Artificial Intelligence-Assisted CRISPR/Cas Systems for Targeting Plant Viruses
Abstract
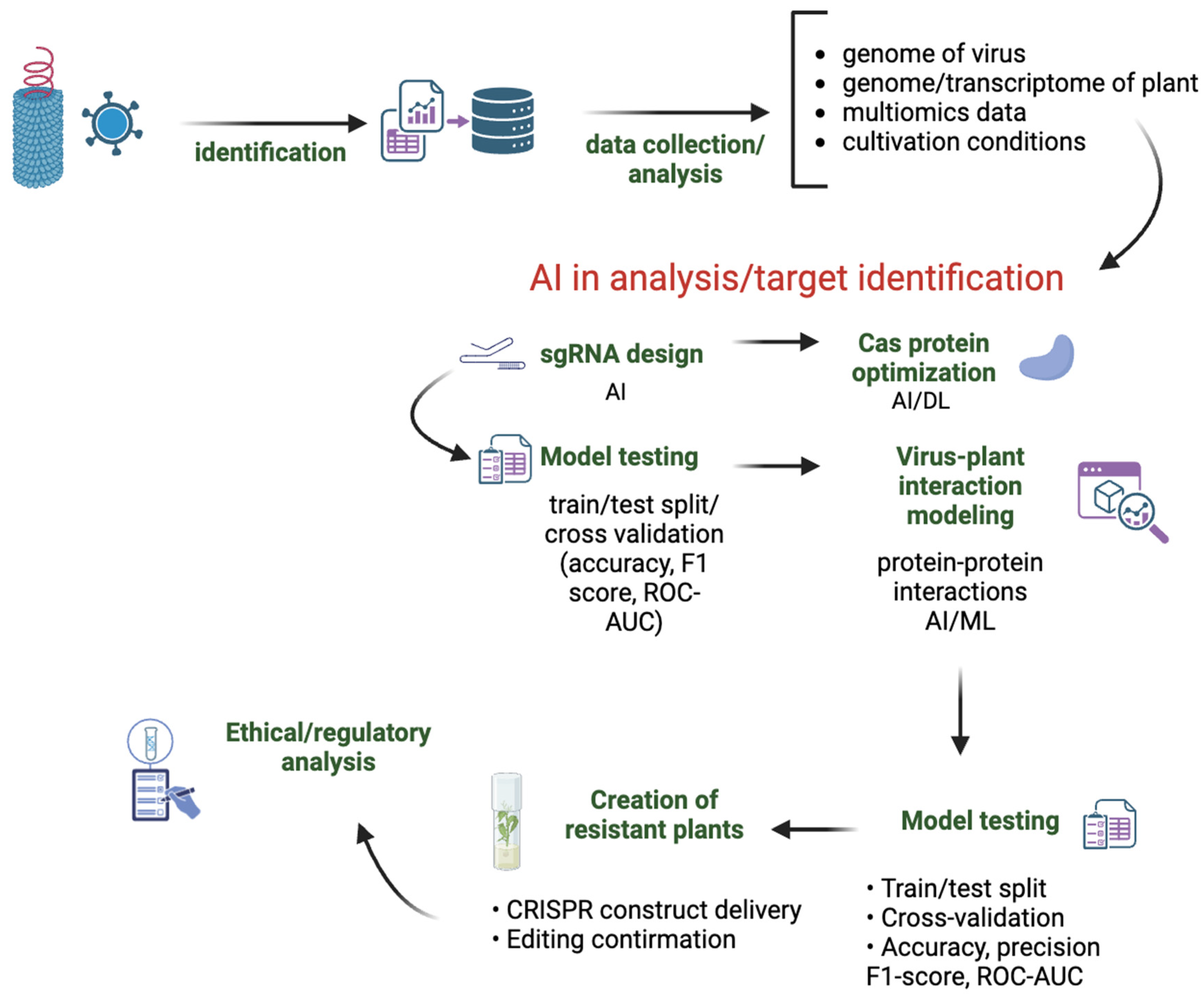
1. Introduction
2. AI-Enhanced CRISPR Strategies Against Plant Viruses
2.1. AI/ML Tools and Algorithms for sgRNA Design
2.2. Modeling and Optimization of Cas Proteins Using AI
2.3. Machine Learning in the Analysis of Virus–Host Interactions
2.4. Using Generative AI Models in CRISPR Design
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.K.; Gupta, O.P.; Pathaw, N.; Sharma, D.; Maibam, A.; Sharma, P.; Sanasam, J.; Karkute, S.G.; Kumar, S.; Bhattacharjee, B. CRISPR-Cas-Led revolution in diagnosis and management of emerging plant viruses: New avenues toward food and nutritional security. Front. Nutr. 2021, 8, 751512. [Google Scholar] [CrossRef]
- Jones, R.A. Global plant virus disease pandemics and epidemics. Plants 2021, 10, 233. [Google Scholar] [CrossRef]
- Ong, S.N.; Taheri, S.; Othman, R.Y.; Teo, C.H. Viral disease of tomato crops (Solanum lycopesicum L.): An overview. J. Plant Dis. Prot. 2020, 127, 725–739. [Google Scholar] [CrossRef]
- Prasad, A.; Sharma, N.; Hari-Gowthem, G.; Muthamilarasan, M.; Prasad, M. Tomato yellow leaf curl virus: Impact, challenges, and management. Trends Plant Sci. 2020, 25, 897–911. [Google Scholar] [CrossRef]
- Brown, J.K.; Khan, Z. Breeding cotton for cotton leaf curl disease resistance. In Cotton Breeding and Biotechnology; CRC Press: Boca Raton, FL, USA, 2020; pp. 171–197. [Google Scholar]
- Shepherd, D.N.; Martin, D.P.; Van der Walt, E.; Dent, K.; Varsani, A.; Rybicki, E.P. Maize streak virus: An old and complex ‘emerging’pathogen. Mol. Plant Pathol. 2010, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iksat, N.; Masalimov, Z.; Omarov, R. Plant virus resistance biotechnological approaches: From genes to the CRISPR/Cas gene editing system. J. Water Land Dev. 2023, 57, 147–158. [Google Scholar] [CrossRef]
- Marqués, M.-C.; Sánchez-Vicente, J.; Ruiz, R.; Montagud-Martínez, R.; Márquez-Costa, R.; Gómez, G.; Carbonell, A.; Daròs, J.-A.; Rodrigo, G. Diagnostics of infections produced by the plant viruses TMV, TEV, and PVX with CRISPR-Cas12 and CRISPR-Cas13. ACS Synth. Biol. 2022, 11, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.E.A.; Tashkandi, M.; Mansoor, S.; Mahfouz, M.M. Engineering plant immunity: Using CRISPR/Cas9 to generate virus resistance. Front. Plant Sci. 2016, 7, 1673. [Google Scholar] [CrossRef]
- Madirov, A.; Iksat, N.; Masalimov, Z. Tomato Bushy Stunt Virus (TBSV): From a Plant Pathogen to a Multifunctional Biotechnology Platform. Viruses 2025, 17, 1268. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant viruses of agricultural importance: Current and future perspectives of virus disease management strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Robertson, G.; Burger, J.; Campa, M. CRISPR/Cas--based tools for the targeted control of plant viruses. Mol. Plant Pathol. 2022, 23, 1701–1718. [Google Scholar] [CrossRef]
- Shahid, M.S.; Sattar, M.N.; Iqbal, Z.; Raza, A.; Al-Sadi, A.M. Next-generation sequencing and the CRISPR-Cas nexus: A molecular plant virology perspective. Front. Microbiol. 2021, 11, 609376. [Google Scholar] [CrossRef]
- Wolter, F.; Puchta, H. The CRISPR/Cas revolution reaches the RNA world: Cas13, a new Swiss Army knife for plant biologists. Plant J. 2018, 94, 767–775. [Google Scholar] [CrossRef]
- Schindele, P.; Wolter, F.; Puchta, H. Transforming plant biology and breeding with CRISPR/Cas9, Cas12 and Cas13. FEBS Lett. 2018, 592, 1954–1967. [Google Scholar] [CrossRef]
- Aksoy, E.; Yildirim, K.; Kavas, M.; Kayihan, C.; Yerlikaya, B.A.; Çalik, I.; Sevgen, I.; Demirel, U. General guidelines for CRISPR/Cas-based genome editing in plants. Mol. Biol. Rep. 2022, 49, 12151–12164. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenkov, G.A.; Rozhnova, N.A.; Kuluev, B.R.; Kiryanova, O.Y.; Gumerova, G.R.; Knyazev, A.V.; Vershinina, Z.R.; Mikhailova, E.V.; Chemeris, D.A.; Matniyazov, R.T.; et al. Design of guide RNA for CRISPR/Cas plant genome editing. Mol. Biol. 2020, 54, 29–50. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, B.; Ding, W.; Liu, X.; Yang, D.-L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y.; et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Koonin, E.V. Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems. Biol. Direct 2011, 6, 38. [Google Scholar] [CrossRef]
