Dirty Ends: Formation, Repair, and Biological Relevance of Non-Canonical DNA Terminal Structures
Abstract
1. Introduction
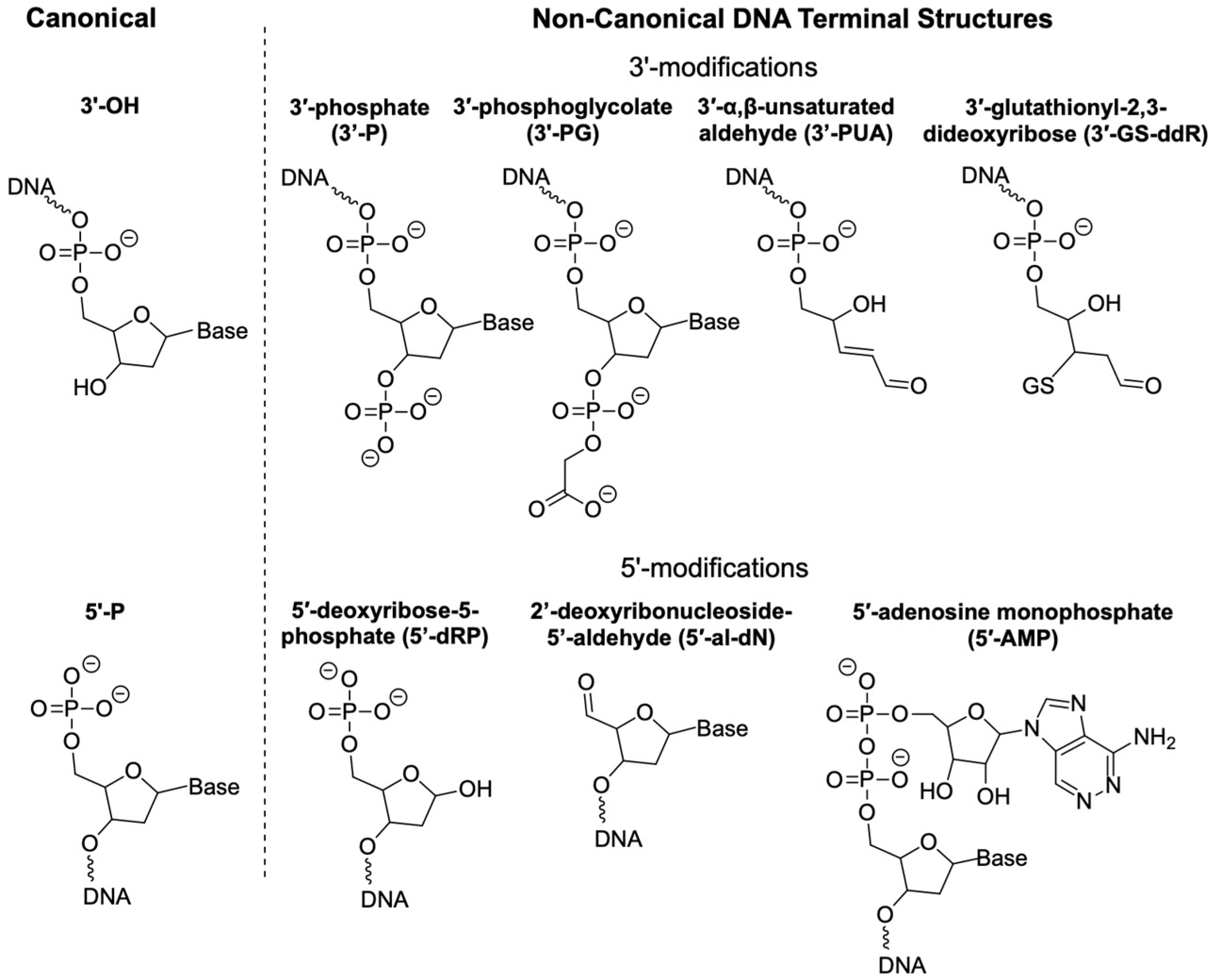
2. Non-Canonical DNA Structures
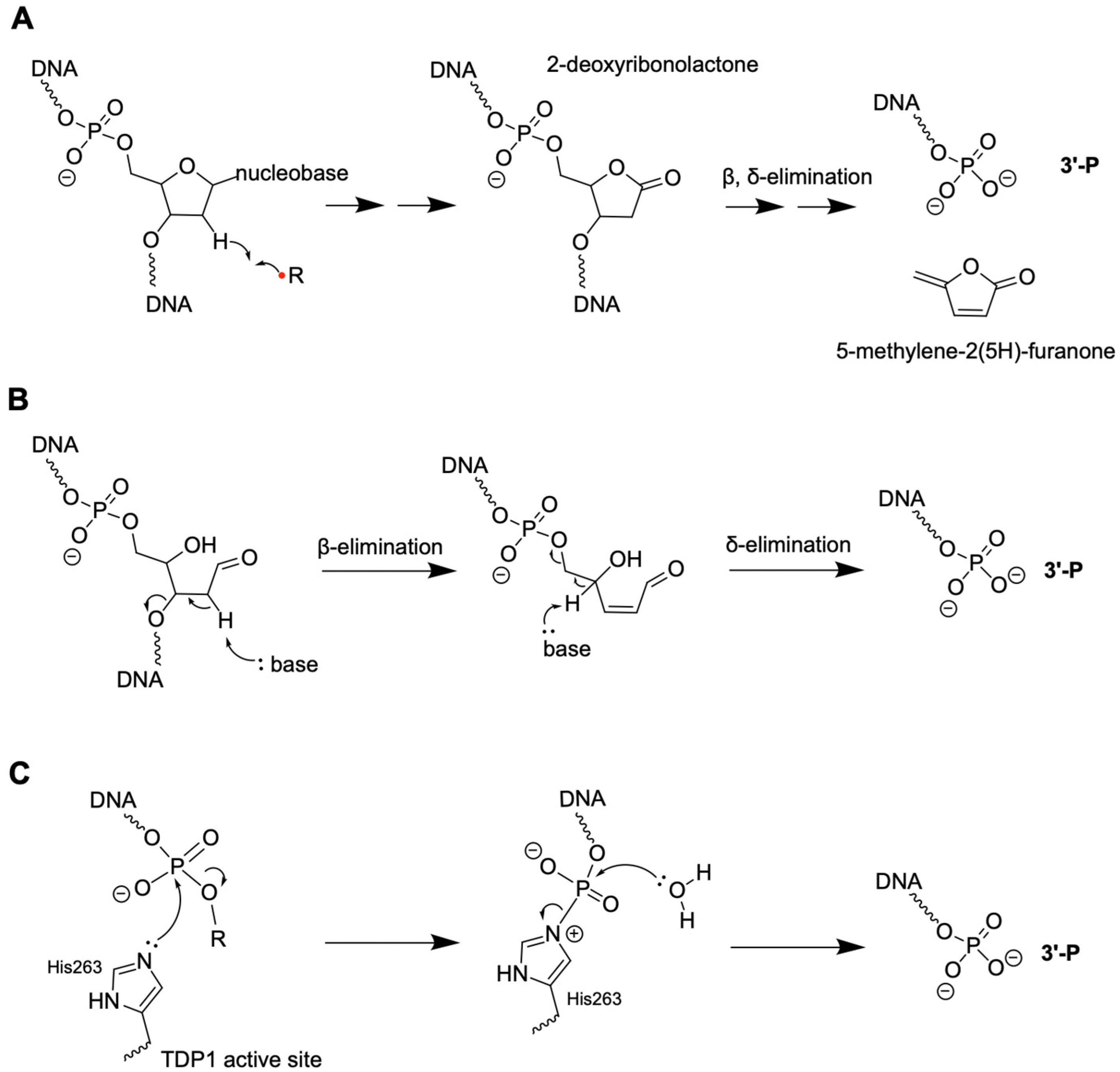
2.1. 3′-P
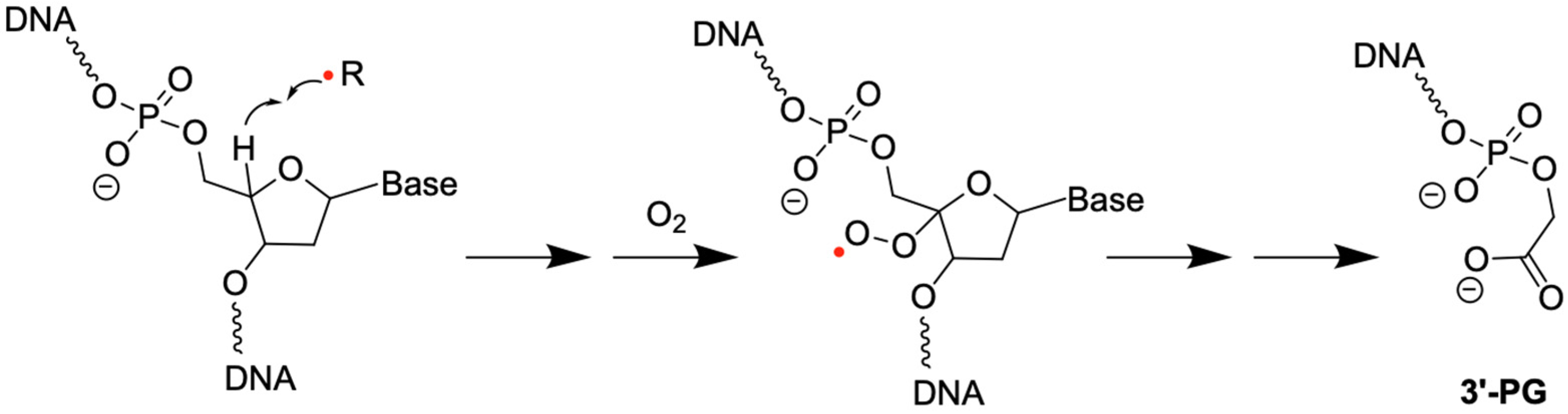
2.2. 3′-PG
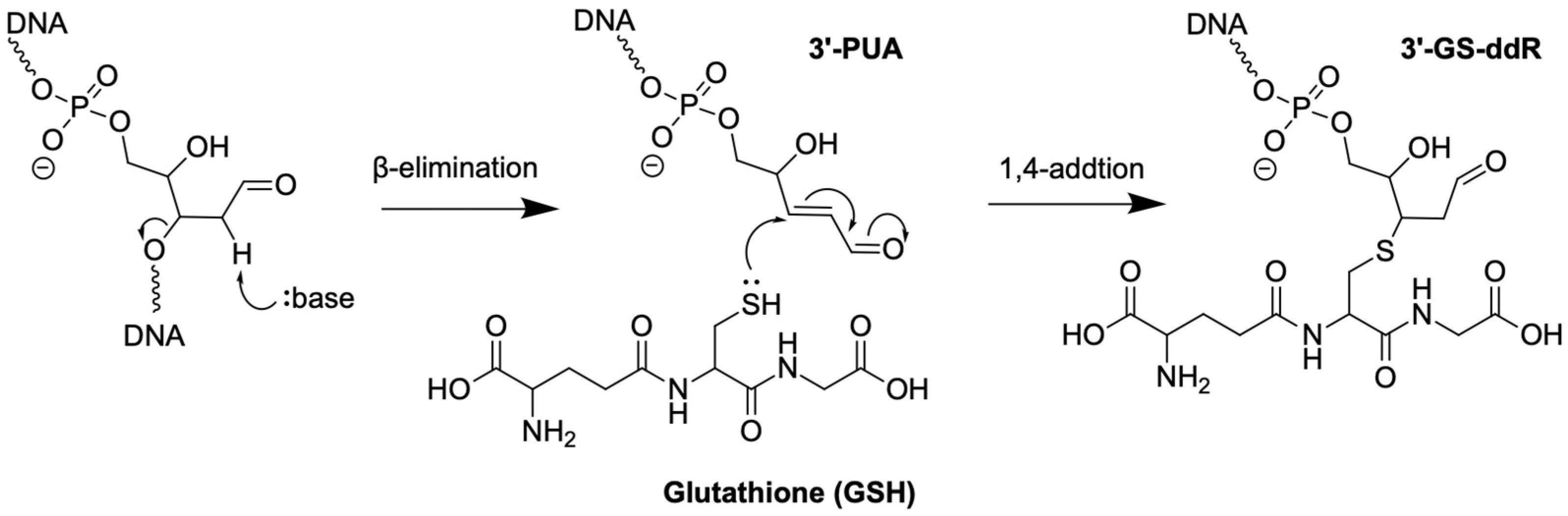
2.3. 3′-PUA and 3′-GS-ddR
2.4. 5′-dRP
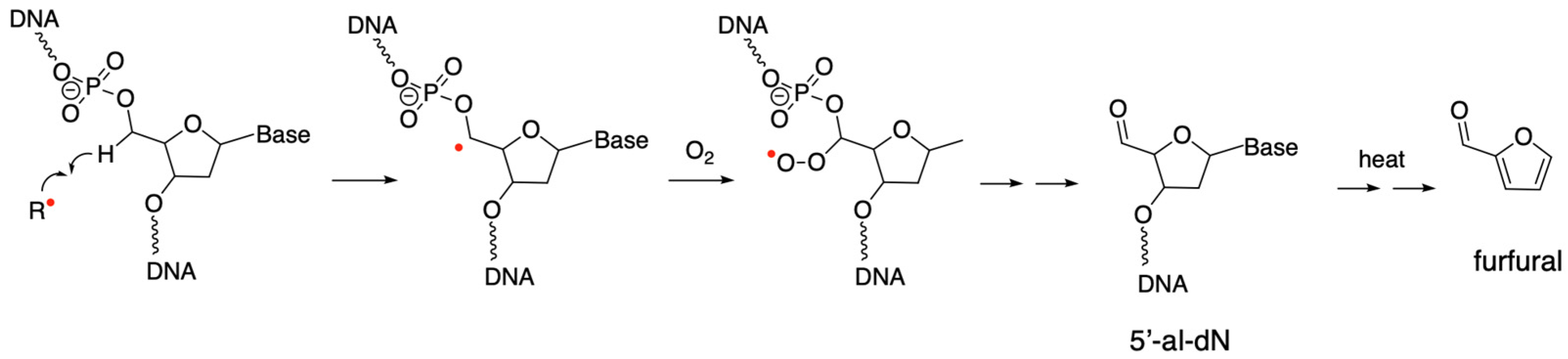
2.5. 5′-al-dN
2.6. 5′-AMP
3. Conclusions
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tiwari, V.; Wilson III, D.M. DNA damage and associated DNA repair defects in disease and premature aging. Am. J. Hum. Genet. 2019, 105, 237–257. [Google Scholar] [CrossRef]
- Andres, S.N.; Schellenberg, M.J.; Wallace, B.D.; Tumbale, P.; Williams, R.S. Recognition and repair of chemically heterogeneous structures at DNA ends. Environ. Mol. Mutagen. 2015, 56, 1–21. [Google Scholar] [CrossRef]
- Nickoloff, J.A.; Sharma, N.; Taylor, L. Clustered DNA double-strand breaks: Biological effects and relevance to cancer radiotherapy. Genes 2020, 11, 99. [Google Scholar] [CrossRef]
- Lomax, M.; Folkes, L.; O’neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef]
- Takashima, H.; Boerkoel, C.F.; John, J.; Saifi, G.M.; Salih, M.A.; Armstrong, D.; Mao, Y.; Quiocho, F.A.; Roa, B.B.; Nakagawa, M. Mutation of TDP1, encoding a topoisomerase I–dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 2002, 32, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Glover, J.M.; Weinfeld, M. Neurological disorders associated with DNA strand-break processing enzymes. Mech. Ageing Dev. 2017, 161, 130–140. [Google Scholar] [CrossRef]
- Kate, W.D.; Fanta, M.; Weinfeld, M. Loss of the DNA repair protein, polynucleotide kinase/phosphatase, activates the type 1 interferon response independent of ionizing radiation. Nucleic Acids Res. 2024, 52, 9630–9653. [Google Scholar] [CrossRef]
- Dalal, S.; Chikova, A.; Jaeger, J.; Sweasy, J.B. The Leu22Pro tumor-associated variant of DNA polymerase beta is dRP lyase deficient. Nucleic Acids Res. 2008, 36, 411–422. [Google Scholar] [CrossRef]
- Zhao, S.; Klattenhoff, A.W.; Thakur, M.; Sebastian, M.; Kidane, D. Mutation in DNA Polymerase Beta Causes Spontaneous Chromosomal Instability and Inflammation-Associated Carcinogenesis in Mice. Cancers 2019, 11, 1160. [Google Scholar] [CrossRef]
- Zhao, S.; Goewey Ruiz, J.A.; Sebastian, M.; Kidane, D. Defective DNA polymerase beta invoke a cytosolic DNA mediated inflammatory response. Front. Immunol. 2022, 13, 1039009. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Peng, Y.; Bryan, C.; Yang, K. Mechanisms of DNA−protein cross-link formation and repair. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140669. [Google Scholar]
- Wojtaszek, J.L.; Williams, R.S. From the TOP: Formation, recognition and resolution of topoisomerase DNA protein crosslinks. DNA Repair 2024, 142, 103751. [Google Scholar] [CrossRef]
- Dedon, P.C. The chemical toxicology of 2-deoxyribose oxidation in DNA. Chem. Res. Toxicol. 2008, 21, 206–219. [Google Scholar]
- Dedon, P.C.; Goldberg, I.H. Free-radical mechanisms involved in the formation of sequence-dependent bistranded DNA lesions by the antitumor antibiotics bleomycin, neocarzinostatin, and calicheamicin. Chem. Res. Toxicol. 1992, 5, 311–332. [Google Scholar] [CrossRef]
- Lindahl, T.; Nyberg, B. Rate of depurination of native deoxyribonucleic acid. Biochemistry 1972, 11, 3610–3618. [Google Scholar] [CrossRef]
- Drinkwater, N.R.; Miller, E.C.; Miller, J.A. Estimation of apurinic/apyrimidinic sites and phosphotriesters in deoxyribonucleic acid treated with electrophilic carcinogens and mutagens. Biochemistry 1980, 19, 5087–5092. [Google Scholar] [CrossRef]
