Abstract
Background: Ethylene is one of the most important plant hormones. ACC oxidase (ACO) plays a vital role in ethylene synthesis and responses to biotic and abiotic stresses in plants. However, its function in Triticum urartu remains unclear. This study aims to systematically identify the members of the TuACO gene family to elucidate its response characteristics and functions under biotic and abiotic stresses. Methods: Through homologous alignment, phylogenetic evolution analysis, and investigations of gene structure and promoter cis-elements, a total of eight TuACO genes were identified in the T. urartu genome based on their homology to OsACO and AtACO protein sequences. Results: These genes were classified into five ACO subfamilies and distributed across chromosomes 1A, 4A, 5A, 6A, and 7A. TuACO gene families contained 0–3 introns and 1–4 exons. The protein sequence contains 10 different conservative motifs. QRT-PCR expression analysis revealed that the transcript levels of TuACO5a, TuACO5b, and TuACO3a were significantly upregulated at 6 and 24 h after infection with powdery mildew, a biotic stress. Under boron deficiency, an abiotic stress, the expression of TuACO6 and TuACO1b increased, whereas the expression of TuACO5b and TuACO3b was markedly induced under high-boron conditions. Conclusions: These results demonstrate that TuACO genes exhibit functional diversification in response to biotic and abiotic stresses, which lays the foundation for elucidating their gene functions.
1. Introduction
Ethylene, one of the most important gaseous hormones, plays an important role in biotic and abiotic stresses responses in plants. The content of ethylene is lower under normal conditions but dramatically elevated during abiotic or biotic stress responses, where downstream genes regulate through the corresponding ethylene signaling pathway, thereby stimulating and initiating a series of cellular responses [1]. For instance, it has been reported that ethylene levels rise rapidly in plants under drought stress [2].
ACC oxidase (ACO) and methionine are key enzymes in the ethylene synthesis and precursors of the ethylene biosynthesis pathways, respectively [3]. Initially, S-adenosyl-L-methionine (SAM) synthase catalyzes the conversion of methionine into SAM under the action of ATP [4]; then, 1-aminocyclopropane-1-carboxylic acid (ACC) does so in the presence of ACC synthase (1-aminocyclopropane-1-carboxylate (ACC) synthase (ACS)) [5,6,7]. Finally, ACC oxidase (1-aminocyclopropane-1-carboxylic acid oxidase, ACO) catalyzes the conversion of ACC into ethylene [8,9]. The cloning of the ACO gene has been widely reported in dicotyledonous plants [10,11,12,13], while progress has been slow in monocotyledonous plants. For instance, seven ACO genes were identified in the barley genome using the ACO gene protein sequence of rice [14]. Additionally, SIACO1, as the first identified ACO gene, has led to the subsequent identification of 9, 13, 5, and 7 ACO genes in the rice, maize, Arabidopsis, and apple genomes, respectively, through its protein sequence. Phylogenetic analysis indicates that these genes are diverged from a common ancestral gene [15].
There are limited reports on the identification and functional studies of ACO genes in wheat and its progenitor species, although ACO genes play an important role in ethylene synthesis and responses to biotic and abiotic stresses. Few examples of the reports include the following: TaACO1, from the wheat cultivar Shanrong 3, had a negative regulatory role in the response to salt stress in Arabidopsis thaliana [16]; and TuACO3 from the T. urartu wheat, in interaction with TuMYB46L, regulated ethylene synthesis and participated in powdery mildew defense response [17]. Therefore, using T. urartu as the experimental material to identify more ACO genes and analyze their functions can deepen our understanding of its stress tolerance mechanisms. In this study, based on the protein sequences of ACO genes from rice and Arabidopsis thaliana, ACO genes in T. urartu wheat will be identified and analyzed for their gene structure, phylogeny, and response patterns to biotic and abiotic stresses. This research will lay the foundation for further investigation into the functions of TuACO genes.