- Sandhya, D.; Jogam, P.; Allini, V.R.; Abbagani, S.; Alok, A. The present and potential future methods for delivering CRISPR/Cas9 components in plants. J. Genet. Eng. Biotechnol. 2020, 18, 25. [Google Scholar] [CrossRef]
- Miller, K.; Eggenberger, A.L.; Lee, K.; Liu, F.; Kang, M.; Drent, M.; Ruba, A.; Kirscht, T.; Wang, K.; Jiang, S. An improved biolistic delivery and analysis method for evaluation of DNA and CRISPR-Cas delivery efficacy in plant tissue. Sci. Rep. 2021, 11, 7695. [Google Scholar] [CrossRef]
- Dixit, S.; Kumar, A.; Srinivasan, K.; Vincent, P.D.R.; Ramu Krishnan, N. Advancing genome editing with artificial intelligence: Opportunities, challenges, and future directions. Front. Bioeng. Biotechnol. 2024, 11, 1335901. [Google Scholar] [CrossRef]
- Fong, J.H.; Wong, A.S. Advancing CRISPR/Cas gene editing with machine learning. Curr. Opin. Biomed. Eng. 2023, 28, 100477. [Google Scholar] [CrossRef]
- Sherkatghanad, Z.; Abdar, M.; Charlier, J.; Makarenkov, V. Using traditional machine learning and deep learning methods for on-and off-target prediction in CRISPR/Cas9: A review. Brief. Bioinform. 2023, 24, bbad131. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Go, M.J.; Kang, S.H.; Jeong, S.H.; Lim, K. Revolutionizing CRISPR technology with artificial intelligence. Exp. Mol. Med. 2025, 57, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Chuai, G.; Ma, H.; Yan, J.; Chen, M.; Hong, N.; Xue, D.; Zhou, C.; Zhu, C.; Chen, K.; Duan, B.; et al. DeepCRISPR: Optimized CRISPR guide RNA design by deep learning. Genome Biol. 2018, 19, 80. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Cheng, L.; Luo, Y. An overview and metanalysis of machine and deep learning-based CRISPR gRNA design tools. RNA Biol. 2020, 17, 13–22. [Google Scholar] [CrossRef]
- Xue, L.; Tang, B.; Chen, W.; Luo, J. Prediction of CRISPR sgRNA activity using a deep convolutional neural network. J. Chem. Inf. Model. 2018, 59, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, W.; Xue, L.; Tang, B. Prediction of activity and specificity of CRISPR-Cpf1 using convolutional deep learning neural networks. BMC Bioinform. 2019, 20, 332. [Google Scholar] [CrossRef]
- Zhang, G.; Dai, Z.; Dai, X. C-RNNCrispr: Prediction of CRISPR/Cas9 sgRNA activity using convolutional and recurrent neural networks. Comput. Struct. Biotechnol. J. 2020, 18, 344–354. [Google Scholar] [CrossRef]
- Toufikuzzaman, M.; Hassan Samee, M.A.; Sohel Rahman, M. CRISPR-DIPOFF: An interpretable deep learning approach for CRISPR Cas-9 off-target prediction. Brief. Bioinform. 2024, 25, bbad530. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Peng, J.; Zhang, Z.; Shang, X. R-CRISPR: A deep learning network to predict off-target activities with mismatch, insertion and deletion in CRISPR-Cas9 system. Genes 2021, 12, 1878. [Google Scholar] [CrossRef]
- Wan, Y.; Jiang, Z. TransCrispr: Transformer based hybrid model for predicting CRISPR/Cas9 single guide RNA cleavage efficiency. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 20, 1518–1528. [Google Scholar] [CrossRef]
- Guan, Z.; Jiang, Z. Transformer-based anti-noise models for CRISPR-Cas9 off-target activities prediction. Brief. Bioinform. 2023, 24, bbad127. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, B.; Devi, P.U.M.; Thanjavur, N.; Buddolla, V. The applications of artificial intelligence (AI)-driven tools in virus-like particles (VLPs) research. Curr. Microbiol. 2024, 81, 234. [Google Scholar] [CrossRef]
- Yakimovich, A. Machine learning and Artificial Intelligence for the prediction of host–pathogen interactions: A viral case. Infect. Drug Resist. 2021, 14, 3319–3326. [Google Scholar] [CrossRef] [PubMed]
- Elste, J.; Saini, A.; Mejia-Alvarez, R.; Mejía, A.; Millán-Pacheco, C.; Swanson-Mungerson, M.; Tiwari, V. Significance of artificial intelligence in the study of virus–host cell interactions. Biomolecules 2024, 14, 911. [Google Scholar] [CrossRef]
- Postnikova, O.A.; Nemchinov, L.G. Comparative analysis of microarray data in Arabidopsis transcriptome during compatible interactions with plant viruses. Virol. J. 2021, 9, 101. [Google Scholar] [CrossRef]
- Ray, M.; Burman, S.; Meshram, S. A Mini Review on Plant Immune System Dynamics: Modern Insights into Biotic and Abiotic Stress. Phyton 2025, 94, 2285. [Google Scholar] [CrossRef]