- Roberts, K.P.; Sobrino, J.A.; Payton, J.; Mason, L.B.; Turesky, R.J. Determination of apurinic/apyrimidinic lesions in DNA with high-performance liquid chromatography and tandem mass spectrometry. Chem. Res. Toxicol. 2006, 19, 300–309. [Google Scholar] [CrossRef]
- Nakamura, J.; Swenberg, J.A. Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues. Cancer Res. 1999, 59, 2522–2526. [Google Scholar]
- Greenberg, M.M. Reactivity of Nucleic Acid Radicals. Adv. Phys. Org. Chem. 2016, 50, 119–202. [Google Scholar] [CrossRef] [PubMed]
- Pogozelski, W.K.; Tullius, T.D. Oxidative strand scission of nucleic acids: Routes initiated by hydrogen abstraction from the sugar moiety. Chem. Rev. 1998, 98, 1089–1108. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef]
- von Sonntag, C. The Chemical Basis of Radiation Biology; Taylor & Francis: Abingdon, UK, 1987. [Google Scholar]
- Guthrie, J.P.; Cossar, J. The pKa values of simple aldehydes determined by kinetics of chlorination. Can. J. Chem. 1986, 64, 2470–2474. [Google Scholar] [CrossRef]
- Wilde, J.A.; Bolton, P.H.; Mazumder, A.; Manoharan, M.; Gerlt, J.A. Characterization of the equilibrating forms of the abasic site in duplex DNA using 17O-NMR. J. Am. Chem. Soc. 1989, 111, 1894–1896. [Google Scholar] [CrossRef]
- Abe, Y.S.; Sasaki, S. DNA cleavage at the AP site via beta-elimination mediated by the AP site-binding ligands. Bioorg. Med. Chem. 2016, 24, 910–914. [Google Scholar] [CrossRef]
- Kawale, A.S.; Povirk, L.F. Tyrosyl-DNA phosphodiesterases: Rescuing the genome from the risks of relaxation. Nucleic Acids Res. 2018, 46, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, N.A.; Rechkunova, N.I.; Lavrik, O.I. AP-site cleavage activity of tyrosyl-DNA phosphodiesterase 1. FEBS Lett. 2011, 585, 683–686. [Google Scholar] [CrossRef]
- Harosh, I.; Binninger, D.M.; Harris, P.V.; Mezzina, M.; Boyd, J.B. Mechanism of action of deoxyribonuclease II from human lymphoblasts. Eur. J. Biochem. 1991, 202, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.J.; Aguilera, R.J. DNase II: Genes, enzymes and function. Gene 2003, 322, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pheiffer, B.H.; Zimmerman, S.B. 3′-Phosphatase activity of the DNA kinase from rat liver. Biochem. Biophys. Res. Commun. 1982, 109, 1297–1302. [Google Scholar] [CrossRef]
- Chappell, C.; Hanakahi, L.A.; Karimi-Busheri, F.; Weinfeld, M.; West, S.C. Involvement of human polynucleotide kinase in double-strand break repair by non-homologous end joining. EMBO J. 2002, 21, 2827–2832. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, L.; Leppard, J.B.; Kedar, P.; Karimi-Busheri, F.; Rasouli-Nia, A.; Weinfeld, M.; Tomkinson, A.E.; Izumi, T.; Prasad, R.; Wilson, S.H.; et al. AP endonuclease-independent DNA base excision repair in human cells. Mol. Cell 2004, 15, 209–220. [Google Scholar] [CrossRef]
- Weinfeld, M.; Mani, R.S.; Abdou, I.; Aceytuno, R.D.; Glover, J.N. Tidying up loose ends: The role of polynucleotide kinase/phosphatase in DNA strand break repair. Trends Biochem. Sci. 2011, 36, 262–271. [Google Scholar] [CrossRef]
- Bernstein, N.K.; Williams, R.S.; Rakovszky, M.L.; Cui, D.; Green, R.; Karimi-Busheri, F.; Mani, R.S.; Galicia, S.; Koch, C.A.; Cass, C.E.; et al. The molecular architecture of the mammalian DNA repair enzyme, polynucleotide kinase. Mol. Cell 2005, 17, 657–670. [Google Scholar] [CrossRef]
- Schellenberg, M.J.; Williams, R.S. DNA end processing by polynucleotide kinase/phosphatase. Proc. Natl. Acad. Sci. USA 2011, 108, 20855–20856. [Google Scholar] [CrossRef]
- Garces, F.; Pearl, L.H.; Oliver, A.W. The structural basis for substrate recognition by mammalian polynucleotide kinase 3′ phosphatase. Mol. Cell 2011, 44, 385–396. [Google Scholar] [CrossRef]