2. Materials and Methods
2.1. Gene Identification and Evolutionary Analysis
According to Hoben’s established research [15], the rice genome contains nine identified ACO genes (OsACO1-OsACO7), with OsACO3 further classified into three subtypes: OsACO3a, OsACO3b, and OsACO3c. In contrast, the Arabidopsis genome comprises five ACO genes (AtACO1-AtACO5). For this study, ACO protein sequences were obtained from the Rice Genome Database (http://rice.uga.edu) and the Arabidopsis Genome Database (https://www.arabidopsis.org/), while the T. urartu ACO gene sequence was sourced from the Wheatomics platform (http://wheatomics.sdau.edu.cn). Using the ACO gene family profile (PF51471) from the Pfam database (http://pfam.xfam.org/) as the seed sequence with an E-value cutoff of <1 × 10−5, candidate ACO proteins were predicted from T. urartu sequences using HMMER 3.0 (http://www.hmmer.org/) under default parameters. Following the removal of incomplete sequences, conserved domain validation was performed using the SMART online tool (http://smart.embl.heidelberg). The molecular weights and isoelectric points of TuACO proteins were analyzed via the ProtParam online platform (https://web.expasy.org), while the subcellular localization of TuACO family members was predicted using Wolfpsort (https://www.genscript.com). Phylogenetic analysis was conducted with MEGA7 software, applying 1000 bootstrap replicates while maintaining default settings for all other parameters.
2.2. Gene Structure, Promoter, Protein Conserved Motif Analysis, Chromosomal Localization, and Expression Patterns
Gene structures were analyzed using GSDS (http://gsds.cbi.pku.edu.cn), while protein conserved motifs were identified with MEME (http://meme-suite.org/tools/meme), configured to detect 10 motifs with lengths of 10–100 amino acids. Cis-acting elements in promoters were predicted using PlantCARE (http://bioinformatics.psb.ugent.Be/webtools/plantcare/html/) and visualized with TBtools (v1.098). Chromosomal locations were mapped using MapInspect. Following Zhang et al. [17], we obtained TuACO gene expression data under powdery mildew induction from Wheatomics (http://wheatomics.sdau.edu.cn) to analyze expression patterns.
2.3. Material Handling
T. urartu wheat was collected from the Department of Plant Pathology, Hebei Agricultural University, China. The inoculation of powdery mildew was conducted referring to the method described by Zhang et al. [17]. The wheat seeds were germinated hydroponically at 22 °C under 16 h of light or 8 h of darkness, followed by inoculation during the one-leaf growth stage. First, the leaves were lightly misted with water. Then, they were uniformly and thoroughly rubbed with seedlings infected with powdery mildew pathotype E09. Samples were collected for qRT-PCR analysis at 0, 6, and 24 h after inoculation with the powdery mildew pathogen.
High- and low-boron stress treatments were conducted using the method described by Leaungthitikanchana et al. [18]. Five days after germination, T. urartu wheat seedlings were transplanted in 2 L brown plastic bottles to be incubated with Hoagland’s culture solution containing 20 nM and 1 m boric acid for 7 days (pH:5.6–6.0), respectively, with artificial climatic chamber conditions of 16 h/day of light, 20–25 °C, and 60–70% relative humidity. The experimental work was set up with a control and three replications for each treatment.
2.4. QRT-PCR Analysis
Total RNA was extracted from T. urartu wheat seedlings and reverse-transcribed into cDNA. SYBR green was used as a fluorescent quantitative dye for quantitative analysis in an ABI ViiA7 (Applied Biosystems, Foster City, CA, USA) instrument. The primers were synthesized by Beijing Qingke Biotechnology Co., Ltd. (Beijing, China). (Table 1). The amplification program was pre-denaturation at 95 °C for 6 min, denaturation at 95 °C for 10 s, and renaturation at 60 °C for 1 min, for 40 cycles. The quantitative fluorescence results were analyzed by 2−∆∆Ct using β-Actin as an internal reference.

Table 1.
Primer sequences for qRT-PCR.
3. Results
3.1. Identification and Physicochemical Properties Analysis of TuACO Gene Family in T. urartu
Based on the protein sequences of nine OsACO genes in rice and five AtACO genes in Arabidopsis thaliana, a total of eight TuACO genes were identified by comparing the T. urartu wheat protein database in Wheatomics (http://wheatomics.sdau.edu.cn) using the Blast P tool (Table 2), including two of TuACO1, TuACO3, and TuACO5 each, and one of TuACO4 and TuACO6 each. Compared with ACO genes in rice and Arabidopsis, TuACO2 and TuACO7 were not identified in the T. urartu genomic sequences of the eight TuACO genes, which varied in length from 927 to 1535 bp, with coding sequence length ranging from 927 to 996 bp, and coding amino acid number ranging from 293 to 331. The isoelectric points of the eight TuACO genes were 4.84–6.56, and the molecular weights of the proteins were 33.55–36.47 kD. All the eight TuACO proteins were hydrophilic proteins located in the cytoplasm.