- Chadoulis, R.T.; Livieratos, I.; Manakos, I.; Spanos, T.; Marouni, Z.; Kalogeropoulos, C.; Kotropoulos, C. 3D-CNN detection of systemic symptoms induced by different Potexvirus infections in four Nicotiana benthamiana genotypes using leaf hyperspectral imaging. Plant Methods 2025, 21, 15. [Google Scholar] [CrossRef]
- Chen, H.; Han, Y.; Liu, Y.; Liu, D.; Jiang, L.; Huang, K.; Wang, H.; Guo, L.; Wang, X.; Wang, J.; et al. Classification models for Tobacco Mosaic Virus and Potato Virus Y using hyperspectral and machine learning techniques. Front. Plant Sci. 2023, 14, 1211617. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, Q.; Lu, H.; Li, Y.; Wang, A.; Tian, Q.; Zhan, Q.; Lu, Y.; Zhang, L.; Huang, T.; et al. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat. Genet. 2018, 50, 278–284. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of multi-omics technologies for crop improvement. Front. Plant Sci. 2021, 12, 563953. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 2020, 29, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Panda, A.K.; Datta, S. Critical considerations and computational tools in plant genome editing. Heliyon 2024, 11, e41135. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yu, T.; Rasheed, A.; Wang, J.; Crossa, J.; Hearne, S.; Li, H. Fast-forwarding plant breeding with deep learning-based genomic prediction. J. Integr. Plant Biol. 2025, 67, 1700–1705. [Google Scholar] [CrossRef]
- Kozlov, K.N.; Bankin, M.P.; Semenova, E.A.; Samsonova, M.G. Genomic prediction of plant traits by popular machine learning methods. Vavilov J. Genet. Breed. 2025, 29, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cheng, Z.; Cao, Y. Artificial Intelligence-Assisted Breeding for Plant Disease Resistance. Int. J. Mol. Sci. 2025, 26, 5324. [Google Scholar] [CrossRef]
- Brock, N.; Kaur, N.; Halford, N.G. Advances in genome editing in plants within an evolving regulatory landscape, with a focus on its application in wheat breeding. J. Plant Biochem. Biotechnol. 2025, 34, 599–614. [Google Scholar] [CrossRef]
- Arndell, T.; Sharma, N.; Langridge, P.; Baumann, U.; Watson-Haigh, N.S.; Whitford, R. gRNA validation for wheat genome editing with the CRISPR-Cas9 system. BMC Biotechnol. 2019, 19, 71. [Google Scholar] [CrossRef]
- Zitnik, M.; Nguyen, F.; Wang, B.; Leskovec, J.; Goldenberg, A.; Hoffman, M.M. Machine Learning for Integrating Data in Biology and Medicine: Principles, Practice, and Opportunities. Inf. Fusion 2019, 50, 71–91. [Google Scholar] [CrossRef]
- Uddin, M.; Wang, Y.; Woodbury-Smith, M. Artificial intelligence for precision medicine in neurodevelopmental disorders. Npj Digit. Med. 2019, 2, 112. [Google Scholar] [CrossRef]
- Niu, M.; Lin, Y.; Zou, Q. sgRNACNN: Identifying sgRNA on-target activity in four crops using ensembles of convolutional neural networks. Plant Mol. Biol. 2021, 105, 483–495. [Google Scholar] [CrossRef]
- Son, H. Harnessing CRISPR/Cas Systems for DNA and RNA Detection: Principles, Techniques, and Challenges. Biosensors 2024, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cheng, X.; Chen, C.; Heidari, A.A.; Liu, J.; Cai, Z.; Chen, H. Apple leaf disease recognition method with improved residual network. Multimed. Tools Appl. 2022, 81, 7759–7782. [Google Scholar] [CrossRef]
- Zhang, G.; Dai, Z.; Dai, X. A Novel Hybrid CNN-SVR for CRISPR/Cas9 Guide RNA Activity Prediction. Front. Genet. 2020, 10, 1303. [Google Scholar] [CrossRef]
- Zhang, G.; Luo, Y.; Dai, X.; Dai, Z. Benchmarking deep learning methods for predicting CRISPR/Cas9 sgRNA on-and off-target activities. Brief. Bioinform. 2023, 24, bbad333. [Google Scholar] [CrossRef] [PubMed]
- Das, B.S.; Mishra, B.S.P.; Tiwar, A.K.; Panda, B. Optimal Trained Deep LSTM Model for Detecting Plant Leave Diseases. Int. J. Eng. Res. Technol. (IJERT) 2023, 12. [Google Scholar] [CrossRef]
- Jasieniecka, A.; Domingues, I. CRISPR-Cas9 and Its Bioinformatics Tools: A Systematic Review. Curr. Issues Mol. Biol. 2025, 47, 307. [Google Scholar] [CrossRef]
- Kim, H.K.; Min, S.; Song, M.; Jung, S.; Choi, J.W.; Kim, Y.; Lee, S.; Yoon, S.; Kim, H. Deep learning improves prediction of CRISPR–Cpf1 guide RNA activity. Nat. Biotechnol. 2018, 36, 239–241. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Y.; Wang, H.; Liu, H.; Zhou, J.; Chen, J.; Lei, J.; Sun, Z.; Paek, C.; Yin, L. Highly parallel profiling of the activities and specificities of Cas12a variants in human cells. Nat. Commun. 2025, 16, 3022. [Google Scholar] [CrossRef]
