Conserved Blood Transcriptome Patterns Highlight microRNA and Hub Gene Drivers of Neurodegeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Dataset
2.2. Data Preprocessing and Quality Control
2.3. Weighted Gene Co-Expression Network Analysis (WGCNA)
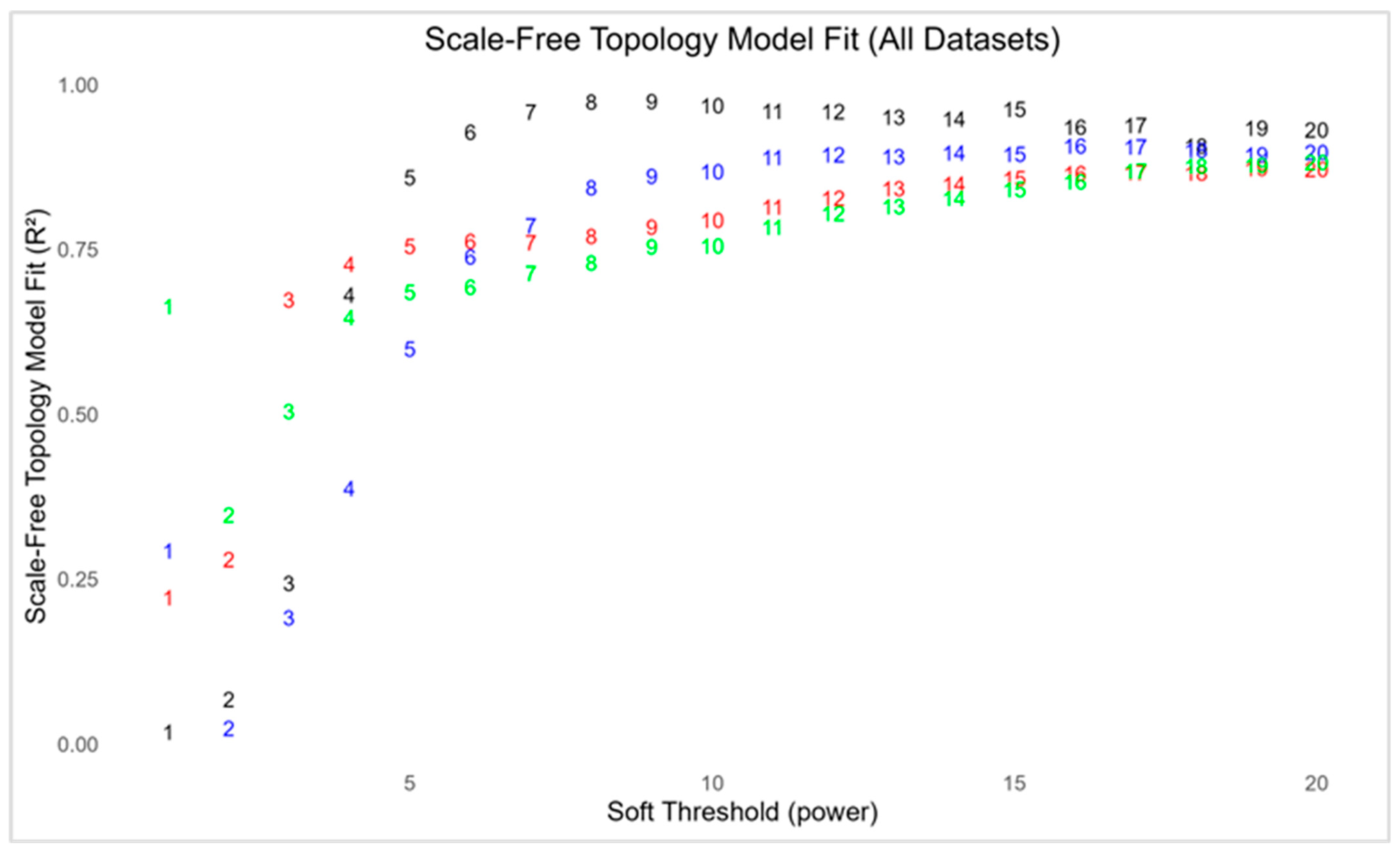
2.3.1. Network Construction and Scale-Free Topology Estimation
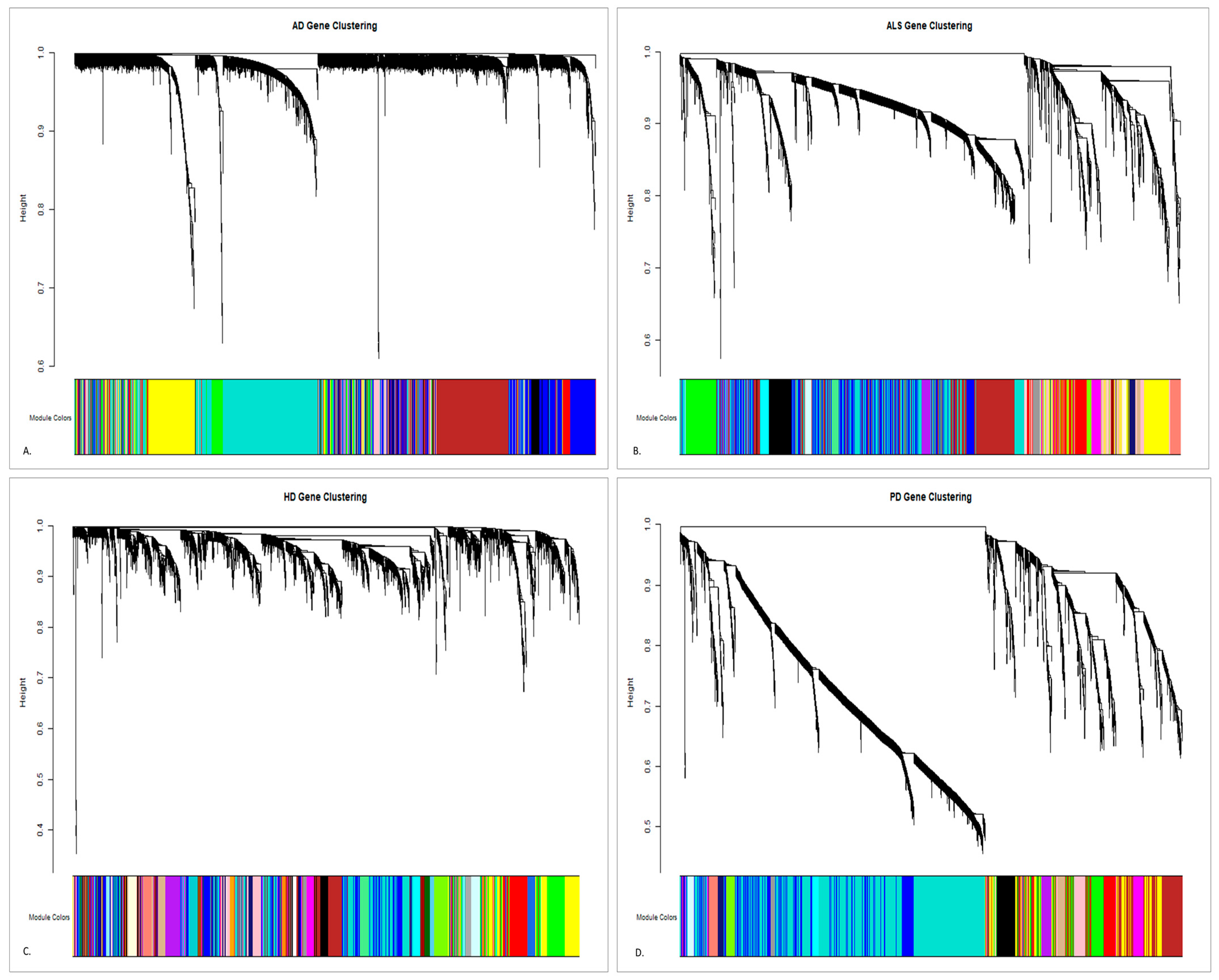
2.3.2. Module Detection and Topological Overlap-Based Clustering
2.3.3. Cross-Dataset Evaluation of Module Preservation
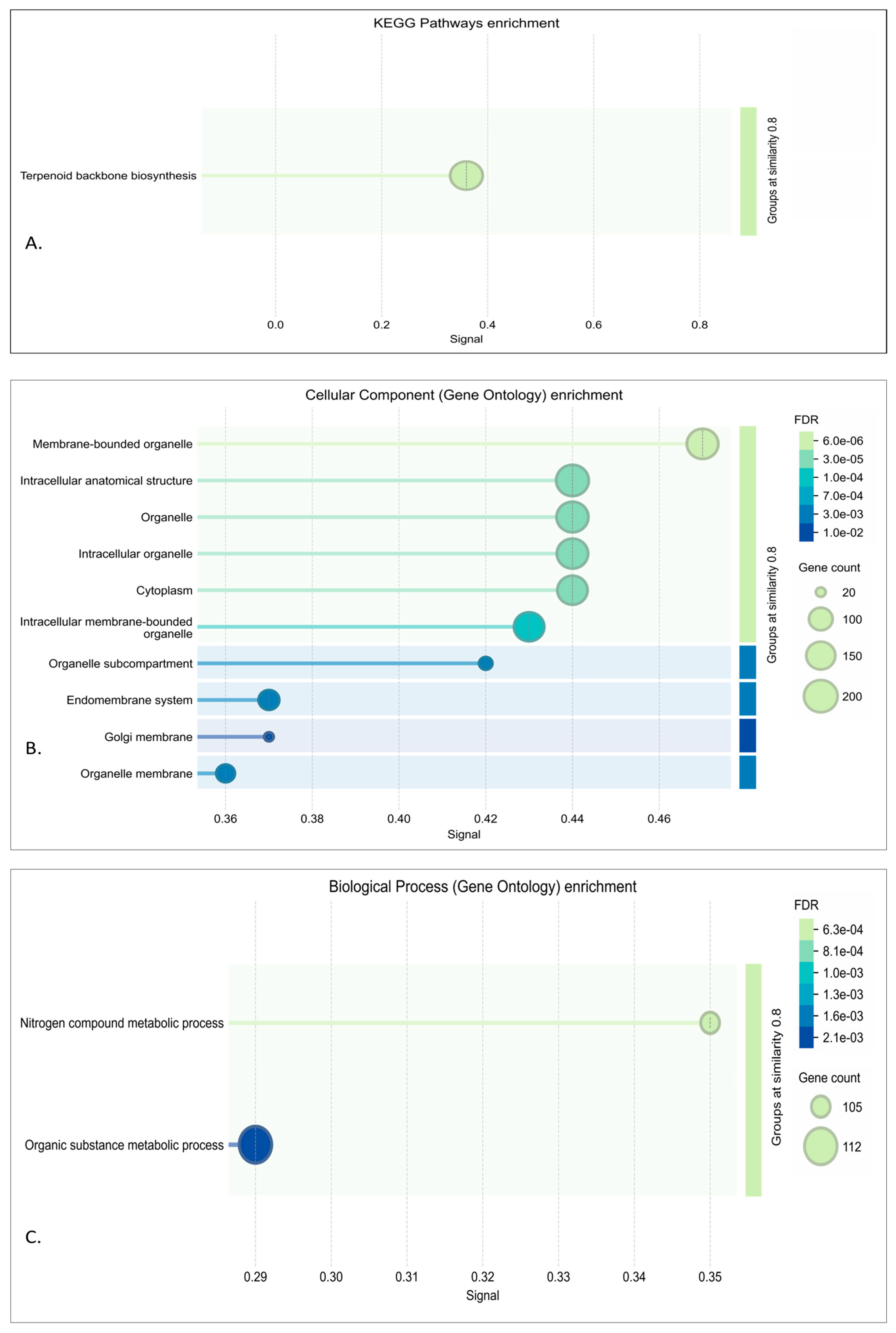
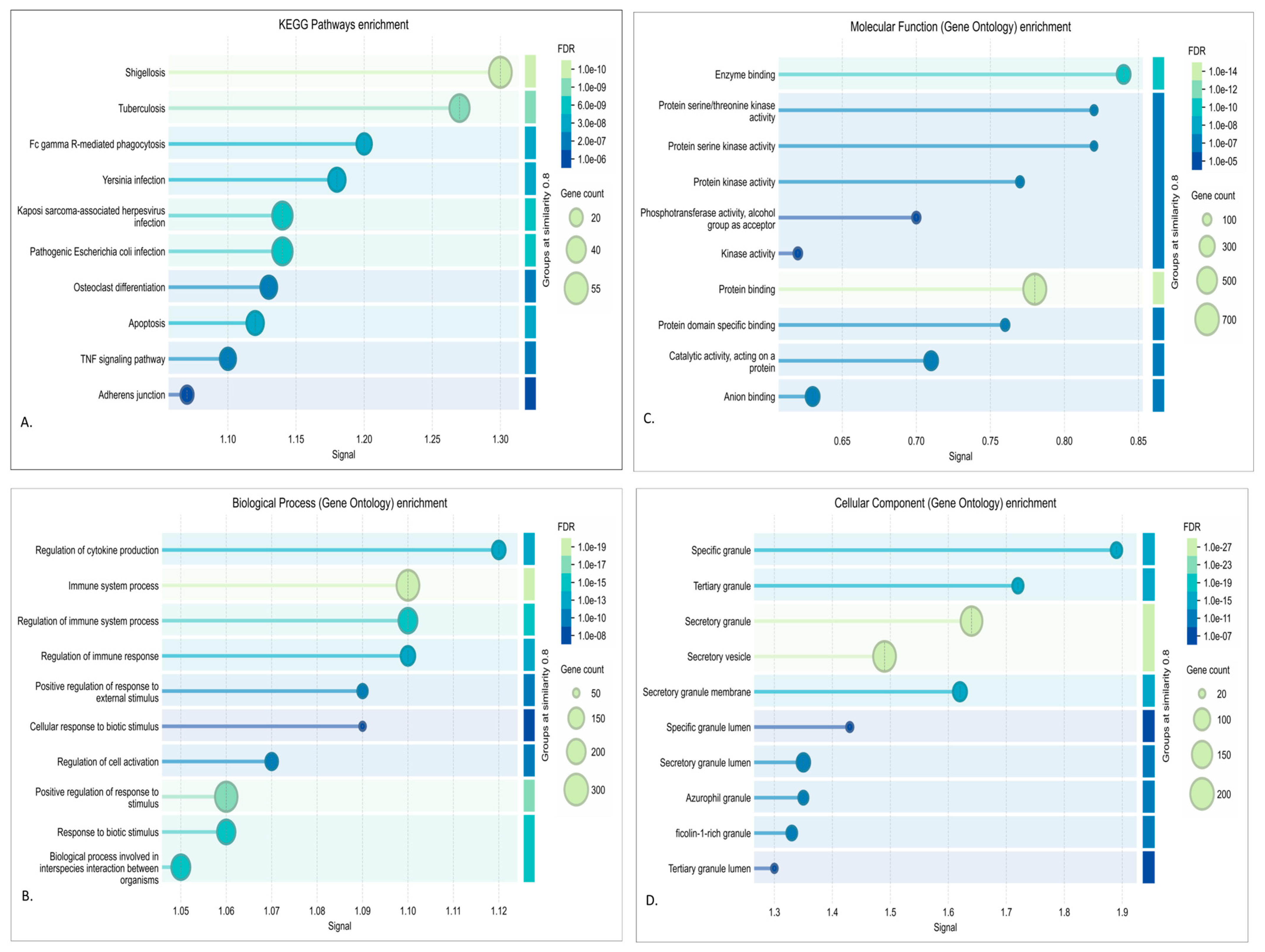
2.4. Function Annotation, Pathway Analysis, and Network Characterization of Modules
2.5. DEA of Hub Genes
| Tier | Criteria | Rationale and Reference |
|---|---|---|
| Tier 1—High Confidence | FDR/p ≤ 0.05 | Commonly used in ND studies, but often misses subtle changes. Utilized by Kurvis et al. [53] and Chen et al. [54] in their papers. |
| Tier 2—Moderate Confidence | Nominal p < 0.05 | Bases are on log2FC. It is seen to be utilized in the paper of Salemi et al. [51] and Lai et al. [52]. |
| Tier 3—Cross Disease | Nominal p < 0.05 in ≥2 diseases/datasets, regardless of FDR | This reflects the replication across datasets or diseases; it prioritizes consistency over statistical stringency [55]. This is seen as used in the paper of Li et al. [56] and Goodwani et al. [57]. |
2.6. miRNA-Module Regulatory Determination and Enrichment Analysis
2.7. Integration of Hub Genes with GWAS-Identified Traits
3. Results
3.1. WGCNA
3.1.1. Data Pre-Processing and Estimation of Scale-Free Network
3.1.2. Gene Co-Expression Module Detection Using TOM Similarity
3.2. Module Preservation Analysis
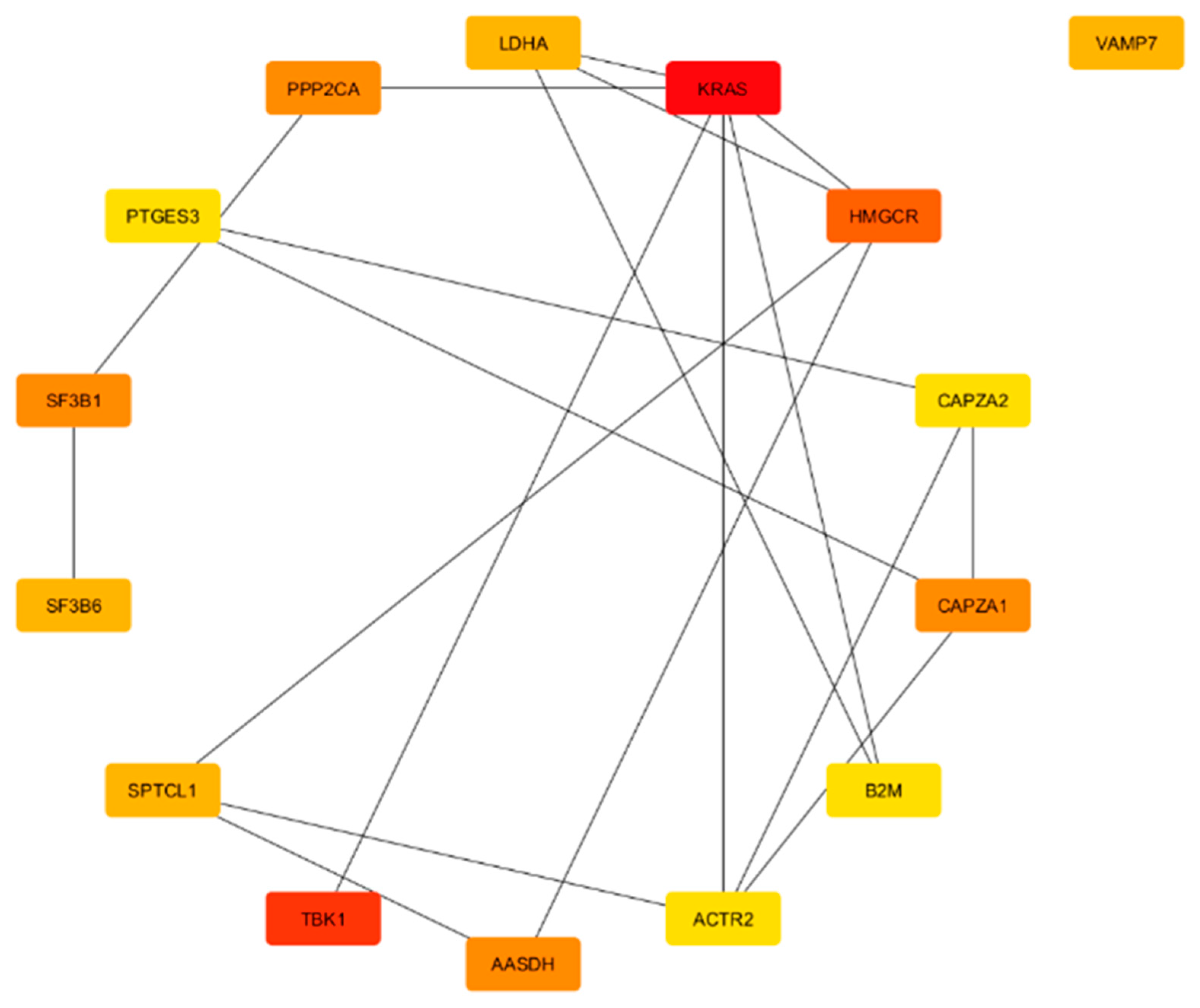
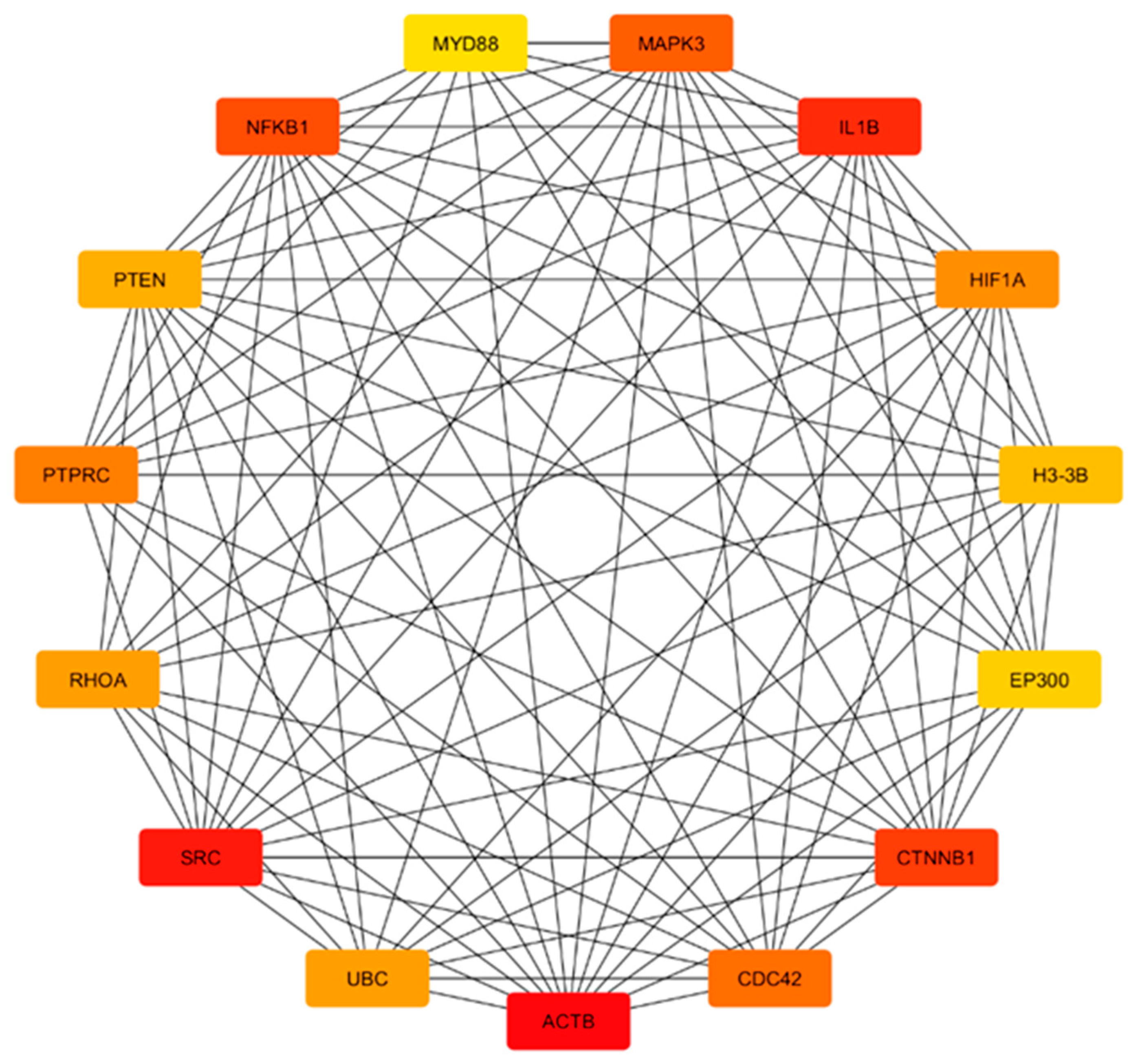
3.3. DEA, Functional Annotation, and PPI Network of Preserved Gene Co-Expression Modules
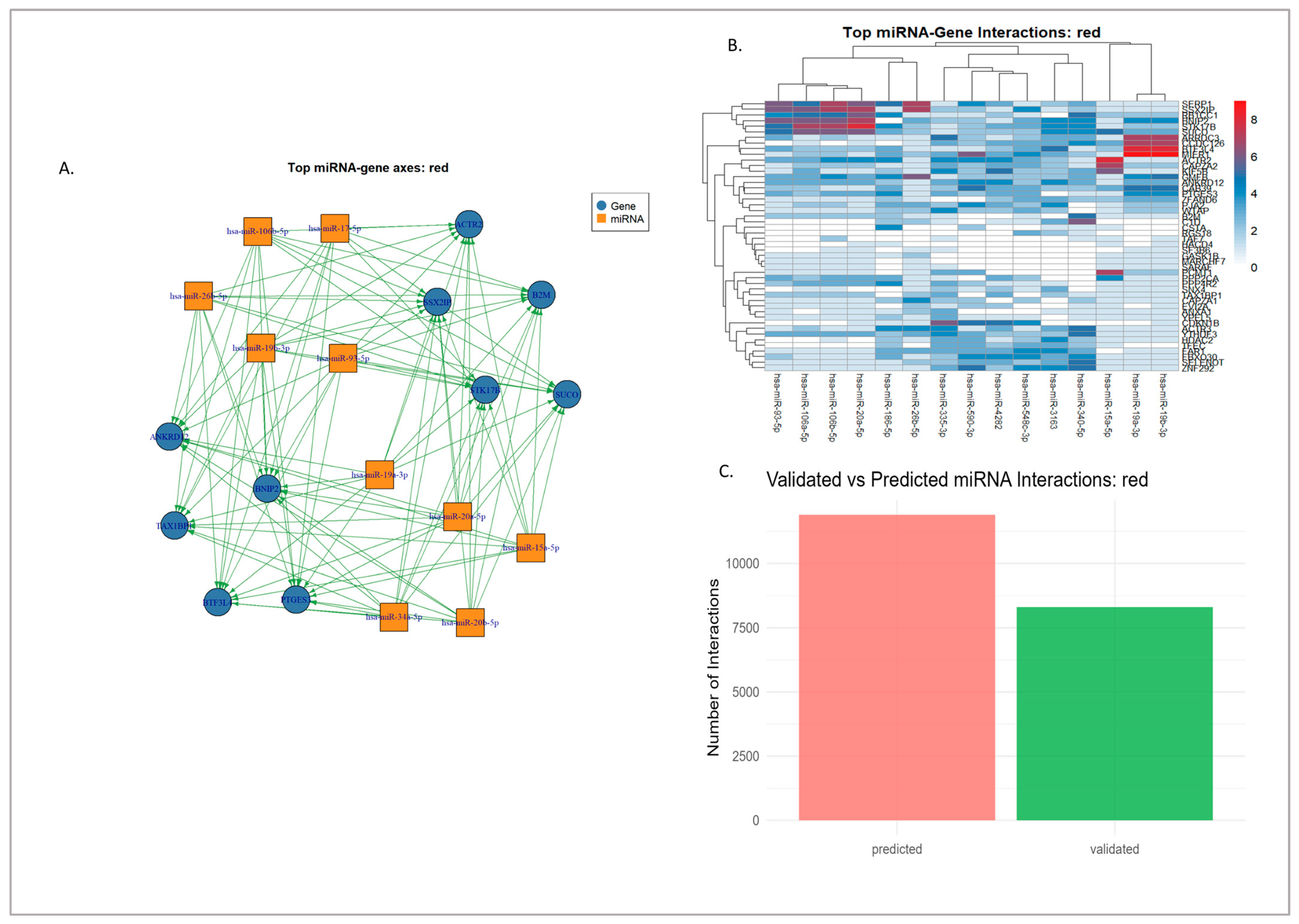
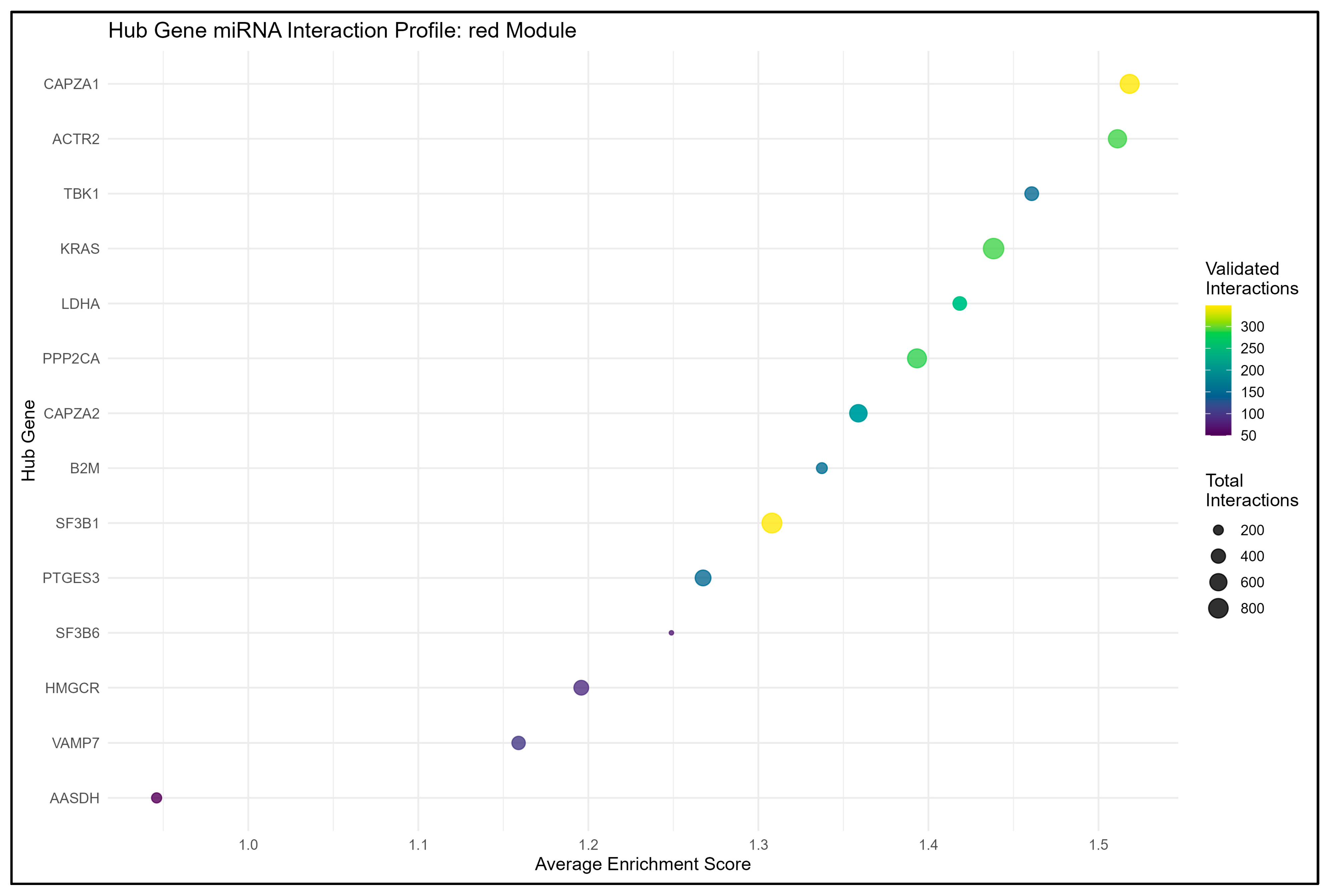
3.3.1. Red Module Result Analysis
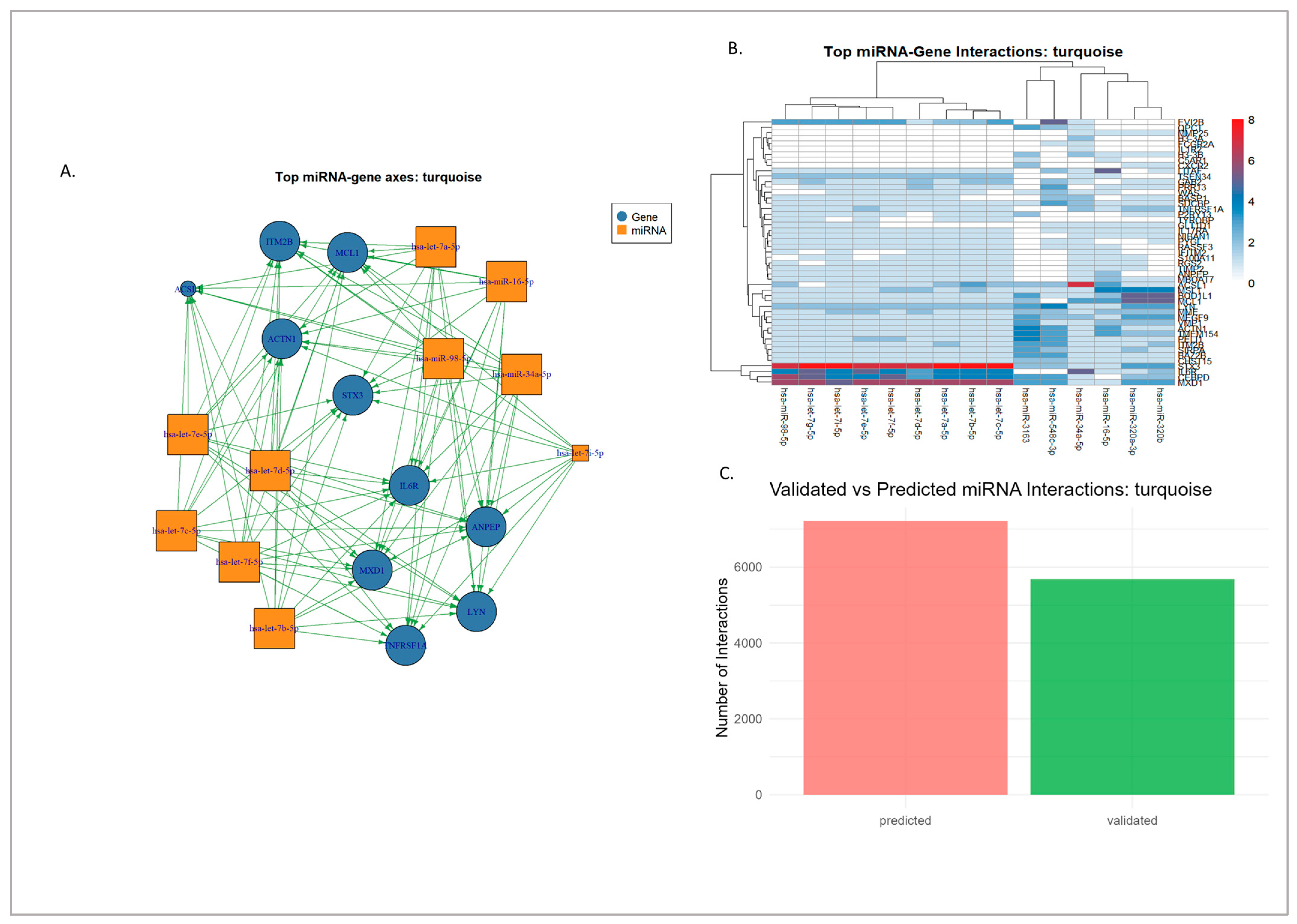
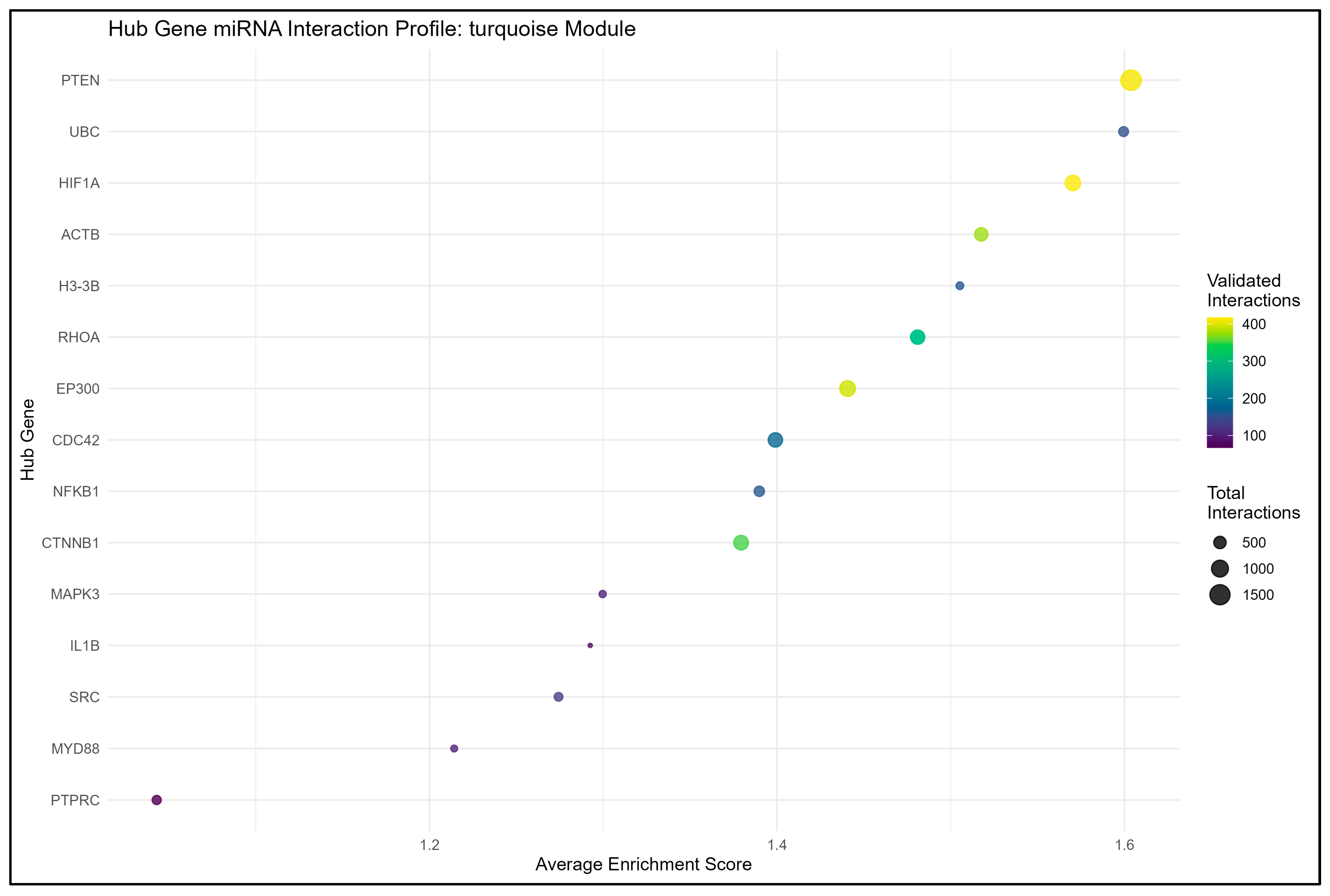
3.3.2. Turquoise Module
3.4. miRNA–Gene Regulatory Interaction of Modules Mapped Through Network Analysis
3.4.1. Experimentally Validated miRNA–Gene Regulatory Networks
3.4.2. Hypothetical Axes from Computationally Predicted Interactions
3.4.3. Synthesis and Prioritized Hub Regulatory Axes
3.5. In Silico Validation of Hub Genes with GWAS Traits
4. Discussion