- Whitaker, A.M.; Freudenthal, B.D. APE1: A skilled nucleic acid surgeon. DNA Repair 2018, 71, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Malfatti, M.C.; Bellina, A.; Antoniali, G.; Tell, G. Revisiting Two Decades of Research Focused on Targeting APE1 for Cancer Therapy: The Pros and Cons. Cells 2023, 12, 1895. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Lin, C.T.; Chang, K.C.; Guo, K.W.; Wang, S.; Chu, J.W.; Hsiao, Y.Y. APE1 distinguishes DNA substrates in exonucleolytic cleavage by induced space-filling. Nat. Commun. 2021, 12, 601. [Google Scholar] [CrossRef]
- Whitaker, A.M.; Stark, W.J.; Freudenthal, B.D. Processing oxidatively damaged bases at DNA strand breaks by APE1. Nucleic Acids Res. 2022, 50, 9521–9533. [Google Scholar] [CrossRef]
- Jordano-Raya, M.; Schrader, C.E.; Ariza, R.R.; Roldán-Arjona, T.; Córdoba-Cañero, D. Divergent evolution of opposite base specificity and single-stranded DNA activity in animal and plant AP endonucleases. Nucleic Acids Res. 2025, 53, gkae1297. [Google Scholar] [CrossRef]
- Mandal, S.M.; Hegde, M.L.; Chatterjee, A.; Hegde, P.M.; Szczesny, B.; Banerjee, D.; Boldogh, I.; Gao, R.; Falkenberg, M.; Gustafsson, C.M. Role of human DNA glycosylase Nei-like 2 (NEIL2) and single strand break repair protein polynucleotide kinase 3′-phosphatase in maintenance of mitochondrial genome. J. Biol. Chem. 2012, 287, 2819–2829. [Google Scholar] [CrossRef]
- Tahbaz, N.; Subedi, S.; Weinfeld, M. Role of polynucleotide kinase/phosphatase in mitochondrial DNA repair. Nucleic Acids Res. 2012, 40, 3484–3495. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 2017, 17, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Guilbaud, E.; Tait, S.W.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Henner, W.D.; Rodriguez, L.O.; Hecht, S.M.; Haseltine, W.A. Gamma Ray induced deoxyribonucleic acid strand breaks. 3′ Glycolate termini. J. Biol. Chem. 1983, 258, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Plastaras, J.P.; Riggins, J.N.; Otteneder, M.; Marnett, L.J. Reactivity and mutagenicity of endogenous DNA oxopropenylating agents: Base propenals, malondialdehyde, and N(epsilon)-oxopropenyllysine. Chem. Res. Toxicol. 2000, 13, 1235–1242. [Google Scholar] [CrossRef]
- Weinfeld, G.W.B.a.M. Influence of nitrogen, oxygen, and nitroimidazole radiosensitizers on DNA damage induced by ionizing radiation. Biochemistry 1993, 32, 2186–2193. [Google Scholar] [CrossRef]
- Christiane Collins, M.M.A.; Zhou, X.; Dedon, P.C. Analysis of 3‘-Phosphoglycolaldehyde Residues in Oxidized DNA by Gas Chromatography/Negative Chemical Ionization/Mass Spectrometry. Chem. Res. Toxicol. 2003, 16, 1560–1566. [Google Scholar] [CrossRef]
- Christiane Collins, X.Z.; Wang, R.; Barth, M.C.; Jiang, T.; Coderre, J.A.; Dedon, P.C. Differential Oxidation of Deoxyribose in DNA by γ and α-Particle Radiation. Radiat. Res. 2005, 163, 654–662. [Google Scholar] [CrossRef]
- Povirk, L.F. Processing of damaged DNA ends for double-strand break repair in mammalian cells. ISRN Mol. Biol. 2012, 2012, 345805. [Google Scholar] [CrossRef]
- Chen, S.; Hannis, J.C.; Flora, J.W.; Muddiman, D.C.; Charles, K.; Yu, Y.; Povirk, L.F. Homogeneous preparations of 3′-phosphoglycolate-terminated oligodeoxynucleotides from bleomycin-treated DNA as verified by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Biochem. 2001, 289, 274–280. [Google Scholar] [CrossRef]
- Winters, T.A.; Henner, W.D.; Russell, P.S.; McCullough, A.; Jorgensen, T.J. Removal of 3′-phosphoglycolate from DNA strand-break damage in an oligonucleotide substrate by recombinant human apurinic/apyrimidinic endonuclease 1. Nucleic Acids Res. 1994, 22, 1866–1873. [Google Scholar] [CrossRef]