Table 2.
Identification and physicochemical properties of TuACO gene in Triticum urartu.
3.2. Clustering Analysis of TuACO Genes
Relationship analysis of the eight TuACO genes in T. urartu, nine OsACO genes of rice, and five AtACO genes of Arabidopsis thaliana was performed. Their protein sequences were used to construct an evolutionary tree (Figure 1). The clustering results showed that TUG1812G0500002671.01 and TUG1812G0500002672.01 clustered with OsACO1 and OsACO2; TuG1812G0600003491.01 and TuG1812G0600003493.01 clustered with OsACO3; TuG1812G0400001021.01 clustered with OsACO4; TuG1812G0100001157.01 and TuG1812G0100001158.01 clustered with OsACO5; TuG1812G0700003990.01 clustered with OsACO6; AtACO2, AtACO3, and AtACO4 formed a distinct cluster; while OsACO7 constituted a separate cluster. Therefore, TUG1812G0500002671.01, TuG1812G0500002672.01, TuG1812G0600003491.01, TuG1812G0600003493.01, TuG1812G0700003990.01, TuG1812G0400001021.01, TuG1812G0100001158.01, and TuG1812G0100001157.01 were designated as TuACO1a, TuACO1b, TuACO3a, TuACO3b, TuACO6, TuACO4, TuACO5a, and TuACO5b, respectively. These results indicate that T. urartu exhibits a closer phylogenetic relationship with rice, while showing a more distant relationship with Arabidopsis thaliana. Additionally, an ortholog of OsACO7 appears to be absent in the T. urartu genome.
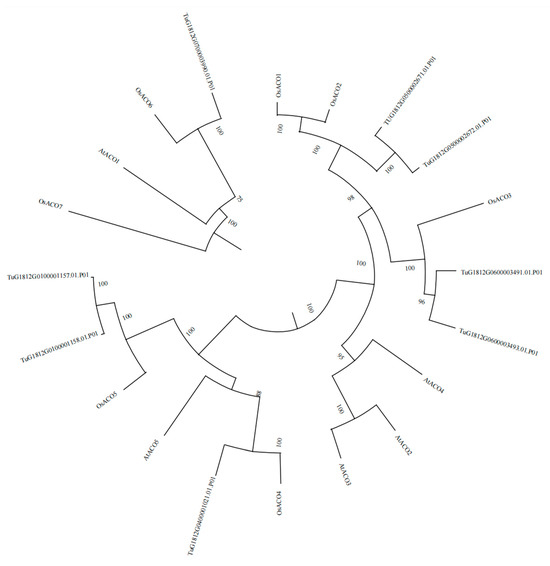
Figure 1.
Analysis of ACO1 gene evolution.
3.3. Chromosomal Localization of TuACO
Based on the physical positions of TuACO genes, eight TuACO genes were mapped to five chromosomes (Figure 2). Specifically, TuACO5a and TuACO5b were located on chromosome 1A; TuACO4 was located on chromosome 4A; TuACO1a and TuACO1b were located on chromosome 5A; TuACO3a and TuACO3b were located on chromosome 6A; and TuACO6 was located on chromosome 7A. The results showed that the distribution of TuACO genes across the chromosomes appeared to be non-uniform. The Ka/Ks ratio (non-synonymous substitution rate/synonymous substitution rate) can reflect a gene’s evolutionary pattern and the selective pressure it has experienced during evolution. In this study, Ka/Ks ratios were used to assess the selection pressure acting on TuACO5a/TuACO5b, TuACO1a/TuACO1b, and TuACO3a/TuACO3b during their evolutionary history. The Ka/Ks values of TuACO5a/TuACO5b, TuACO1a/TuACO1b, and TuACO3a/TuACO3b were 0.10, 0.13, and 0.36, respectively. These results indicated that TuACO5, TuACO2, and TuACO1 underwent strong purifying selection throughout their evolutionary process.
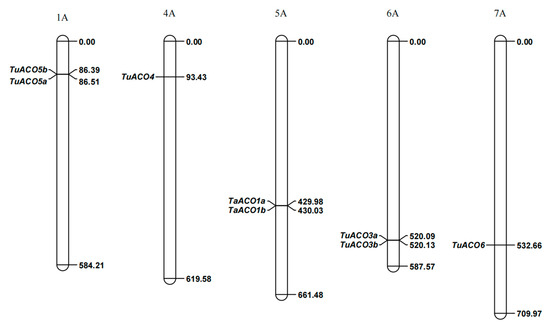
Figure 2.