4.1. Hub Genes as Central Regulators of Pathways in Neurodegeneration
Integration of Hub Genes with Genome-Wide Association Studies
4.2. miRNA–mRNA Regulatory Collapse and Pathway Disinhibition
4.3. Clinical and Translational Relevance of Hub Genes and miRNAs
4.4. Blood vs. Brain Transcriptomic Signatures
4.5. Limitations and Future Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ALS | Amyotrophic Lateral Sclerosis |
| HD | Huntington’s Disease |
| ND | Neurodegenerative Diseases |
| PD | Parkinson’s Disease |
| WGCNA | Weighted Gene Co-Expression Network Analysis |
References
- Hurtley, S.; Alderton, G. Neurodegeneration. Science 2020, 370, 48–49. [Google Scholar] [CrossRef]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Chi, H.; Chang, H.-Y.; Sang, T.-K. Neuronal Cell Death Mechanisms in Major Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Brisbane, Australia, 2018; pp. 3–26. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, J.; Cooper, A.; Morris, M.J. Parkinson’s disease: From genetics to molecular dysfunction and targeted therapeutic approaches. Genes. Dis. 2023, 10, 786–798. [Google Scholar] [CrossRef]
- Coukos, R.; Krainc, D. Key genes and convergent pathogenic mechanisms in Parkinson disease. Nat. Rev. Neurosci. 2024, 25, 393–413. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. Alzheimer’s Disease: Exploring the Landscape of Cognitive Decline. ACS Chem. Neurosci. 2024, 15, 3800–3827. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A. Alzheimer’s disease: The role of proteins in formation, mechanisms, and new therapeutic approaches. Neurosci. Lett. 2023, 817, 137532. [Google Scholar] [CrossRef]
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Petri, S. Targeting C9orf72 in people with ALS. Lancet Neurol. 2024, 23, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Peggion, C.; Scalcon, V.; Massimino, M.L.; Nies, K.; Lopreiato, R.; Rigobello, M.P.; Bertoli, A. SOD1 in ALS: Taking Stock in Pathogenic Mechanisms and the Role of Glial and Muscle Cells. Antioxidants 2022, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, P.B.; Kanhere, H.S.; Bansinge, P.C.; Rathod, S.K.; Khandare, V.S.; Das, R.K. Huntington’s disease: Pathophysiology and therapeutic intervention. GSC Biol. Pharm. Sci. 2021, 15, 171–184. [Google Scholar] [CrossRef]