- Li, C.; Zou, Q.; Li, J.; Feng, H. Prediction of CRISPR-Cas9 on-target activity based on a hybrid neural network. Comput. Struct. Biotechnol. J. 2025, 27, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Zou, Q.; Ruan, Y.; Feng, H. Prediction of CRISPR-Cas9 off-target activities with mismatches and indels based on hybrid neural network. Comput. Struct. Biotechnol. J. 2023, 21, 5039–5048. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Anju, T.; Kumar, S.; Chhapekar, S.S.; Sreedharan, S.; Singh, S.; Choi, S.R.; Ramchiary, N.; Lim, Y.P. Integrating omics and gene editing tools for rapid improvement of traditional food plants for diversified and sustainable food security. Int. J. Mol. Sci. 2021, 22, 8093. [Google Scholar] [CrossRef]
- Chari, R.; Yeo, N.C.; Chavez, A.; Church, G.M. sgRNA Scorer 2.0: A Species-Independent Model To Predict CRISPR/Cas9 Activity. ACS Synth. Biol. 2017, 6, 902–904. [Google Scholar] [CrossRef]
- Naim, F.; Shand, K.; Hayashi, S.; O’Brien, M.; McGree, J.; Johnson, A.A.T.; Dugdale, B.; Waterhouse, P.M. Are the current gRNA ranking prediction algorithms useful for genome editing in plants? PLoS ONE 2020, 15, e0227994. [Google Scholar] [CrossRef]
- Ali, Z.; Mahfouz, M.M. CRISPR/Cas systems versus plant viruses: Engineering plant immunity and beyond. Plant Physiol. 2021, 186, 1770–1785. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, L.; Ntui, V.O.; Tripathi, J.N.; Kumar, P.L. Application of CRISPR/Cas for diagnosis and management of viral diseases of banana. Front. Microbiol. 2021, 11, 609784. [Google Scholar] [CrossRef]
- Ali, Z.; Abulfaraj, A.; Idris, A.; Ali, S.; Tashkandi, M.; Mahfouz, M.M. CRISPR/Cas9-mediated viral interference in plants. Genome Biol. 2015, 16, 238. [Google Scholar] [CrossRef]
- Maree, H.J.; Gardner, H.F.; Freeborough, M.J.; Burger, J.T. Mapping of the 5′ terminal nucleotides of Grapevine leafroll-associated virus 3 sgRNAs. Virus Res. 2010, 151, 252–255. [Google Scholar] [CrossRef]
- Liu, J.; Carino, E.; Bera, S.; Gao, F.; May, J.P.; Simon, A.E. Structural analysis and whole genome mapping of a new type of plant virus subviral RNA: Umbravirus-like associated RNAs. Viruses 2021, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Simón, A.; García, C.; Velasco, L.; Janssen, D. First natural crossover recombination between two distinct species of the family Closteroviridae leads to the emergence of a new disease. PLoS ONE 2018, 13, e0198228. [Google Scholar] [CrossRef]
- Zaidi, S.S.E.A.; Mahas, A.; Vanderschuren, H.; Mahfouz, M.M. Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol. 2020, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Wessels, H.-H.; Stirn, A.; Méndez-Mancilla, A.; Kim, E.J.; Hart, S.K.; Knowles, D.A.; Sanjana, N.E. Prediction of on-target and off-target activity of CRISPR–Cas13d guide RNAs using deep learning. Nat. Biotechnol. 2024, 42, 628–637. [Google Scholar] [CrossRef]
- Montague, T.G.; Cruz, J.M.; Gagnon, J.A.; Church, G.M.; Valen, E. CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014, 42, W401–W407. [Google Scholar] [CrossRef]
- Shashikala, T.; Nagesha, S.; Asokan, R.; Venkataravanappa, V.; Shyamalamma, S. Designing and Validation of sgRNA for CRISPR/Cas12a-based Diagnosis of Tomato Leaf Curl Virus (ToLCV). Mysore J. Agric. Sci. 2025, 59, 302–310. [Google Scholar]
- Bae, S.; Park, J.; Kim, J.-S. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef]
- Wang, X.; Tu, M.; Wang, Y.; Yin, W.; Zhang, Y.; Wu, H.; Gu, Y.; Li, Z.; Xi, Z.; Wang, X. Whole-genome sequencing reveals rare off-target mutations in CRISPR/Cas9-edited grapevine. Hortic. Res. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Q.; Zhong, Z.; Liu, G.; Han, Y.; Bao, Y.; Liu, L.; Xiang, S.; Liu, S.; Tang, X.; et al. Genome-wide analyses of PAM-relaxed Cas9 genome editors reveal substantial off-target effects by ABE8e in rice. Plant Biotechnol. J. 2022, 20, 1670–1682. [Google Scholar] [CrossRef]