- Parsons, J.L.; Dianova, I.I.; Dianov, G.L. APE1 is the major 3′-phosphoglycolate activity in human cell extracts. Nucleic Acids Res. 2004, 32, 3531–3536. [Google Scholar] [CrossRef]
- Suh, D.; Wilson III, D.M.; Povirk, L.F. 3′-phosphodiesterase activity of human apurinic/apyrimidinic endonuclease at DNA double-strand break ends. Nucleic Acids Res. 1997, 25, 2495–2500. [Google Scholar] [CrossRef]
- Inamdar, K.V.; Pouliot, J.J.; Zhou, T.; Lees-Miller, S.P.; Rasouli-Nia, A.; Povirk, L.F. Conversion of phosphoglycolate to phosphate termini on 3′ overhangs of DNA double strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. J. Biol. Chem. 2002, 277, 27162–27168. [Google Scholar] [CrossRef]
- Zhou, T.; Lee, J.W.; Tatavarthi, H.; Lupski, J.R.; Valerie, K.; Povirk, L.F. Deficiency in 3′-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1). Nucleic Acids Res. 2005, 33, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Povirk, L.F.; Zhou, T.; Zhou, R.; Cowan, M.J.; Yannone, S.M. Processing of 3′-phosphoglycolate-terminated DNA double strand breaks by Artemis nuclease. J. Biol. Chem. 2007, 282, 3547–3558. [Google Scholar] [CrossRef]
- Fisher, L.A.; Samson, L.; Bessho, T. Removal of reactive oxygen species-induced 3′-blocked ends by XPF-ERCC1. Chem. Res. Toxicol. 2011, 24, 1876–1881. [Google Scholar] [CrossRef]
- Haldar, T.; Jha, J.S.; Yang, Z.; Nel, C.; Housh, K.; Cassidy, O.J.; Gates, K.S. Unexpected complexity in the products arising from NaOH-, heat-, amine-, and glycosylase-induced strand cleavage at an abasic site in DNA. Chem. Res. Toxicol. 2022, 35, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Rahimoff, R.; Kosmatchev, O.; Kirchner, A.; Pfaffeneder, T.; Spada, F.; Brantl, V.; Muller, M.; Carell, T. 5-Formyl- and 5-Carboxydeoxycytidines Do Not Cause Accumulation of Harmful Repair Intermediates in Stem Cells. J. Am. Chem. Soc. 2017, 139, 10359–10364. [Google Scholar] [CrossRef]
- Wei, X.; Yang, K. PARP1 Incises abasic sites and covalently cross-links to 3′-DNA termini via cysteine addition not reductive amination. Biochemistry 2023, 62, 1527–1530. [Google Scholar] [CrossRef]
- Wei, X.; Person, M.D.; Yang, K. Tyrosyl-DNA phosphodiesterase 1 excises the 3′-DNA-ALKBH1 cross-link and its application for 3′-DNA-ALKBH1 cross-link characterization by LC-MS/MS. DNA Repair 2022, 119, 103391. [Google Scholar] [CrossRef]
- Xu, W.; Tang, J.; Zhao, L. DNA-protein cross-links between abasic DNA damage and mitochondrial transcription factor A (TFAM). Nucleic Acids Res. 2023, 51, 41–53. [Google Scholar] [CrossRef]
- Esterbauer, H.; Zollner, H.; Scholz, N. Reaction of glutathione with conjugated carbonyls. Z. Naturforsch C Biosci. 1975, 30, 466–473. [Google Scholar] [CrossRef]
- Jumpathong, W.; Chan, W.; Taghizadeh, K.; Babu, I.R.; Dedon, P.C. Metabolic fate of endogenous molecular damage: Urinary glutathione conjugates of DNA-derived base propenals as markers of inflammation. Proc. Natl. Acad. Sci. USA 2015, 112, E4845–E4853. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.; Miners, J.O. The effects of buthionine sulphoximine (BSO) on glutathione depletion and xenobiotic biotransformation. Biochem. Pharmacol. 1984, 33, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Bailly, V.; Verly, W.G. Importance of thiols in the repair mechanisms of DNA containing AP (apurinic or apyrimidinic) sites. Nucleic Acids Res. 1988, 16, 9489–9496. [Google Scholar] [CrossRef]
- Jha, J.S.; Yin, J.; Haldar, T.; Yang, Z.; Wang, Y.; Gates, K.S. Reconsidering the Chemical Nature of Strand Breaks Derived from Abasic Sites in Cellular DNA: Evidence for 3′-Glutathionylation. J. Am. Chem. Soc. 2022, 144, 10471–10482. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Gates, K.S.; Wang, Y. N-Methyl-N-Nitrosourea Induced 3′-Glutathionylated DNA Cleavage Products in Mammalian Cells. Anal. Chem. 2022, 94, 15595–15603. [Google Scholar] [CrossRef]