- Testa, C.M.; Jankovic, J. Huntington disease: A quarter century of progress since the gene discovery. J. Neurol. Sci. 2019, 396, 52–68. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Sugandhi, V.V.; Jha, S.K.; Nangare, S.N.; Gupta, G.; Singh, S.K.; Dua, K.; Cho, H.; Hansbro, P.M.; Paudel, K.R. Neurodegenerative disorders: Mechanisms of degeneration and therapeutic approaches with their clinical relevance. Ageing Res. Rev. 2024, 99, 102357. [Google Scholar] [CrossRef]
- Smail, C.; Montgomery, S.B. RNA Sequencing in Disease Diagnosis. Annu. Rev. Genom. Hum. Genet. 2024, 25, 353–367. [Google Scholar] [CrossRef]
- Zhang, H.; He, L.; Cai, L. Transcriptome Sequencing: RNA-Seq; Springer: New York, NY, USA, 2018; pp. 15–27. [Google Scholar] [CrossRef]
- Lake, J.; Storm, C.S.; Makarious, M.B.; Bandres-Ciga, S. Genetic and Transcriptomic Biomarkers in Neurodegenerative Diseases: Current Situation and the Road Ahead. Cells 2021, 10, 1030. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Li, J.; Zhou, D.; Qiu, W.; Shi, Y.; Yang, J.; Chen, S.; Wang, Q.; Pan, H. Application of Weighted Gene Co-expression Network Analysis for Data from Paired Design. Sci. Rep. 2018, 8, 622. [Google Scholar] [CrossRef]
- Santiago, J.A.; Bottero, V.; Potashkin, J.A. Dissecting the Molecular Mechanisms of Neurodegenerative Diseases through Network Biology. Front. Aging Neurosci. 2017, 9, 166. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Kumar, A. Paradigm shift: microRNAs interact with target gene promoters to cause transcriptional gene activation or silencing. Exp. Cell Res. 2025, 444, 114372. [Google Scholar] [CrossRef]
- de Souza, P.C.; Bezerra, T.P.W.; de Oliveira, I.L.R.; Gomes, P.A.T.d.M.; Rêgo, M.J.B.d.M.; Pereira, M.C.; Pitta, M.G.d.R.; da Rosa, M.M. MicroRNAs in neuroplasticity: A comprehensive review of mechanisms and therapeutic strategies for neurodegenerative diseases. Neuroscience 2025, 585, 97–106. [Google Scholar] [CrossRef]
- Kapplingattu, S.V.; Bhattacharya, S.; Adlakha, Y.K. MiRNAs as major players in brain health and disease: Current knowledge and future perspectives. Cell Death Discov. 2025, 11, 7. [Google Scholar] [CrossRef]
- Clough, E.; Barrett, T. The Gene Expression Omnibus Database; Springer: New York, NY, USA, 2016; pp. 93–110. [Google Scholar] [CrossRef]
- Mastrokolias, A.; Ariyurek, Y.; Goeman, J.J.; van Duijn, E.; Roos, R.A.C.; van der Mast, R.C.; van Ommen, G.; den Dunnen, J.T.; ‘t Hoen, P.A.C.; van Roon-Mom, W.M.C. Huntington’s disease biomarker progression profile identified by transcriptome sequencing in peripheral blood. Eur. J. Hum. Genet. 2015, 23, 1349–1356. [Google Scholar] [CrossRef]