- Xu, W.; Fu, W.; Zhu, P.; Li, Z.; Wang, C.; Wang, C.; Zhang, Y.; Zhu, S. Comprehensive analysis of CRISPR/Cas9-mediated mutagenesis in arabidopsis thaliana by genome-wide sequencing. Int. J. Mol. Sci. 2019, 20, 4125. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Kumar, S.; Mishra, D.C.; Chaturvedi, K.K.; Paul, R.K.; Kairi, A. Machine learning in the estimation of CRISPR-Cas9 cleavage sites for plant system. Front. Genet. 2023, 13, 1085332. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. Deep learning in CRISPR-Cas systems: A review of recent studies. Front. Bioeng. Biotechnol. 2023, 11, 1226182. [Google Scholar] [CrossRef] [PubMed]
- Murmu, S.; Chaurasia, H.; Guha Majumdar, S.; Rao, A.R.; Rai, A.; Archak, S. Prediction of protein–protein interactions between anti-CRISPR and CRISPR-Cas using machine learning technique. J. Plant Biochem. Biotechnol. 2023, 32, 818–830. [Google Scholar] [CrossRef]
- Fang, Z.; Feng, T.; Zhou, H.; Chen, M. DeePVP: Identification and classification of phage virion proteins using deep learning. GigaScience 2022, 11, giac076. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Q.; Yi, X.; An, H.; Zhao, Y.; Ma, S.; Zhou, G. Establishing RNA virus resistance in plants by harnessing CRISPR immune system. Plant Biotechnol. J. 2018, 16, 1415–1423. [Google Scholar] [CrossRef]
- Yin, K.; Han, T.; Xie, K.; Zhao, J.; Song, J.; Liu, Y. Engineer complete resistance to Cotton Leaf Curl Multan virus by the CRISPR/Cas9 system in Nicotiana benthamiana. Phytopathol. Res. 2019, 1, 9. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, H.; Zhou, X.; Li, F. Conferring resistance to plant RNA viruses with the CRISPR/CasRx system. Virol. Sin. 2021, 36, 814–817. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, H.; Zhang, Y.; Wang, Y.; Gao, C. Establishing a CRISPR–Cas-like immune system conferring DNA virus resistance in plants. Nat. Plants 2015, 1, 15144. [Google Scholar] [CrossRef]
- Ghorbani Faal, P.; Farsi, M.; Seifi, A.; Mirshamsi Kakhki, A. Virus-induced CRISPR-Cas9 system improved resistance against tomato yellow leaf curl virus. Mol. Biol. Rep. 2020, 47, 3369–3376. [Google Scholar] [CrossRef]
- Xiang, Y.; Dong, X. Translational Regulation of Plant Stress Responses: Mechanisms, Pathways, and Applications in Bioengineering. Annu. Rev. Phytopathol. 2025, 63, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Zorzatto, C.; Machado, J.P.B.; Lopes, K.V.G.; Nascimento, K.J.T.; Pereira, W.A.; Brustolini, O.J.B.; Reis, P.A.B.; Calil, I.P.; Deguchi, M.; Sachetto-Martins, G.; et al. NIK1-mediated translation suppression functions as a plant antiviral immunity mechanism. Nature 2015, 520, 679–682. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, M.; Sun, Y.; Chen, Z.; Chen, Z.; Wang, L.; Cui, J.-R.; Zhang, F.; Lv, D.; Chen, X.; et al. protects the receptor-like kinase BIR2 from SNIPER2a/b-mediated degradation to promote pattern-triggered immunity in Nicotiana benthamiana. Plant Cell 2023, 35, 3566–3584. [Google Scholar] [CrossRef]
- Wen, Z.; Hu, R.; Pi, Q.; Zhang, D.; Duan, J.; Li, Z.; Li, Q.; Zhao, X.; Yang, M.; Zhao, X.; et al. DEAD-box RNA helicase RH20 positively regulates RNAi-based antiviral immunity in plants by associating with SGS3/RDR6 bodies. Plant Biotechnol. J. 2024, 22, 3295–3311. [Google Scholar] [CrossRef] [PubMed]
- Uranga, M.; Daròs, J.A. Tools and targets: The dual role of plant viruses in CRISPR–Cas genome editing. Plant Genome 2023, 16, e20220. [Google Scholar] [CrossRef]
- Ghosh, D.; Chakraborty, S.; Kodamana, H.; Chakraborty, S. Application of machine learning in understanding plant virus pathogenesis: Trends and perspectives on emergence, diagnosis, host-virus interplay and management. Virol. J. 2022, 19, 42. [Google Scholar] [CrossRef]
- Peng, Y.; Dallas, M.M.; Ascencio-Ibáñez, J.T.; Hoyer, J.S.; Legg, J.; Hanley-Bowdoin, L.; Bruce, G.; Yin, H. Early detection of plant virus infection using multispectral imaging and spatial–spectral machine learning. Sci. Rep. 2022, 12, 3113. [Google Scholar] [CrossRef] [PubMed]
- Breves, S.S.; Silva, F.A.; Euclydes, N.C.; Saia, T.F.; Jean-Baptiste, J.; Andrade Neto, E.R.; Fontes, E.P. Begomovirus–host interactions: Viral proteins orchestrating intra and intercellular transport of viral DNA while suppressing host defense mechanisms. Viruses 2023, 15, 1593. [Google Scholar] [CrossRef]
- Yadav, S.; Chhibbar, A.K. Plant–virus interactions. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–77. [Google Scholar]
- Hipper, C.; Brault, V.; Ziegler-Graff, V.; Revers, F. Viral and cellular factors involved in phloem transport of plant viruses. Front. Plant Sci. 2013, 4, 154. [Google Scholar] [CrossRef] [PubMed]