Chromosome location of TuACO genes.
3.4. The Structure and Protein Conserved Motifs of TuACO
The TuACO gene structure analysis revealed that the number of introns and exons varied significantly. The number of introns ranged from 0 to 3, while the number of exons ranged from 1 to 4 (Figure 3). TuACO4 contained three introns and four exons; TuACO3b, and TuACO6 each contained two introns and three exons; TuACO1a and TuACO1b each contained two exons and one intron; and TuACO3a, TuACO5a and TuACO5b each contained one exon with no intron. These findings indicate substantial structural variation among TuACO genes. On the other hand, the protein conserved motif analysis revealed that the TuACO protein family contained a total of 10 different conserved motifs, with all eight genes containing motifs 1–8, whereas motif 9 was present in TuACO1a and TuACO1b, and motif 10 was only present in TuACO3a and TuACO3b (Figure 4). The functional annotation also showed that motifs 1, 5 and 7 belonged to the 2OG-Fe (II) oxidase superfamily and were typical domains of the ACO gene family. Motif and domain analyses reveal that TuACO gene sequences are relatively conserved.
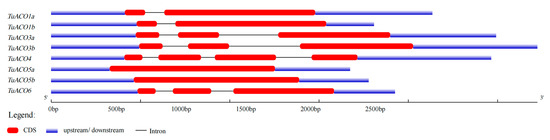
Figure 3.
Intron and exon organization of TuACO.
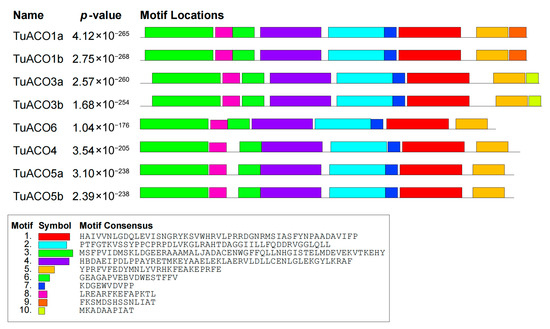
Figure 4.
Conserved motif analysis of TuACO protein.
3.5. Promoter Cis-Acting Primitive Analysis
The cis-element analysis was conducted on the 2000 bp sequence upstream of the start codon of the TuACO gene using the online analysis software PlantCARE (Figure 5). The results indicated that the predicted promoters include ABRE (ABA response), LTR (low-temperature stress), ARE (anaerobic induced correlation), MBS (myb transcription factor binding site), G-box, ACE, MRE, Sp1, and Box4 (photoresponsive element). This result demonstrates the gene’s association with a stress response. ABA-responsive elements are the most abundant, followed by light-responsive elements such as G-box (Figure 6). Among the 8 TuACO genes, TuACO1b contained 16 cis-acting elements, with the ABA response element and myb transcription factor binding sites being the most abundant, while the TuACO6 and TuACO5b had the fewest, with only 4 cis-acting elements (Figure 5). The results suggest potential functional divergence among different TuACO genes.
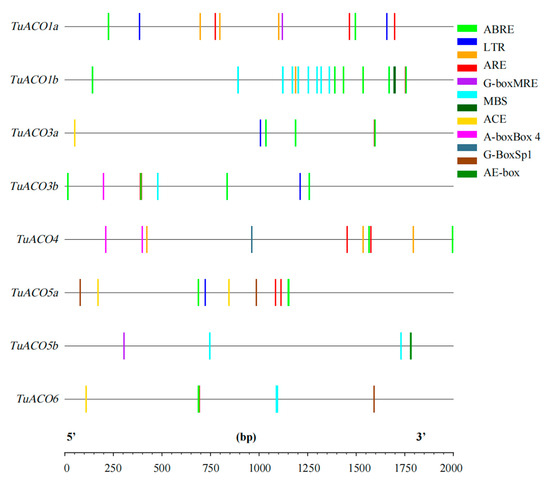
Figure 5.
Cis-elements analysis in the promoter of TuACO gene.