- Chen, Y.H.; Esparza Sanchez, M.; Hung, T.I.; Tang, J.; Xu, W.; Yin, J.; Wang, Y.; Chang, C.-E.A.; Zhang, H.; Chen, J.; et al. Glutathionylated DNA Adducts Accumulate in Mitochondrial DNA and Are Regulated by AP Endonuclease 1 and Tyrosyl-DNA Phosphodiesterase 1. Proc. Natl. Acad. Sci. USA, 2025; in press. [Google Scholar]
- Wei, X.; Wang, Z.; Hinson, C.; Yang, K. Human TDP1, APE1 and TREX1 repair 3′-DNA-peptide/protein cross-links arising from abasic sites in vitro. Nucleic Acids Res. 2022, 50, 3638–3657. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kim, K. Excision of deoxyribose phosphate residues by DNA polymerase β during DNA repair. Science 1995, 269, 699–702. [Google Scholar] [CrossRef]
- Garcıa-Dıaz, M.; Bebenek, K.; Kunkel, T.A.; Blanco, L. Identification of an intrinsic 5′-deoxyribose-5-phosphate lyase activity in human DNA polymerase λ: A possible role in base excision repair. J. Biol. Chem. 2001, 276, 34659–34663. [Google Scholar] [PubMed]
- Beard, W.A.; Horton, J.K.; Prasad, R.; Wilson, S.H. Eukaryotic base excision repair: New approaches shine light on mechanism. Annu. Rev. Biochem. 2019, 88, 137. [Google Scholar] [CrossRef] [PubMed]
- Schermerhorn, K.M.; Delaney, S. A chemical and kinetic perspective on base excision repair of DNA. Acc. Chem. Res. 2014, 47, 1238–1246. [Google Scholar]
- Kauppila, J.H.; Bonekamp, N.A.; Mourier, A.; Isokallio, M.A.; Just, A.; Kauppila, T.E.; Stewart, J.B.; Larsson, N.-G. Base-excision repair deficiency alone or combined with increased oxidative stress does not increase mtDNA point mutations in mice. Nucleic Acids Res. 2018, 46, 6642–6669. [Google Scholar] [PubMed]
- Mullins, E.A.; Rodriguez, A.A.; Bradley, N.P.; Eichman, B.F. Emerging roles of DNA glycosylases and the base excision repair pathway. Trends Biochem. Sci. 2019, 44, 765–781. [Google Scholar] [CrossRef]
- Fairlamb, M.S.; Spies, M.; Washington, M.T.; Freudenthal, B.D. Visualizing the coordination of apurinic/apyrimidinic endonuclease (APE1) and DNA polymerase β during base excision repair. J. Biol. Chem. 2023, 299, 104636. [Google Scholar] [CrossRef]
- Bebenek, K.; Tissier, A.; Frank, E.G.; McDonald, J.P.; Prasad, R.; Wilson, S.H.; Woodgate, R.; Kunkel, T.A. 5′-Deoxyribose Phosphate Lyase Activity of Human DNA Polymerase ɩ in Vitro. Science 2001, 291, 2156–2159. [Google Scholar] [CrossRef]
- Prasad, R.; Longley, M.J.; Sharief, F.S.; Hou, E.W.; Copeland, W.C.; Wilson, S.H. Human DNA polymerase θ possesses 5′-dRP lyase activity and functions in single-nucleotide base excision repair in vitro. Nucleic Acids Res. 2009, 37, 1868–1877. [Google Scholar] [CrossRef]
- Roberts, S.A.; Strande, N.; Burkhalter, M.D.; Strom, C.; Havener, J.M.; Hasty, P.; Ramsden, D.A. Ku is a 5′-dRP/AP lyase that excises nucleotide damage near broken ends. Nature 2012, 464, 1214. [Google Scholar] [CrossRef] [PubMed]
- Yamtich, J.; Sweasy, J.B. DNA polymerase family X: Function, structure, and cellular roles. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.J.; Horton, J.K.; Zhao, M.-L.; Wilson, S.H. Lysines in the lyase active site of DNA polymerase β destabilize nonspecific DNA binding, facilitating searching and DNA gap recognition. J. Biol. Chem. 2020, 295, 12181–12187. [Google Scholar] [CrossRef]
- Weaver, T.M.; Ryan, B.J.; Thompson, S.H.; Hussen, A.S.; Spencer, J.J.; Xu, Z.; Schnicker, N.J.; Freudenthal, B.D. Structural basis of gap-filling DNA synthesis in the nucleosome by DNA Polymerase β. Nat. Commun. 2025, 16, 2607. [Google Scholar] [CrossRef]