- Grima, N.; Liu, S.; Southwood, D.; Henden, L.; Smith, A.; Lee, A.; Rowe, D.B.; D’Silva, S.; Blair, I.P.; Williams, K.L. RNA sequencing of peripheral blood in amyotrophic lateral sclerosis reveals distinct molecular subtypes: Considerations for biomarker discovery. Neuropathol. Appl. Neurobiol. 2023, 49, 6. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.R.; Wang, Q.; Meechoovet, B.; Siniard, A.L.; Naymik, M.; De Both, M.; Huentelman, M.J.; Caselli, R.J.; Driver-Dunckley, E.; Dunckley, T. DNA Methylation and Expression Profiles of Whole Blood in Parkinson’s Disease. Front. Genet. 2021, 12, 640266. [Google Scholar] [CrossRef]
- Iga, J.; Yoshino, Y.; Ozaki, T.; Tachibana, A.; Kumon, H.; Funahashi, Y.; Mori, H.; Ueno, M.; Ozaki, Y.; Yamazaki, K.; et al. Blood RNA transcripts show changes in inflammation and lipid metabolism in Alzheimer’s disease and mitochondrial function in mild cognitive impairment. J. Alzheimers Dis. Rep. 2024, 8, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Zogopoulos, V.L.; Saxami, G.; Malatras, A.; Papadopoulos, K.; Tsotra, I.; Iconomidou, V.A.; Michalopoulos, I. Approaches in Gene Coexpression Analysis in Eukaryotes. Biology 2022, 11, 1019. [Google Scholar] [CrossRef]
- Wang, N.; Langfelder, P.; Stricos, M.; Ramanathan, L.; Richman, J.B.; Vaca, R.; Plascencia, M.; Gu, X.; Zhang, S.; Tamai, T.K.; et al. Mapping brain gene coexpression in daytime transcriptomes unveils diurnal molecular networks and deciphers perturbation gene signatures. Neuron 2022, 110, 3318–3338.e9. [Google Scholar] [CrossRef] [PubMed]
- Talubo, N.D.D.; Tsai, P.-W.; Tayo, L.L. Comprehensive RNA-Seq Gene Co-Expression Analysis Reveals Consistent Molecular Pathways in Hepatocellular Carcinoma across Diverse Risk Factors. Biology 2024, 13, 765. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Tsai, T.-H.; Shen, Z.-Q.; Chan, Y.-H.; Tu, C.-W.; Tung, C.-Y.; Wang, P.-N.; Tsai, T.-F. Transcriptomic predictors of rapid progression from mild cognitive impairment to Alzheimer’s disease. Alzheimers Res. Ther. 2025, 17, 3. [Google Scholar] [CrossRef]
- Park, M.-K.; Ahn, J.; Lim, J.-M.; Han, M.; Lee, J.-W.; Lee, J.-C.; Hwang, S.-J.; Kim, K.-C. A Transcriptomics-Based Machine Learning Model Discriminating Mild Cognitive Impairment and the Prediction of Conversion to Alzheimer’s Disease. Cells 2024, 13, 1920. [Google Scholar] [CrossRef]