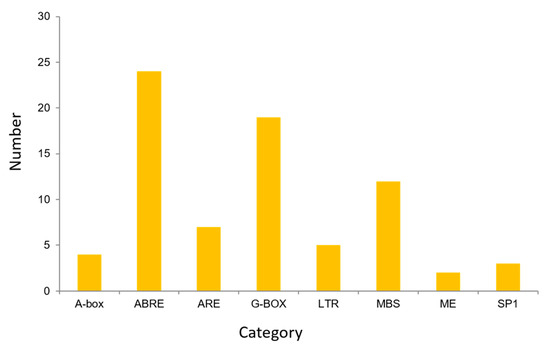
Figure 6.
Cis-elements categories in TuACO gene promoter.
3.6. TuACO Response to Biotic Stress
In order to analyze the response of TuACO gene to biological stress, transcriptome expression data of the TuACO genes in six T. urartu wheat varieties infected by powdery mildew pathogen was obtained from the Wheatomcis website (http://wheatomics.sdau.edu.cn) [17]. The results showed that the expression patterns of the eight TuACO genes were generally similar across the six varieties. At 0 h post-inoculation (hpi), 4 hpi, and 24 hpi with powdery mildew, TuACO5a and TuACO5b consistently exhibited the highest expression levels, while TuACO6, TuACO3b, and TuACO4 showed the lowest expression levels, respectively. The expression levels of different genes varied among different varieties. For example, in variety PI428196, the expression levels of TuACO5a and TuACO5b were significantly upregulated by 4 hpi. In contrast, in varieties PI428193 and PI42888, their expression levels decreased by 4 hpi. In three varieties PI428193, PI428196, and PI428288, TuACO1b was significantly upregulated by 4 hpi, while in the other three varieties, the changes were relatively minor. Specifically, TuACO5a was downregulated in PI428288 4 hpi (Figure 7). The results indicate that TuACO5a and TuACO5b are involved in the powdery mildew stress response in T. urartu.
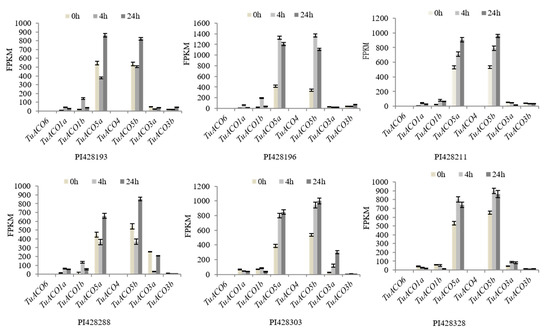
Figure 7.
TuACO response to powdery mildew.
At 0, 6, and 24 hpi, the expression levels of 8 TuACO genes were analyzed using quantitative real-time PCR (qRT-PCR). The qRT-PCR analysis validated the transcriptomic data, showing consistent expression trends for all eight TuACO genes under the conditions of β-actin normalization and primer efficiencies >95% [17]. Following the inoculation of powdery mildew at 6 and 24 h, the expression levels of TuACO5a, TuACO5b, and TuACO3a were significantly upregulated, whereas no significant differences were detected in the other 5 genes (Figure 8). These findings revealed that TuACO5a, TuACO5b, and TuACO3a were involved in the response of T. urartu to powdery mildew. This finding is consistent with the transcriptomic analysis results.
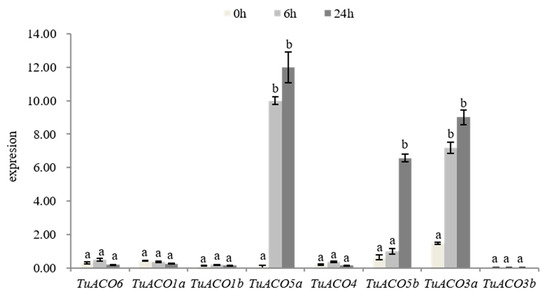
Figure 8.
Expression of TuACO after infection by powdery mildew. Different letters represent statistical difference p < 0.05.
3.7. TuACO Response to Abiotic Stress
The findings demonstrate that ACO1 plays a critical role in plant stress adaptation [15]. Boron is an essential micronutrient in plant growth and development. Elevated boron levels induce toxicity that impairs normal development, while boron deficiency causes infertility in plants. Analysis of the expression of TuACO in T. urartu under high- and low-boron stresses revealed the consistent upregulation of all eight genes under both boron-excess and boron-deficient conditions (Figure 9). Under boron-deficient conditions, TuACO6 and TuACO1b exhibited markedly higher expression levels than under boron-excess conditions, indicating a highly significant differential expression. In contrast, TuACO5b and TuACO3b exhibited the opposite pattern, showing markedly higher expression under high-boron conditions compared to low-boron conditions, and these differences were also highly significant. For TuACO1a, TuACO5a, TuACO4, and TuACO3a, there was no significant difference in the expression levels between the high- and low-boron conditions. The results demonstrated functional divergence among TuACO genes under boron stress: TuACO6 and TuACO1b respond to boron deficiency, while TuACO5b and TuACO3b are induced under high-boron conditions.