- Kumar, A.; Reed, A.J.; Zahurancik, W.J.; Daskalova, S.M.; Hecht, S.M.; Suo, Z. Interlocking activities of DNA polymerase β in the base excision repair pathway. Proc. Natl. Acad. Sci. USA 2022, 119, e2118940119. [Google Scholar] [CrossRef]
- DeMott, M.S.; Beyret, E.; Wong, D.; Bales, B.C.; Hwang, J.-T.; Greenberg, M.M.; Demple, B. Covalent trapping of human DNA polymerase β by the oxidative DNA lesion 2-deoxyribonolactone. J. Biol. Chem. 2002, 277, 7637–7640. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, J.L.; Thapar, U.; Yu, K.; Fang, Q.; Sobol, R.W.; Demple, B. Enzyme mechanism-based, oxidative DNA–protein cross-links formed with DNA polymerase β in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 8602–8607. [Google Scholar] [CrossRef]
- Podlutsky, A.J.; Dianova, I.I.; Podust, V.N.; Bohr, V.A.; Dianov, G.L. Human DNA polymerase β initiates DNA synthesis during long-patch repair of reduced AP sites in DNA. EMBO J. 2001, 20, 1477–1482. [Google Scholar] [CrossRef]
- Hou, E.W.; Prasad, R.; Asagoshi, K.; Masaoka, A.; Wilson, S.H. Comparative assessment of plasmid and oligonucleotide DNA substrates in measurement of in vitro base excision repair activity. Nucleic Acids Res. 2007, 35, e112. [Google Scholar] [CrossRef]
- Longley, M.J.; Prasad, R.; Srivastava, D.K.; Wilson, S.H.; Copeland, W.C. Identification of 5′-deoxyribose phosphate lyase activity in human DNA polymerase γ and its role in mitochondrial base excision repair in vitro. Proc. Natl. Acad. Sci. USA 1998, 95, 12244–12248. [Google Scholar] [CrossRef]
- Sykora, P.; Kanno, S.; Akbari, M.; Kulikowicz, T.; Baptiste, B.A.; Leandro, G.S.; Lu, H.; Tian, J.; May, A.; Becker, K.A. DNA polymerase beta participates in mitochondrial DNA repair. Mol. Cell. Biol. 2017, 37, e00237-17. [Google Scholar] [CrossRef]
- Prasad, R.; Çağlayan, M.; Dai, D.-P.; Nadalutti, C.A.; Zhao, M.-L.; Gassman, N.R.; Janoshazi, A.K.; Stefanick, D.F.; Horton, J.K.; Krasich, R. DNA polymerase β: A missing link of the base excision repair machinery in mammalian mitochondria. DNA Repair 2017, 60, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hussen, A.S.; Freudenthal, B.D.; Suo, Z.; Zhao, L. Mitochondrial transcription factor A (TFAM) has 5′-deoxyribose phosphate lyase activity in vitro. DNA Repair 2024, 137, 103666. [Google Scholar] [CrossRef]
- Cejka, P.; Symington, L.S. DNA end resection: Mechanism and control. Annu. Rev. Genet. 2021, 55, 285–307. [Google Scholar] [CrossRef] [PubMed]
- Chandramouly, G.; Jamsen, J.; Borisonnik, N.; Tyagi, M.; Calbert, M.L.; Tredinnick, T.; Ozdemir, A.Y.; Kent, T.; Demidova, E.V.; Arora, S. Polλ promotes microhomology-mediated end-joining. Nat. Struct. Mol. Biol. 2023, 30, 107–114. [Google Scholar] [CrossRef]
- Bailly, V.; Verly, W. The multiple activities of Escherichia coli endonuclease IV and the extreme lability of 5′-terminal base-free deoxyribose 5-phosphates. Biochem. J. 1989, 259, 761–768. [Google Scholar] [CrossRef]
- Price, A.; Lindahl, T. Enzymic release of 5′-terminal deoxyribose phosphate residues from damaged DNA in human cells. Biochemistry 1991, 30, 8631–8637. [Google Scholar] [CrossRef]
- Admiraal, S.J.; O’Brien, P.J. Reactivity and cross-linking of 5′-terminal abasic sites within DNA. Chem. Res. Toxicol. 2017, 30, 1317–1326. [Google Scholar] [CrossRef]
- Simonelli, V.; Narciso, L.; Dogliotti, E.; Fortini, P. Base excision repair intermediates are mutagenic in mammalian cells. Nucleic Acids Res. 2005, 33, 4404–4411. [Google Scholar] [CrossRef]
- Sobol, R.W.; Prasad, R.; Evenski, A.; Baker, A.; Yang, X.P.; Horton, J.K.; Wilson, S.H. The lyase activity of the DNA repair protein beta-polymerase protects from DNA-damage-induced cytotoxicity. Nature 2000, 405, 807–810. [Google Scholar] [CrossRef]