- Mathys, H.; Peng, Z.; Boix, C.A.; Victor, M.B.; Leary, N.; Babu, S.; Abdelhady, G.; Jiang, X.; Ng, A.P.; Ghafari, K.; et al. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer’s disease pathology. Cell 2023, 186, 4365–4385.e27. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zeng, L.; Haure-Mirande, J.-V.; Wang, M.; Huffman, D.M.; Haroutunian, V.; Ehrlich, M.E.; Zhang, B.; Tu, Z. Transcriptomic Changes Highly Similar to Alzheimer’s Disease Are Observed in a Subpopulation of Individuals During Normal Brain Aging. Front Aging Neurosci 2021, 13, 711524. [Google Scholar] [CrossRef]
- Orda, M.A.; Fowler, P.M.P.T.; Tayo, L.L. Modular Hub Genes in DNA Microarray Suggest Potential Signaling Pathway Interconnectivity in Various Glioma Grades. Biology 2024, 13, 206. [Google Scholar] [CrossRef]
- Suratos, K.S.; Orda, M.A.; Tsai, P.-W.; Tayo, L.L. Signaling Pathways in Clear Cell Renal Cell Carcinoma and Candidate Drugs Unveiled through Transcriptomic Network Analysis of Hub Genes. Appl. Sci. 2024, 14, 8768. [Google Scholar] [CrossRef]
- Barretto, A.J.B.; Orda, M.A.; Tsai, P.; Tayo, L.L. Analysis of Modular Hub Genes and Therapeutic Targets across Stages of Non-Small Cell Lung Cancer Transcriptome. Genes. 2024, 15, 1248. [Google Scholar] [CrossRef]
- Pasamba, E.C.; Orda, M.A.; Villanueva, B.H.A.; Tsai, P.-W.; Tayo, L.L. Transcriptomic Analysis of Hub Genes Reveals Associated Inflammatory Pathways in Estrogen-Dependent Gynecological Diseases. Biology 2024, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Manuel, M.T.A.; Tayo, L.L. Navigating the Gene Co-Expression Network and Drug Repurposing Opportunities for Brain Disorders Associated with Neurocognitive Impairment. Brain Sci. 2023, 13, 1564. [Google Scholar] [CrossRef]
- Garcia, J.P.T.; Tayo, L.L. Theoretical Studies of DNA Microarray Present Potential Molecular and Cellular Interconnectivity of Signaling Pathways in Immune System Dysregulation. Genes. 2024, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Xiao, Y.; Hsiao, T.-H.; Suresh, U.; Chen, H.-I.H.; Wu, X.; Wolf, S.E.; Chen, Y. A novel significance score for gene selection and ranking. Bioinformatics 2014, 30, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Tuly, K.; Hossen, M.B.; Islam, M.A.; Kibria, M.K.; Alam, M.S.; Harun-Or-Roshid, M.; Begum, A.A.; Hasan, S.; Mahumud, R.A.; Mollah, M.N.H. Robust Identification of Differential Gene Expression Patterns from Multiple Transcriptomics Datasets for Early Diagnosis, Prognosis, and Therapies for Breast Cancer. Medicina 2023, 59, 1705. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Lanza, G.; Mogavero, M.P.; Cosentino, F.I.I.; Borgione, E.; Iorio, R.; Ventola, G.M.; Marchese, G.; Salluzzo, M.G.; Ravo, M.; et al. A Transcriptome Analysis of mRNAs and Long Non-Coding RNAs in Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 1535. [Google Scholar] [CrossRef]