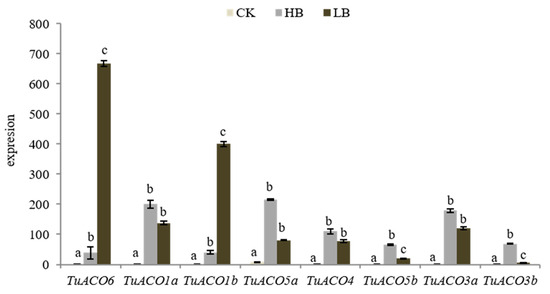
Figure 9.
Expression of TuACO under high B and low B. Different letters represent statistical difference p < 0.05.
4. Discussion
4.1. The TuACO Genes Exhibit Significant Structural Diversity During Evolution While Maintaining Conserved Sequence Architecture
The ACO genes belong to the heme-dependent dioxygenase (2OGD) superfamily. Genomic analyses revealed the presence of 9, 13, 5, 7, and 7 ACO genes in rice (Oryza sativa), maize (Zea mays), Arabidopsis (Arabidopsis thaliana), apple (Malus domestica), and barley (Hordeum vulgare), respectively. Notably, three isoforms of OsACO3 exist in rice [14,15]. Based on the ACO protein sequences from rice and Arabidopsis, this study identified eight TuACO genes in the T. urartu genome. Gene structure analysis revealed that TuACO genes contain 0–2 introns and 1–4 exons, indicating structural divergence during evolution. Clustering analysis demonstrated that TuACO1, TuACO3, and TuACO5 each have two subtypes, consistent with the OsACO3 pattern in rice, suggesting gene duplication events in ACO evolution. Phylogenetic analysis classified T. urartu TuACO genes into five subgroups, compared to six subgroups for rice OsACO and three for Arabidopsis AtACO genes, revealing higher variation diversity in T. urartu and rice than in Arabidopsis, which aligns with previous findings [18]. Protein motif prediction confirmed that all eight TuACO genes contain multiple characteristic sequences belonging to the 2OG-Fe(II) oxygenase superfamily. Notably, TuACO1a/TuACO1b and TuACO3a/TuACO3b possess motifs 9 and 10, respectively, unlike TuACO4, TuACO6, TuACO1a and TuACO1b, demonstrating the conservation of core motifs and structural domains during evolution.
4.2. The Distribution of Core Promoter Elements Varies Significantly Among Different TuACO Genes
In rice, OsACO1, OsACO2, OsACO3, OsACO5, and OsACO7 are induced by light, ethylene, auxin, cytokinin, gibberellin, and abscisic acid to participate in diverse biological developmental processes and abiotic stress responses [19,20,21]. Promoter cis-acting element analysis revealed that TuACO gene promoters contained multiple stress-associated core regulatory elements, including the hormone-responsive element, ABRE; low-temperature stress element, LTR; and light-responsive elements, G-box, ACE, MRE, Sp1, and Box 4 (Figure 5). MYB transcription factors, known for their broad involvement in plant development and stress regulation, were found to have abundant binding sites in TuACO promoters. Additionally, anaerobic response elements (ARE) were identified in this trial, and the presence of multiple elements in the promoter is consistent with the TuACO gene’s broad involvement in responses to both biotic and abiotic stresses. Among the eight TuACO genes, TuACO1b contains up to 16 cis-acting elements, with the highest abundance of ABA-responsive elements and MYB transcription factor binding sites. In contrast, TuACO6 and TuACO5b possess only four elements each. This differential distribution of core promoter elements among TuACO genes aligns with their involvement in diverse stress responses.