- Iksat, N.; Madirov, A.; Artykbayeva, D.; Shevchenko, O.; Zhanassova, K.; Baikarayev, Z.; Masalimov, Z. Heat Stress Induces Partial Resistance to Tomato Bushy Stunt Virus in Nicotiana benthamiana Via Combined Stress Pathways. Viruses 2025, 17, 1250. [Google Scholar] [CrossRef]
- Kamal, H.; Minhas, F.A.; Tripathi, D.; Abbasi, W.A.; Hamza, M.; Mustafa, R.; Khan, M.Z.; Mansoor, S.; Pappu, H.R.; Amin, I. βC1, pathogenicity determinant encoded by Cotton leaf curl Multan betasatellite, interacts with calmodulin-like protein 11 (Gh-CML11) in Gossypium hirsutum. PLoS ONE 2019, 14, e0225876. [Google Scholar] [CrossRef]
- Shukla, D.; Alanazi, A.M.; Panda, S.P.; Dwivedi, V.D.; Kamal, M.A. Unveiling the antiviral potential of Plant compounds from the Meliaceae family against the Zika virus through QSAR modeling and MD simulation analysis. J. Biomol. Struct. Dyn. 2024, 42, 11064–11079. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.N.; Fazeel, A.; Ibrahim, M.A.; Dengel, A.; Ahmed, S. MP-VHPPI: Meta predictor for viral host protein-protein interaction prediction in multiple hosts and viruses. Front. Med. 2022, 9, 1025887. [Google Scholar] [CrossRef] [PubMed]
- Murmu, S.; Chaurasia, H.; Rao, A.; Rai, A.; Jaiswal, S.; Bharadwaj, A.; Yadav, R.; Archak, S. PlantPathoPPI: An Ensemble-based Machine Learning Architecture for Prediction of Protein-Protein Interactions between Plants and Pathogens. J. Mol. Biol. 2025, 437, 169093. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Liu, T.; Feng, H.; He, Z.; Li, F.; Zhao, J.; Liu, H. CBIL-VHPLI: A model for predicting viral-host protein-lncRNA interactions based on machine learning and transfer learning. Sci. Rep. 2024, 14, 17549. [Google Scholar] [CrossRef]
- Gu, Q.; Sheng, L.; Zhang, T.; Lu, Y.; Zhang, Z.; Zheng, K.; Hu, H.; Zhou, H. Early detection of tomato spotted wilt virus infection in tobacco using the hyperspectral imaging technique and machine learning algorithms. Comput. Electron. Agric. 2019, 167, 105066. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, J.; Chen, J.; Shao, T.; Ni, H.; Cai, H. Deep transfer learning-based computer vision for real-time harvest period classification and impurity detection of Porphyra haitnensis. Aquac. Int. 2024, 32, 5171–5198. [Google Scholar] [CrossRef]
- Bhaskar, N.; Tupe-Waghmare, P.; Shetty, P.; Shetty, S.S.; Rai, T. A deep learning hybrid approach for automated leaf disease identification in paddy crops. In Proceedings of the 2024 International Conference on Distributed Computing and Optimization Techniques (ICDCOT), Bangalore, India, 15–16 March 2024; pp. 1–5. [Google Scholar]
- Soni, A.; Kushvaha, R.P.; Snehi, S.K. Current strategies for management of plant viruses and future perspectives: Enhancing crop health, yield and productivity. Asian J. Biochem. Genet. Mol. Biol. 2021, 16, 21–34. [Google Scholar] [CrossRef]
- Kasi Viswanath, K.; Hamid, A.; Ateka, E.; Pappu, H.R. CRISPR/Cas, multiomics, and RNA interference in virus disease management. Phytopathology 2023, 113, 1661–1676. [Google Scholar] [CrossRef]
- Tugrul, B.; Elfatimi, E.; Eryigit, R. Convolutional neural networks in detection of plant leaf diseases: A review. Agriculture 2022, 12, 1192. [Google Scholar] [CrossRef]
- Ashtagi, R.; Mane, D.; Deore, M.; Maranur, J.R.; Hosmani, S. Combined deep learning and machine learning models for the prediction of stages of melanoma. J. Auton. Intell. 2024, 7. [Google Scholar] [CrossRef]
- Ghosh, D.; Malavika, M.; Chakraborty, S. Impact of viral silencing suppressors on plant viral synergism: A global agro-economic concern. Appl. Microbiol. Biotechnol. 2021, 105, 6301–6313. [Google Scholar] [CrossRef] [PubMed]
- Zhanassova, K.; Satkanov, M.; Samat, A.; Iksat, N.; Bekturova, A.; Zhamanbayeva, M.; Kurmanbayeva, A.; Masalimov, Z. Short-term high temperature stress in plants: Stress markers and cell signaling. Casp. J. Environ. Sci. 2025, 23, 805–844. [Google Scholar]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Applying and improving AlphaFold at CASP14. Proteins Struct. Funct. Bioinform. 2021, 89, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; Anishchenko, I.; Humphreys, I.R.; Cong, Q.; Baker, D.; DiMaio, F. Efficient and accurate prediction of protein structure using RoseTTAFold2. BioRxiv 2023. preprint. [Google Scholar] [CrossRef]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wei, H.; You, H.; Liu, J.; Wu, H.; Liu, Y. Engineering CRISPR/Cas Systems for High-Specific Nucleic Acid Detection: Innovations, Challenges and Opportunities. Anal. Sens. 2025. [Google Scholar] [CrossRef]