- Lai, Y.; Lin, H.; Chen, M.; Lin, X.; Wu, L.; Zhao, Y.; Lin, F.; Lin, C. Integration of bulk RNA sequencing and single-cell analysis reveals a global landscape of DNA damage response in the immune environment of Alzheimer’s disease. Front. Immunol. 2023, 14, 1115202. [Google Scholar] [CrossRef]
- Kurvits, L.; Lättekivi, F.; Reimann, E.; Kadastik-Eerme, L.; Kasterpalu, K.M.; Kõks, S.; Taba, P.; Planken, A. Transcriptomic profiles in Parkinson’s disease. Exp. Biol. Med. 2021, 246, 584–595. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Luo, Z.; Chen, X.; Wang, Y.; Qi, B.; Lin, J.; Lin, W.-W.; Sun, C.; Zhou, Y.; et al. Exercise Modifies the Transcriptional Regulatory Features of Monocytes in Alzheimer’s Patients: A Multi-Omics Integration Analysis Based on Single Cell Technology. Front Aging Neurosci 2022, 14, 881488. [Google Scholar] [CrossRef]
- Ghandikota, S.; Sharma, M.; Jegga, A.G. Computational workflow for functional characterization of COVID-19 through secondary data analysis. STAR Protoc. 2021, 2, 100873. [Google Scholar] [CrossRef]
- Li, D.; Han, X.; Farrer, L.A.; Stein, T.D.; Jun, G.R. Transcriptome Signatures for Cognitive Resilience Among Individuals with Pathologically Confirmed Alzheimer Disease. Alzheimer’s Dement. 2024, 20, e090972. [Google Scholar] [CrossRef]
- Goodwani, S.; Lightfoot, Y.L.; Ford, B.G.; Calderon, O.; McReynolds, M.; Allton, K.; Ma, J.; Botte, A.; Trinh, R.T.P.; Pearson, T. RIP Kinase 1 (RIPK1) modulates disease associated microglial response during demyelination. Alzheimer’s Dement. 2023, 19, e077538. [Google Scholar] [CrossRef]
- Buniello, A.; Suveges, D.; Cruz-Castillo, C.; Llinares, M.B.; Cornu, H.; Lopez, I.; Tsukanov, K.; Roldán-Romero, J.M.; Mehta, C.; Fumis, L.; et al. Open Targets Platform: Facilitating therapeutic hypotheses building in drug discovery. Nucleic Acids Res. 2025, 53, D1467–D1475. [Google Scholar] [CrossRef]
- Perier, C.; Vila, M. Mitochondrial Biology and Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2019, 2, a009332. [Google Scholar] [CrossRef]