4.3. Functional Divergence of TuACO Genes in Response to Biotic and Abiotic Stresses
Ethylene, as a gaseous plant hormone, plays a vital role in the regulation of plant abiotic stress adaptation [22,23]. The ACO is the key gene to the conversion of ACC to ethylene [24]. In deepwater rice, the levels of OsACO1 transcripts and enzyme activity were increased by flooding [25]. In etiolated seedlings, the expression levels of OsACO3 and OsACO2 were affected by auxin and ethylene [20]. Increasing the expression levels of LeACO1 and LeACO4 effectively enhances the brassinosteroid stress response in peach (Prunus persica) [18]. TaACO1 negatively regulates the response of salt stress in Arabidopsis thaliana [13]. TuACO3 plays a significant role in the regulation of powdery mildew resistance [17]. The expression of OsACO2 in rice increases upon infection with Magnaporthe oryzae, enhancing ethylene biosynthesis and consequently improving resistance to rice blast [26]. Following powdery mildew (Blumeria graminis) inoculation, TuACO3 expression increases across diverse T. urartu accessions, correlating with elevated ethylene levels. CRISPR-mediated TuACO3 knockout reduces both ethylene content and powdery mildew resistance. In this study, the expression levels of TuACO5a, TuACO5b, and TuACO1b were upregulated at 0 h post-powdery mildew inoculation (hpi) and at 4 h and 24 h post-inoculation. For instance, TuACO6, TuACO3b, and TuACO4 do not appear to be involved in the response to the powdery mildew pathogen. Further validation of expression patterns demonstrated consistent transcriptional profiles at 0, 6, and 24 h post-inoculation with powdery mildew, aligning with previously reported findings [17].
Boron is an essential nutrient element for plant growth and development [18,27]. This study analyzed the expression profiles of 8 TuACO genes under high-boron and low-boron stress. The results showed that all 8 TuACO genes were upregulated under both high-boron and low-boron conditions, but they exhibited different responses to high-boron and low-boron stress. Under low-boron conditions, TuACO6 and TuACO1b displayed higher expression levels, indicating their sensitivity to low-boron stress. Under high-boron conditions, TuACO5b and TuACO3b exhibited higher expression levels, indicating their sensitivity to high-boron stress. The expression levels of TuACO1a, TuACO5a, TuACO4, and TuACO3a increased under both high-boron and low-boron conditions, though the differences were not statistically significant, suggesting that these four genes are involved in the response to both high-boron and low-boron stress. The differential expression patterns of various TuACO genes under high-boron and low-boron stress imply that changes in their coding sequences may lead to functional divergence; however, this hypothesis requires further experimental validation.
In this study, eight TuACO genes were identified in T. urartu, and their functions in response to biotic and abiotic stresses were validated. Given that T. urartu serves as the A-genome donor of common wheat, this research will establish an important theoretical foundation and provide valuable genetic resources for future disease resistance and stress tolerance breeding.
Author Contributions
Design and supervision: M.L. and Z.L.; Experimental design and execution: X.L. and S.W.; Data analysis: X.W. and P.G.; Manuscript revision: T.W.G. and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32161143007, 32372111 and 32572342) and the Henan Provincial Science and Technology Tackling Project (232102110239).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We sincerely appreciate the dedicated efforts of all individuals involved in the experiments. Special thanks are extended to Zhao Xinyang, Li Yueting, Niu Shijie, and Li Jiawang from the College of Plant Protection, Hebei Agricultural University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, J.; Li, C.Y. Progress of phytohormone research in the 70 years since the founding of New China. Sci. China Life Sci. 2019, 49, 1227–1281. [Google Scholar]
- Pierik, R.; Sasidharan, R.; Voesenek, L.A.C.J. Growth control by ethylene: Adjusting phenotypes to the environment. J. Plant Growth Regul. 2007, 26, 188–200. [Google Scholar] [CrossRef]
- Lieberman, M.; Kunishi, A.; Mapson, L.; Wardale, D.A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966, 44, 376–382. [Google Scholar] [CrossRef]
- Adams, D.O.; Yang, S.O. Methionine metabolism in apple tissue: Implication of s-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol. 1977, 60, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Murr, D.P.; Yang, S.F. Conversion of 5′-methylthioadenosine to methionine by apple tissue. Phytochemistry 1975, 14, 1291–1292. [Google Scholar] [CrossRef]