- Trivedi, R.N.; Almeida, K.H.; Fornsaglio, J.L.; Schamus, S.; Sobol, R.W. The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res. 2005, 65, 6394–6400. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.B.; Svilar, D.; Trivedi, R.N.; Wang, X.H.; Goellner, E.M.; Moore, B.; Hamilton, R.L.; Banze, L.A.; Brown, A.R.; Sobol, R.W. N-methylpurine DNA glycosylase and DNA polymerase beta modulate BER inhibitor potentiation of glioma cells to temozolomide. Neuro Oncol. 2011, 13, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, A.; Becker, D.; Palmer, B.J.; Heizer, A.N.; Sevilla, M.D. Direct Formation of the C5′-Radical in the Sugar–Phosphate Backbone of DNA by High-Energy Radiation. J. Phys. Chem. B 2012, 116, 5900–5906. [Google Scholar] [CrossRef] [PubMed]
- Kappen, L.S.; Goldberg, I.H.; Liesch, J.M. Identification of thymidine-5′-aldehyde at DNA strand breaks induced by neocarzinostatin chromophore. Proc. Natl. Acad. Sci. USA 1982, 79, 744–748. [Google Scholar] [CrossRef]
- Pitié, M.; Pratviel, G.; Bernadou, J.; Meunier, B. Preferential hydroxylation by the chemical nuclease meso-tetrakis-(4-N-methylpyridiniumyl) porphyrinatomanganeseIII pentaacetate/KHSO5 at the 5′carbon of deoxyriboses on both 3′sides of three contiguous AT base pairs in short double-stranded oligonucleotides. Proc. Natl. Acad. Sci. USA 1992, 89, 3967–3971. [Google Scholar]
- Kodama, T.; Greenberg, M.M. Preparation and analysis of oligonucleotides containing lesions resulting from C5 ‘-oxidation. J. Org. Chem. 2005, 70, 9916–9924. [Google Scholar] [CrossRef]
- Rana, A.; Yang, K.; Greenberg, M.M. Reactivity of the Major Product of C5′-Oxidative DNA Damage in Nucleosome Core Particles. ChemBioChem 2019, 20, 672–676. [Google Scholar] [CrossRef]
- Pratviel, G.; Pitié, M.; Bernadou, J.; Meunier, B. Furfural as a marker of DNA cleavage by hydroxylation at the 5′ carbon of deoxyribose. Angew. Chem. Int. Ed. Engl. 1991, 30, 702–704. [Google Scholar] [CrossRef]
- Chan, W.; Chen, B.; Wang, L.; Taghizadeh, K.; Demott, M.S.; Dedon, P.C. Quantification of the 2-deoxyribonolactone and nucleoside 5′-aldehyde products of 2-deoxyribose oxidation in DNA and cells by isotope-dilution gas chromatography mass spectrometry: Differential effects of gamma-radiation and Fe2+-EDTA. J. Am. Chem. Soc. 2010, 132, 6145–6153. [Google Scholar] [CrossRef]
- Robert, G.; Wagner, J.R. Radical Oxidation of the 2-Deoxyribosyl Moiety of DNA: Characterization of Unique Strand Breaks with 5′-Carboxylate Termini. J. Am. Chem. Soc. 2025, 147, 20189–20193. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.; Sczepanski, J.T.; Greenberg, M.M. Excision of a lyase-resistant oxidized abasic lesion from DNA. Chem. Res. Toxicol. 2010, 23, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.C.; Kreller, C.R.; Greenberg, M.M. Long patch base excision repair compensates for DNA polymerase beta inactivation by the C4′-oxidized abasic site. Biochemistry 2011, 50, 136–143. [Google Scholar] [CrossRef]
- Ahel, I.; Rass, U.; El-Khamisy, S.F.; Katyal, S.; Clements, P.M.; McKinnon, P.J.; Caldecott, K.W.; West, S.C. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature 2006, 443, 713–716. [Google Scholar] [CrossRef]
- Date, H.; Onodera, O.; Tanaka, H.; Iwabuchi, K.; Uekawa, K.; Igarashi, S.; Koike, R.; Hiroi, T.; Yuasa, T.; Awaya, Y. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new HIT superfamily gene. Nat. Genet. 2001, 29, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.-C.; Barbot, C.; Tachi, N.; Kozuka, N.; Uchida, E.; Gibson, T.; Mendonça, P.; Costa, M.; Barros, J.; Yanagisawa, T. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat. Genet. 2001, 29, 189–193. [Google Scholar] [CrossRef]