- Álvarez-Luquín, D.D.; González-Fernández, R.R.; Torres-Velasco, M.E.; Ichikawa-Escamilla, E.; Arce-Sillas, A.; Martínez-Martínez, E.; Miranda-Narvaez, C.L.; Rodríguez-Ramírez, J.F.; Adalid-Peralta, L. Neurodegeneration models in Parkinson’s disease: Cellular and molecular paths to neuron death. Behav. Brain Funct. 2025, 21, 14. [Google Scholar] [CrossRef]
- Vengatharajuloo, V.; Goh, H.-H.; Hassan, M.; Govender, N.; Sulaiman, S.; Afiqah-Aleng, N.; Harun, S.; Mohamed-Hussein, Z.-A. Gene Co-Expression Network Analysis Reveals Key Regulatory Genes in Metisa plana Hormone Pathways. Insects 2023, 14, 503. [Google Scholar] [CrossRef]
- Wijesooriya, K.; Jadaan, S.A.; Perera, K.L.; Kaur, T.; Ziemann, M. Urgent need for consistent standards in functional enrichment analysis. PLoS Comput. Biol. 2022, 18, e1009935. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef]
- Huo, J.; Wang, L.; Tian, Y.; Sun, W.; Zhang, G.; Zhang, Y.; Liu, Y.; Zhang, J.; Yang, X.; Liu, Y. Gene Co-Expression Analysis Identified Preserved and Survival-Related Modules in Severe Blunt Trauma, Burns, Sepsis, and Systemic Inflammatory Response Syndrome. Int. J. Gen. Med. 2021, 14, 7065–7076. [Google Scholar] [CrossRef]
- Nouri, N.; Gaglia, G.; Mattoo, H.; de Rinaldis, E.; Savova, V. GENIX enables comparative network analysis of single-cell RNA sequencing to reveal signatures of therapeutic interventions. Cell Rep. Methods 2024, 4, 100794. [Google Scholar] [CrossRef]
- Benn, J.A.; Mukadam, A.S.; McEwan, W.A. Targeted protein degradation using intracellular antibodies and its application to neurodegenerative disease. Semin. Cell Dev. Biol. 2022, 126, 138–149. [Google Scholar] [CrossRef]
- Bal, M.; Leitz, J.; Reese, A.L.; Ramirez, D.M.O.; Durakoglugil, M.; Herz, J.; Monteggia, L.M.; Kavalali, E.T. Reelin Mobilizes a VAMP7-Dependent Synaptic Vesicle Pool and Selectively Augments Spontaneous Neurotransmission. Neuron 2013, 80, 934–946. [Google Scholar] [CrossRef]
- Lázaro-Mixteco, P.E.; González-Coronel, J.M.; Hernández-Padilla, L.; Martínez-Alcantar, L.; Martínez-Carranza, E.; López-Bucio, J.S.; Guevara-García, Á.A.; Campos-García, J. Transcriptomics Reveals the Mevalonate and Cholesterol Pathways Blocking as Part of the Bacterial Cyclodipeptides Cytotoxic Effects in HeLa Cells of Human Cervix Adenocarcinoma. Front. Oncol. 2022, 12, 790537. [Google Scholar] [CrossRef]
- Wlodarchak, N.; Xing, Y. PP2A as a master regulator of the cell cycle. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 162–184. [Google Scholar] [CrossRef]