- Adams, D.O.; Yang, S.F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 1979, 76, 170–174. [Google Scholar] [CrossRef]
- Boller, T.; Herner, R.C.; Kende, H. Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid. Planta 1979, 145, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.J.; Lycett, G.W.; Grierson, D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 1990, 346, 284–287. [Google Scholar] [CrossRef]
- Ververidis, P.; John, P. Complete recovery in vitro of ethylene-forming enzyme activity. Phytochemistry 1991, 30, 725–727. [Google Scholar] [CrossRef]
- Xu, Z.C. Differential expression of ACC oxidase gene in different tissues of ripe kiwifruit. J. Univ. Sci. Technol. China 2001, 31, 235–240. [Google Scholar]
- Zhang, X.S.; Zhong, H.W.; Lu, C.; Huang, X.; Cao, Z.X. Pollination-induced ethylene synthesis and ACC oxidase gene expression in Phalaenopsis pistils. Acta Bot. Sin. 1996, 38, 375–378. [Google Scholar]
- Binnie, J.E.; McManus, M.T. Characterization of the 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase multigene family of Malus domestica Borkh. Phytochemistry 2009, 70, 348–360. [Google Scholar] [CrossRef]
- Chen, B.C.; McManus, M.T. Expression of 1-aminocyclopropane-1-carboxylate (ACC) oxidase genes during the development of vegetative tissues in white clover (Trifolium repens L.) is regulated by ontological cues. Plant Mol. Biol. 2006, 60, 451–467. [Google Scholar] [CrossRef]
- Dahleen, L.S.; Tyagi, N.; Bregitzer, P.; Ryan, H.B.; William, C.M. Developing tools for investigating the multiple roles of ethylene: Identification and mapping genes for ethylene biosynthesis and reception in barley. Mol. Genet. Genom. 2012, 287, 793–802. [Google Scholar] [CrossRef]
- Houben, M.; Poel, B.V. 1-aminocyclopropane-1-carboxylic acid oxidase (ACO): The enzyme that makes the plant hormone ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef]
- Chen, D.H.; Ma, X.Y.; Li, C.L.; Zhang, W.; Xia, G.M.; Wang, M. A wheat aminocyclopropane-1-carboxylate oxidase gene, TaACO1, negatively regulates salinity stress in Arabidopsis thaliana. Plant Cell Rep. 2014, 33, 1815–1827. [Google Scholar] [CrossRef]
- Zhang, J.C.; Zheng, H.Y.; Li, Y.W.; Li, H.J.; Liu, X.; Qin, H.J.; Dong, L.L.; Wang, D.W. Coexpression network analysis of the genes regulated by two types of resistance responses to powdery mildew in wheat. Sci. Rep. 2016, 6, 23805. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Tan, W.R.; Deng, X.G.; Zheng, T.; Zhang, D.W.; Lin, H.H. Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biol. Tec. 2015, 100, 196–204. [Google Scholar] [CrossRef]
- Leaungthitikanchana, S.; Fujibe, T.; Tanaka, M.; Wang, S.; Sotta, N.; Takano, J.; Fujiwara, T. Differential expression of three BOR1 genes corresponding to different genomes in response to boron conditions in hexaploid wheat (Triticum Aestivum L). Plant Cell Physiol. 2013, 54, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Cho, Y.G.; Park, M.Y.; Lee, M.C.; Eun, M.Y.; Kang, B.G.; Kim, W.T. Hormonal cross-talk between auxin and ethylene differentially regulates the expression of two members of the 1-aminocyclopropane-1-carboxylate oxidase gene family in rice (Oryza sativa L.). Plant Cell Physiol. 2000, 41, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Rzewuski, G.; Sauter, M. Ethylene biosynthesis and signaling in rice. Plant Sci. 2008, 175, 32–42. [Google Scholar] [CrossRef]
- Vande, P.B.; Smet, D.; Van Der, S.D. Ethylene and hormonal cross talk in vegetative growth and development. Plant Physiol. 2015, 169, 61–72. [Google Scholar] [CrossRef]
- Hu, X.F.; Xiang, X.B.; Xiong, L.J.; Zhu, Y.F.; Deng, Z.J. Differences in seed germination rates of different rice varieties and their responses to exogenous phytohormones. Seed 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Ann. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Mekhedov, S.L.; Kende, H. Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant Cell Physiol. 1996, 37, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, W.; Cao, J.; Meng, F.W.; Yu, Y.Q.; Huang, J.K.; Jiang, L.; Liu, M.X.; Zhang, Z.G.; Chen, X.W.; et al. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. 2017, 89, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Ahmadizadeh, M.; Chen, J.T.; Hasanzadeh, S.; Ahmar, S.; Heidari, P. Insights into the genes involved in the ethylene biosynthesis pathway in Arabidopsis thaliana and Oryza sativa. J. Genet. Eng. Biotechnol. 2020, 18, 62. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).