- Li, C.X.; Tan, X.R.; Wei, W.; Li, M.Q.; Zhang, W.N.; Gong, Z.C.; Zhang, Y.; Zhao, H.R. A radiobiological perspective on radioresistance or/and radiosensitivity of head and neck squamous cell carcinoma. Rep. Pract. Oncol. Radiother. 2024, 28, 809–822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Li, P.; Zhang, Y.; Zheng, L. Transcriptome combined with single-cell sequencing explored prognostic markers associated with T cell exhaustion characteristics in head and neck squamous carcinoma. Sci. Rep. 2025, 15, 8209. [Google Scholar] [CrossRef]
- Rupar, M.J.; Golusinski, P.; Golusinski, W.; Masternak, M.M. Human Papillomavirus and the use of nanoparticles for immunotherapy in HPV-related cancer: A review. Rep. Pract. Oncol. Radiother. 2019, 24, 544–550. [Google Scholar] [CrossRef]
- Roszkowski, S.; Durczyńska, Z.; Szablewska, S. Targeted nanodelivery systems for personalized cancer therapy. Rep. Pract. Oncol. Radiother. 2025, 29, 776–788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wojtera, B.; Ostrowska, K.; Szewczyk, M.; Masternak, M.M.; Golusiński, W. Chloride intracellular channels in oncology as potential novel biomarkers and personalized therapy targets: A systematic review. Rep. Pract. Oncol. Radiother. 2024, 29, 258–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jose, S.R.; Timothy, P.B.; Suganthy, J.; Backianathan, S.; Amirtham, S.M.; Rani, S.; Singh, R. Determination of dose-response calibration curves for gamma radiation using gamma-H2AX immunofluorescence based biodosimetry. Rep. Pract. Oncol. Radiother. 2024, 29, 164–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maćkowiak, B.; Ostrowska, K.; Kulcenty, K.; Kaźmierska, J.; Ostapowicz, J.; Nowicka, H.; Szewczyk, M.; Książek, K.; Suchorska, W.M.; Golusiński, W. The impact of XPC gene single nucleotide polymorphism rs2228001 on head and neck cancer patients’ response to radiotherapy treatment. Rep. Pract. Oncol. Radiother. 2024, 29, 148–154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bass, B.L.; Weintraub, H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell 1988, 55, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Nishikura, K. Functions and regulation of RNA editing by ADAR deaminases. Annu. Rev. Biochem. 2010, 79, 321–349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, L.D.; Öhman, M. ADAR1 Editing and its Role in Cancer. Genes 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, U.; Wang, Y.; Sanford, T.; Zeng, Y.; Nishikura, K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc. Natl. Acad. Sci. USA 1994, 91, 11457–11461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Connell, M.A.; Krause, S.; Higuchi, M.; Hsuan, J.J.; Totty, N.F.; Jenny, A. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol. Cell. Biol. Marzec 1995, 15, 1389–1397. [Google Scholar] [CrossRef]
- Chen, C.X.; Cho, D.S.; Wang, Q.; Lai, F.; Carter, K.C.; Nishikura, K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA 2000, 6, 755–767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashley, C.N.; Broni, E.; Miller, W.A. ADAR Family Proteins: A Structural Review. Curr. Issues Mol. Biol. 2024, 46, 3919–3945. [Google Scholar] [CrossRef]
- Paz-Yaacov, N.; Bazak, L.; Buchumenski, I.; Porath, H.T.; Danan-Gotthold, M.; Knisbacher, B.A.; Eisenberg, E.; Levanon, E.Y. Elevated RNA Editing Activity is a Major Contributor to Transcriptomic Diversity in Tumors. Cell Rep. 2015, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, D.; Gacquer, D.; Rothé, F.; Lefort, A.; Libert, F.; Brown, D.; Kheddoumi, N.; Shlien, A.; Konopka, T.; Salgado, R.; et al. Principles Governing A-to-I RNA Editing in the Breast Cancer Transcriptome. Cell Rep. 2015, 13, 277–289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Li, Y.; Lin, C.H.; Chan, T.H.; Chow, R.K.; Song, Y.; Liu, M.; Yuan, Y.F.; Fu, L.; Kong, K.L.; et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat. Med. 2013, 19, 209–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, Y.R.; Qiao, J.J.; Chan, T.H.; Zhu, Y.H.; Li, F.F.; Liu, H.; Fei, J.; Li, Y.; Guan, X.Y.; Chen, L. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma. Cancer Res. 2014, 74, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moya, J.; Baker, A.R.; Slack, F.J.; Santisteban, P. ADAR1-mediated RNA editing is a novel oncogenic process in thyroid cancer and regulates miR-200 activity. Oncogene 2020, 39, 3738–3753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zipeto, M.A.; Court, A.C.; Sadarangani, A.; Delos Santos, N.P.; Balaian, L.; Chun, H.J.; Pineda, G.; Morris, S.R.; Mason, C.N.; Geron, I.; et al. ADAR1 Activation Drives Leukemia Stem Cell Self-Renewal by Impairing Let-7 Biogenesis. Cell Stem Cell. 2016, 19, 177–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melcher, T.; Maas, S.; Herb, A.; Sprengel, R.; Seeburg, P.H.; Higuchi, M. A mammalian RNA editing enzyme. Nature 1996, 379, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Brusa, R.; Zimmermann, F.; Koh, D.S.; Feldmeyer, D.; Gass, P.; Seeburg, P.H.; Sprengel, R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science 1995, 270, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.; Patt, S.; Schrey, M.; Rich, A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc. Natl. Acad. Sci. USA 2001, 98, 14687–14692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbieri, I.; Kouzarides, T. Role of RNA modifications in cancer. Nat. Rev. Cancer 2020, 20, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Galeano, F.; Rossetti, C.; Tomaselli, S.; Cifaldi, L.; Lezzerini, M.; Pezzullo, M.; Boldrini, R.; Massimi, L.; Di Rocco, C.M.; Locatelli, F.; et al. ADAR2-editing activity inhibits glioblastoma growth through the modulation of the CDC14B/Skp2/p21/p27 axis. Oncogene 2013, 32, 998–1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gan, Z.; Zhao, L.; Yang, L.; Huang, P.; Zhao, F.; Li, W.; Liu, Y. RNA editing by ADAR2 is metabolically regulated in pancreatic islets and beta-cells. J. Biol. Chem. 2006, 281, 33386–33394. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.P.; Maggi LBJr Weber, J.D. The Role of RNA Editing in Cancer Development and Metabolic Disorders. Front. Endocrinol. 2018, 9, 762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Wang, K.; Zhao, Z.; Sun, S.; Zhang, K.; Huang, R.; Zeng, F.; Hu, H. ADAR3 expression is an independent prognostic factor in lower-grade diffuse gliomas and positively correlated with the editing level of GRIA2Q607R. Cancer Cell Int. 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamperska, K.M.; Kozlowski, P.; Kolenda, T.; Teresiak, A.; Blizniak, R.; Przybyla, W.; Masternak, M.M.; Golusinski, P.; Golusinski, W. Unpredictable changes of selected miRNA in expression profile of HNSCC. Cancer Biomark. Sect. A Dis. Markers 2016, 16, 55–64. [Google Scholar] [CrossRef]
- World Health Organization. WHO Classification of Head and Neck Tumours, 5th ed.; IARC Press: Lyon, France, 2022. [Google Scholar]
- Brierley, J.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell on behalf of UICC: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kolenda, T.; Poter, P.; Guglas, K.; Kozłowska-Masłoń, J.; Braska, A.; Kazimierczak, U.; Teresiak, A. Biological role and diagnostic utility of ribosomal protein L23a pseudogene 53 in cutaneous melanoma. Rep. Pract. Oncol. Radiother. 2023, 28, 255–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. (Eds.) Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Zhang, Y.; Qian, H.; Xu, J.; Gao, W. ADAR, the carcinogenesis mechanisms of ADAR and related clinical applications. Ann. Transl. Med. 2019, 7, 686. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cesarini, V.; Silvestris, D.A.; Galeano, F.; Tassinari, V.; Martini, M.; Locatelli, F.; Gallo, A. ADAR2 Protein Is Associated with Overall Survival in GBM Patients and Its Decrease Triggers the Anchorage-Independent Cell Growth Signature. Biomolecules 2022, 12, 1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alberto, C.V.C.; Troiano, G.; Botti, G.; Pedicillo, M.C.; Russo, L.L.; Mastrangelo, F.; Ciavarella, D.; Losito, N.S.; Aquino, G.; Nocini, R.; et al. Overexpression of ADAR1 into the cytoplasm correlates with a better prognosis of patients with oral squamous cells carcinoma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2019, 48, 108–114. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.; Huang, J.; Wu, M.; Zhang, Z.; Xu, R.; Zhang, P.; Zhao, S.; Liu, L.; Jiang, H. ADAR1 promotes the epithelial-to-mesenchymal transition and stem-like cell phenotype of oral cancer by facilitating oncogenic microRNA maturation. J. Exp. Clin. Cancer Res. 2019, 38, 315. [Google Scholar] [CrossRef]
- Velazquez-Torres, G.; Shoshan, E.; Ivan, C.; Huang, L.; Fuentes-Mattei, E.; Paret, H.; Kim, S.J.; Rodriguez-Aguayo, C.; Xie, V.; Brooks, D.; et al. A-to-I miR-378a-3p editing can prevent melanoma progression via regulation of PARVA expression. Nat. Commun. 2018, 9, 461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiga, K.; Ogawa, T.; Katagiri, K.; Yoshida, F.; Tateda, M.; Matsuura, K.; Kobayashi, T. Differences between oral cancer and cancers of the pharynx and larynx on a molecular level. Oncol. Lett. 2012, 3, 238–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shevchenko, G.; Morris, K.V. All I’s on the RADAR: Role of ADAR in gene regulation. FEBS Lett. 2018, 592, 2860–2873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kokkat, T.J.; Patel, M.S.; McGarvey, D.; LiVolsi, V.A.; Baloch, Z.W. Archived formalin-fixed paraffin-embedded (FFPE) blocks: A valuable underexploited resource for extraction of DNA, RNA, and protein. Biopreserv. Biobanking 2013, 11, 101–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, H.; Zhu, M.; Cui, F.; Wang, S.; Gao, X.; Lu, S.; Wu, Y.; Zhu, H. Quantitative assessment of short amplicons in FFPE-derived long-chain RNA. Sci. Rep. 2014, 4, 7246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, G.; Huang, K.; Luan, Y.; Cao, Z.; Chen, S.; Ma, B.; Yuan, J.; Wu, X.; Chen, G.; Wang, T.; et al. Human papillomavirus infection among head and neck squamous cell carcinomas in southern China. PLoS ONE 2019, 14, e0221045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, F.; Zeng, Q.; Yu, G.; Li, S.; Wang, C.Y. Wnt/beta-catenin signaling inhibits death receptor-mediated apoptosis and promotes invasive growth of HNSCC. Cell Signal. 2006, 18, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Alamoud, K.A.; Kukuruzinska, M.A. Emerging Insights into Wnt/β-catenin Signaling in Head and Neck Cancer. J. Dent. Res. 2018, 97, 665–673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukusumi, T.; Califano, J.A. The NOTCH Pathway in Head and Neck Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 645–653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Z.L.; Zhang, L.; Huang, C.F.; Ma, S.R.; Bu, L.L.; Liu, J.F.; Yu, G.T.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; et al. NOTCH1 inhibition enhances the efficacy of conventional chemotherapeutic agents by targeting head neck cancer stem cell. Sci. Rep. 2016, 6, 24704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, X.; Sheng, T.; Zhang, Y.; Zhang, X.; He, J.; Huang, S.; Chen, K.; Sultz, J.; Adegboyega, P.A.; Zhang, H.; et al. Hedgehog signaling is activated in subsets of esophageal cancers. Int. J. Cancer 2006, 118, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Tang, Y.L.; Liang, X.H. Transforming growth factor-β signaling in head and neck squamous cell carcinoma: Insights into cellular responses. Oncol. Lett. 2018, 16, 4799–4806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Parameter | Mean ± SEM | p | |
|---|---|---|---|
| Age | <60 | 0.144 ± 0.034 | 0.1374 |
| ≥60 | 0.211 ± 0.029 | ||
| Sex | Female | 0.269 ± 0.042 | 0.0179 |
| Male | 0.149 ± 0.021 | ||
| Alcohol consumption | Yes | 0.186 ± 0.029 | 0.9523 |
| No | 0.189 ± 0.036 | ||
| Smoking | Yes | 0.158 ± 0.029 | 0.1645 |
| No | 0.223 ± 0.036 | ||
| Degree of tumor cell differentiation | G1 + G2 | 0.182 ± 0.026 | 0.7805 |
| G3 + G4 | 0.196 ± 0.047 | ||