- Clements, P.M.; Breslin, C.; Deeks, E.D.; Byrd, P.J.; Ju, L.; Bieganowski, P.; Brenner, C.; Moreira, M.-C.; Taylor, A.M.R.; Caldecott, K.W. The ataxia–oculomotor apraxia 1 gene product has a role distinct from ATM and interacts with the DNA strand break repair proteins XRCC1 and XRCC4. DNA Repair 2004, 3, 1493–1502. [Google Scholar] [CrossRef]
- Gueven, N.; Becherel, O.J.; Kijas, A.W.; Chen, P.; Howe, O.; Rudolph, J.H.; Gatti, R.; Date, H.; Onodera, O.; Taucher-Scholz, G. Aprataxin, a novel protein that protects against genotoxic stress. Hum. Mol. Genet. 2004, 13, 1081–1093. [Google Scholar] [CrossRef]
- Mosesso, P.; Piane, M.; Palitti, F.; Pepe, G.; Penna, S.; Chessa, L. The novel human gene aprataxin is directly involved in DNA single-strand-break repair. Cell. Mol. Life Sci. CMLS 2005, 62, 485–491. [Google Scholar]
- Sykora, P.; Croteau, D.L.; Bohr, V.A.; Wilson III, D.M. Aprataxin localizes to mitochondria and preserves mitochondrial function. Proc. Natl. Acad. Sci. USA 2011, 108, 7437–7442. [Google Scholar]
- Akbari, M.; Sykora, P.; Bohr, V.A. Slow mitochondrial repair of 5′-AMP renders mtDNA susceptible to damage in APTX deficient cells. Sci. Rep. 2015, 5, 12876. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Croteau, D.L.; Bohr, V.A.; Akbari, M. Diminished OPA1 expression and impaired mitochondrial morphology and homeostasis in Aprataxin-deficient cells. Nucleic Acids Res. 2019, 47, 4086–4110. [Google Scholar] [CrossRef] [PubMed]
- Madsen, H.B.; Pease, L.I.; Scanlan, R.-L.; Akbari, M.; Rasmussen, L.J.; Shanley, D.P.; Bohr, V.A. The DNA repair enzyme, aprataxin, plays a role in innate immune signaling. Front. Aging Neurosci. 2023, 15, 1290681. [Google Scholar] [CrossRef] [PubMed]
| Enzyme Name (Gene) | Substrate | Product | Activity | Biological Relevance * |
|---|---|---|---|---|
| tyrosyl-DNA phosphodiesterase 1 (TDP1) | AP sites, 3′-DPCs, 3′-PG, 3′-PUA, 3′-GS-ddR | 3′-P | AP endonuclease, phosphodiesterase, limited 3′-exonuclease | spinocerebellar ataxia with axonal neuropathy (SCAN1) |
| deoxyribonuclease II (DNASE2) | DNA, RNA | 3′-P | endonuclease | autoinflammatory-pancytopenia syndrome and dyskeratosis congenita, autosomal dominant 6 |
| polynucleotide kinase phosphatase (PNKP) | 3′-P, 3′-OH | 3′-OH, 3′-P | 5′-kinase, 3′-phosphatase | Alzheimer’s, microcephaly, seizures, developmental delay, ataxia-ocular motor apraxia 4 (AOA4) |
| AP endonuclease 1 (APEX1) | AP sites, 3′-P, 3′-PG, 3′-PUA, 3′-GS-ddR | 5′-dRP (from AP sites) and 3′-OH (from 3′-lesions) | AP endonuclease, 3′-phosphodiesterase, 3′-5′-exonuclease | malignancies and neurodegenerative diseases |
| Artemis nuclease (DCLRE1C) | 3′-PG | 3′-OH | exonuclease, endonuclease | Severe combined immunodeficiency with sensitivity to ionizing radiation, Omenn syndrome |
| DNA polymerase β (POLB) | 5′-dRP | 5′-P | dRP lyase, DNA polymerase | Werner syndrome, esophageal cancer |
| DNA Polymerase λ (POLL) | 5′-dRP | 5′-P | dRP lyase, DNA polymerase | Adams-Oliver syndrome, Xeroderma Pigmentosum, variant type |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sothy, S.; Zhao, L. Dirty Ends: Formation, Repair, and Biological Relevance of Non-Canonical DNA Terminal Structures. Genes 2025, 16, 1188. https://doi.org/10.3390/genes16101188
Sothy S, Zhao L. Dirty Ends: Formation, Repair, and Biological Relevance of Non-Canonical DNA Terminal Structures. Genes. 2025; 16(10):1188. https://doi.org/10.3390/genes16101188
Chicago/Turabian StyleSothy, Seanmory, and Linlin Zhao. 2025. "Dirty Ends: Formation, Repair, and Biological Relevance of Non-Canonical DNA Terminal Structures" Genes 16, no. 10: 1188. https://doi.org/10.3390/genes16101188
APA StyleSothy, S., & Zhao, L. (2025). Dirty Ends: Formation, Repair, and Biological Relevance of Non-Canonical DNA Terminal Structures. Genes, 16(10), 1188. https://doi.org/10.3390/genes16101188