- Tang, N.; Ji, Q. Miniature CRISPR-Cas12 systems: Mechanisms, engineering, and genome editing applications. ACS Chem. Biol. 2024, 19, 1399–1408. [Google Scholar] [CrossRef]
- Jin, S.; Wu, Q.; Fu, G.; Lu, D.; Wang, F.; Deng, L.; Nie, K. Breaking Evolution’s Ceiling: AI-Powered Protein Engineering. Catalysts 2025, 15, 842. [Google Scholar] [CrossRef]
- Chen, G.; Hou, L.; Li, Z.; Xie, B.; Liu, Y. A new strategy for Cas protein recognition based on graph neural networks and SMILES encoding. Sci. Rep. 2025, 15, 15236. [Google Scholar] [CrossRef]
- Burman, N.; Belukhina, S.; Depardieu, F.; Wilkinson, R.A.; Skutel, M.; Santiago-Frangos, A.; Graham, A.B.; Livenskyi, A.; Chechenina, A.; Morozova, N.; et al. A virally encoded tRNA neutralizes the PARIS antiviral defence system. Nature 2024, 634, 424–431. [Google Scholar] [CrossRef]
- Karimi, M.; Ghorbani, A.; Niazi, A.; Rostami, M.; Tahmasebi, A. CRISPR-Cas13a as a next-generation tool for rapid and precise plant RNA virus diagnostics. Plant Methods 2025, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.G.; Choi, J.; Fuchs, M.F. Predictive modeling of proteins encoded by a plant virus sheds a new light on their structure and inherent multifunctionality. Biomolecules 2024, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, X. Artificial intelligence in de novo protein design. Med. Nov. Technol. Devices 2025, 26, 100366. [Google Scholar] [CrossRef]
| Name | Goal | Model | Learning Type | + | — | Organisms | Metrics | Metrics (Plants) |
|---|---|---|---|---|---|---|---|---|
| sgRNACNN | Prediction of sgRNA efficiency | Hybrid CNN | Deep Learning (Keras/TensorFlow) | Trained on plant data, high accuracy | Domain sensitive, requires transfer learning | A. thaliana, O. sativa, Z. mays, S. lycopersicum | ROC-AUC ~0.85, Spearman ~0.70 | ROC-AUC ~0.85, Spearman ~0.70 |
| Positional classifiers | On-target activity | Gradient Boosting | Classical ML | Interpretability, low computational requirements | They do not take into account 3D chromatin and epigenetics. | O. sativa, Z. mays, A. thaliana | Spearman ~0.55 | Spearman ~0.45–0.55 |
| DeepCpf1 | Prediction of Cas12a activity | CNN + FC Layers | Deep Learning (TensorFlow) | Accuracy for Cas12a | No plant data, reduced accuracy | O. sativa | ROC-AUC ~0.82, R2 ~0.65 | AUC ~0.55–0.60 |
| sgRNA Scorer 2.0 | Predicting gRNA efficiency for Cas9 in different genomes | Gradient Boosting | Classical ML | Simplicity, accessibility | Heuristic model, no plant data | O. sativa, N. benthamiana | Correlation ~0.70 | Correlation ~0.30–0.40 |
| E-CRISP | sgRNA generation and scoring | Rule-based | Rule-based | Simplicity, speed | Does not take epigenetics into account | A. thaliana, O. sativa | Corr ~0.35 | ~0.30–0.35 |
| CHOPCHOP | A universal web tool for sgRNA | Rule-based | Rule-based | Support for many Cas, simplicity | Primitive on-target metrics | N. benthamiana, S. lycopersicum, O. sativa | Corr ~0.30 | Correlation ~0.30 |
| Cas-OFFinder | Off-target search | Exhaustive search algorithm | no ML | Flexibility, PAM support, speed | No consideration of the biocontext | Z. mays, G. max, N. benthamiana | - | Greatly overestimates the risks |
| CRISPR Genome-wide analysis | Genomic screening | Pipeline-based | no ML | Genomic coverage | No ML, not adapted to plants | C. annuum L., O. Sativa, A. thaliana | - | - |
| Cas Protein | Target Virus | Plant | AI | Efficiency | + | — |
|---|---|---|---|---|---|---|
| FnCas9 | CMV, TMV | N. benthamiana, A. thaliana | CNN for assessing FnCas9-RNA interactions | Decreased viral RNA levels, inherited | RNA viruses, no need for DNA editing | Potential off-target for RNA |
| SpCas9 | CLCuMuV | N. benthamiana, A. thaliana | Deep-RPA (1D Convolutional Neural Network) | 99% accuracy in predicting off-target sites | Targeting multiple sites | Risk of mosaic mutation, Limited to model plants |
| SaCas9 (modified) | TYLCV, TMV | S. lycopersicum, N. benthamiana | GUIDE-seq + Deep Learning (CNN for off-target) | Off-target reduction without loss of activity (~80–90%) | Improved specificity; suitable for vectors | Not tested in plants; obtained in animal models |