| Tumor size | T1 + T2 | 0.197 ± 0.034 | 0.6659 |
| T3 + T4 | 0.176 ± 0.03 | ||
| Presence of cancer cells in lymph nodes | N0 | 0.194 ± 0.031 | 0.6965 |
| N1 + N2 + N3 | 0.176 ± 0.034 | ||
| Perineural space invasion | Yes | 0.215 ± 0.039 | 0.1411 |
| No | 0.135 ± 0.038 | ||
| Degree of cancer cell spread | I + II | 0.185 ± 0.044 | 0.9648 |
| III + IV | 0.183 ± 0.026 | ||
| Cervical lymph node dissection | Yes | 0.147 ± 0.025 | 0.0043 |
| No | 0.31 ± 0.043 | ||
| HPV infection status | Positive | 0.088 ± 0.079 | 0.4388 |
| Negative | 0.175 ± 0.068 | ||
| Parameter | Mean ± SEM | p | |
|---|---|---|---|
| Age | <60 | −0.195 ± 0.057 | 0.6462 |
| ≥60 | −0.146 ± 0.053 | ||
| Sex | Female | 0.066 ± 0.072 | 0.0006 |
| Male | −0.251 ± 0.045 | ||
| Alcohol consumption | Yes | −0.186 ± 0.046 | 0.3195 |
| No | −0.124 ± 0.077 | ||
| Smoking | Yes | −0.283 ± 0.048 | 0.0005 |
| No | 0.01 ± 0.066 | ||
| Degree of tumor cell differentiation | G1 + G2 | −0.112 ± 0.044 | 0.0379 |
| G3 + G4 | −0.264 ± 0.078 | ||
| Tumor size | T1 + T2 | −0.221 ± 0.068 | 0.342 |
| T3 + T4 | −0.134 ± 0.048 | ||
| Presence of cancer cells in lymph nodes | N0 | −0.117 ± 0.057 | 0.2001 |
| N1 + N2 + N3 | −0.211 ± 0.055 | ||
| Perineural space invasion | Yes | −0.08 ± 0.064 | 0.1919 |
| No | −0.221 ± 0.068 | ||
| Degree of cancer cell spread | I + II | −0.147 ± 0.081 | 0.7109 |
| III + IV | −0.174 ± 0.045 | ||
| Cervical lymph node dissection | Yes | −0.148 ± 0.042 | 0.3653 |
| No | −0.247 ± 0.104 | ||
| HPV infection status | Positive | −0.726 ± 0.152 | <0.0001 |
| Negative | 0.004 ± 0.096 | ||
| Parameter | Mean ± SEM | p | |
|---|---|---|---|
| Age | <60 | −0.064 ± 0.139 | 0.613 |
| ≥60 | −0.101 ± 0.11 | ||
| Sex | Female | −0.573 ± 0.123 | 0.0077 |
| Male | 0.086 ± 0.108 | ||
| Alcohol consumption | Yes | 0.04 ± 0.108 | 0.0298 |
| No | −0.339 ± 0.15 | ||
| Smoking | Yes | −0.01 ± 0.109 | 0.1097 |
| No | −0.2 ± 0.149 | ||
| Degree of tumor cell differentiation | G1 + G2 | −0.408 ± 0.087 | 0.0002 |
| G3 + G4 | 0.57 ± 0.208 | ||
| Tumor size | T1 + T2 | −0.276 ± 0.153 | 0.0035 |
| T3 + T4 | −0.269 ± 0.106 | ||
| Presence of cancer cells in lymph nodes | N0 | −0.288 ± 0.114 | 0.099 |
| N1 + N2 + N3 | 0.132 ± 0.136 | ||
| Perineural space invasion | Yes | −0.533 ± 0.109 | 0.1327 |
| No | −0.048 ± 0.146 | ||
| Degree of cancer cell spread | I + II | −0.048 ± 0.18 | 0.791 |
| III + IV | −0.07 ± 0.101 | ||
| Cervical lymph node dissection | Yes | −0.248 ± 0.09 | 0.0054 |
| No | 0.585 ± 0.242 | ||
| HPV infection status | Positive | 2.084 ± 0.338 | <0.0001 |
| Negative | −0.143 ± 0.181 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolenda, T.; Białas, P.; Poter, P.; Janiczek-Polewska, M.; Zapłata, A.; Guglas, K.; Mantaj, P.; Przybyła, A.; Kazimierczak, U.; Leporowska, E.; et al. Expression Profile and Clinical Relevance of ADAR Family Genes in Head and Neck Squamous Cell Carcinoma. Genes 2025, 16, 1316. https://doi.org/10.3390/genes16111316
Kolenda T, Białas P, Poter P, Janiczek-Polewska M, Zapłata A, Guglas K, Mantaj P, Przybyła A, Kazimierczak U, Leporowska E, et al. Expression Profile and Clinical Relevance of ADAR Family Genes in Head and Neck Squamous Cell Carcinoma. Genes. 2025; 16(11):1316. https://doi.org/10.3390/genes16111316
Chicago/Turabian StyleKolenda, Tomasz, Piotr Białas, Paulina Poter, Marlena Janiczek-Polewska, Anna Zapłata, Kacper Guglas, Patrycja Mantaj, Anna Przybyła, Urszula Kazimierczak, Ewa Leporowska, and et al. 2025. "Expression Profile and Clinical Relevance of ADAR Family Genes in Head and Neck Squamous Cell Carcinoma" Genes 16, no. 11: 1316. https://doi.org/10.3390/genes16111316
APA StyleKolenda, T., Białas, P., Poter, P., Janiczek-Polewska, M., Zapłata, A., Guglas, K., Mantaj, P., Przybyła, A., Kazimierczak, U., Leporowska, E., Cybulski, Z., & Teresiak, A. (2025). Expression Profile and Clinical Relevance of ADAR Family Genes in Head and Neck Squamous Cell Carcinoma. Genes, 16(11), 1316. https://doi.org/10.3390/genes16111316