| CasRx (RfxCas13d) | ssRNA viruses | N. benthamiana | Attention network for RNA structure | Suppression of viral expression by more than 80% | High specificity, small size | HEPN domain activity |
| Cas13a (codon-optimized) | TuMV | N. benthamiana | DeepCodon (DL for codon optimization) | Cas13a expression increased 2.3-fold; viral load decreased | Enhanced expression through DL optimization | Limited to plants with PVX infection |
| Cas12a (modified) | TYLCV | S.lycopersicum | CRISPR-GAN (DL for generating Cas12a variants) | Cas12a activity increased by 40% compared to wild type | Extended PAM profiles | High computational costs |
| Name | Type of Learning | Application | Plante | Target Virus | + | — | Metrics (Accuracy) |
|---|---|---|---|---|---|---|---|
| CBIL-VHPLI | CNN + BiLSTM + Transfer Learning | Prediction of viral protein PPIs from lncRNA | G. hirsutum | CLCuMB | High accuracy, generalizability, adaptability to new data | Requires large training samples, high computational load | Accuracy: 91.6%, Precision: ~93% |
| SVM + Gradient Boosting | ML, transcriptome classification | Determination of resistance markers at the expression level | S.lycopersicum | TSWV | Biomarker detection, integration with climate factors | Sensitive to sampling, requires annotated data | Accuracy: ~89% |
| CNN + Random Forest | Hybrid Deep Learning + ML | Classification of images of viral infection symptoms | N. tabacum | TMV | Resistance to visual noise, high F1 metric | Dependence on image quality and domain | Accuracy: 95%, F1: ~0.94 |
| Decision Tree + SVM | ML on genomic SNP data | Detection of susceptibility alleles (eIF(iso)4G) | O.sativa | RTBV | Detection of functionally significant mutations | Further validation is needed (CRISPR) | F1: ~0.88 |
| AI Model | Goal | Virus/Plant | Efficiency | + | — |
|---|---|---|---|---|---|
| AlphaFold2 | Prediction of 3D structures of Cas proteins (Cas9, Cas12a) and TYLCV virus proteins for the selection of interaction interfaces | TYLCV/S. lycopersicum | RMSD < 2Å, high structural accuracy; used to create stable complexes with targeted mutations | Allows for clarification of Cas protein interactions with viral DNA, speeding up the design cycle | Does not predict time-scale dynamics; subsequent molecular dynamics simulations are required |
| RoseTTAFold | Engineering Cas13a for RNA viruses, modeling the complex structure of Cas13-viral RNA | RTBV/O. sativa | RMSD 1.8–2.3Å; the accuracy of the complex prediction has been confirmed experimentally | Accounting for intermolecular interfaces, support for multi-chain modeling | High computational load, limited training set of plant viruses |
| ProGen2 | Generation of new Cas protein variants with altered PAM recognition | CLCuV/G. hirsutum | ~30% of generated sequences retained functionality in vitro | The possibility of creating non-standard Cas proteins with new specificities | Low accuracy without filtering; further structure validation required |
| ESMFold (Meta AI) | Rapid structure prediction of modified Cas proteins to improve stability in plant cytoplasm | TMV/N. tabacum | MSD ~2.1Å; models were used to screen proteins with high resistance to degradation | high speed, no alignment required | No accurate accounting of interactions with DNA/RNA; requires supplementation with molecular dynamics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iksat, N.; Madirov, A.; Zhanassova, K.; Masalimov, Z. Artificial Intelligence-Assisted CRISPR/Cas Systems for Targeting Plant Viruses. Genes 2025, 16, 1258. https://doi.org/10.3390/genes16111258
Iksat N, Madirov A, Zhanassova K, Masalimov Z. Artificial Intelligence-Assisted CRISPR/Cas Systems for Targeting Plant Viruses. Genes. 2025; 16(11):1258. https://doi.org/10.3390/genes16111258
Chicago/Turabian StyleIksat, Nurgul, Almas Madirov, Kuralay Zhanassova, and Zhaksylyk Masalimov. 2025. "Artificial Intelligence-Assisted CRISPR/Cas Systems for Targeting Plant Viruses" Genes 16, no. 11: 1258. https://doi.org/10.3390/genes16111258
APA StyleIksat, N., Madirov, A., Zhanassova, K., & Masalimov, Z. (2025). Artificial Intelligence-Assisted CRISPR/Cas Systems for Targeting Plant Viruses. Genes, 16(11), 1258. https://doi.org/10.3390/genes16111258