- Sodero, A.O.; Barrantes, F.J. Pleiotropic effects of statins on brain cells. Biochim. Biophys. Acta BBA Biomembr. 2020, 1862, 183340. [Google Scholar] [CrossRef]
- Stradal, T.E.B.; Sanders, M.B.; Bieling, P. Arp2/3-complex regulation—Novel insights and open questions. Curr. Opin. Cell Biol. 2025, 95, 102565. [Google Scholar] [CrossRef]
- Wurz, A.I.; Schulz, A.M.; O’Bryant, C.T.; Sharp, J.F.; Hughes, R.M. Cytoskeletal dysregulation and neurodegenerative disease: Formation, monitoring, and inhibition of cofilin-actin rods. Front. Cell. Neurosci. 2022, 16, 982074. [Google Scholar] [CrossRef]
- Oakes, J.A.; Davies, M.C.; Collins, M.O. TBK1: A new player in ALS linking autophagy and neuroinflammation. Mol. Brain 2017, 10, 5. [Google Scholar] [CrossRef]
- Harding, O.; Evans, C.S.; Ye, J.; Cheung, J.; Maniatis, T.; Holzbaur, E.L.F. ALS- and FTD-associated missense mutations in TBK1 differentially disrupt mitophagy. Proc. Natl. Acad. Sci. USA 2021, 118, e2025053118. [Google Scholar] [CrossRef]
- Nikom, D.; Zheng, S. Alternative splicing in neurodegenerative disease and the promise of RNA therapies. Nat. Rev. Neurosci. 2023, 24, 457–473. [Google Scholar] [CrossRef]
- Bergot, T.; Lippert, E.; Douet-Guilbert, N.; Commet, S.; Corcos, L.; Bernard, D.G. Human Cancer-Associated Mutations of SF3B1 Lead to a Splicing Modification of Its Own RNA. Cancers 2020, 12, 652. [Google Scholar] [CrossRef]
- Guo, M.; Liu, L.; Mao, X.; Xiao, M.; He, X.; Pan, X.; Chen, Y.; Yi, W.; Li, Q.; Piao, X.; et al. Deciphering the role of CAPZA2 in neurodevelopmental disorders: Insights from mouse models. Commun. Biol. 2025, 8, 1048. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Tang, F.; Yang, J.-Z.; Chen, X.; Wang, Z.-F.; Li, Z.-Q. The Role of Beta2-Microglobulin in Central Nervous System Disease. Cell Mol. Neurobiol. 2024, 44, 46. [Google Scholar] [CrossRef]
- Hotulainen, P.; Hoogenraad, C.C. Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol. 2010, 189, 619–629. [Google Scholar] [CrossRef]
- Myers, K.R.; Fan, Y.; McConnell, P.; Cooper, J.A.; Zheng, J.Q. Actin capping protein regulates postsynaptic spine development through CPI-motif interactions. Front. Mol. Neurosci. 2022, 15, 1020949. [Google Scholar] [CrossRef]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- AbdAllah, N.B.; Toraih, E.A.; Al Ageeli, E.; Elhagrasy, H.; Gouda, N.S.; Fawzy, M.S.; Helal, G.M. MYD88, NFKB1, and IL6 transcripts overexpression are associated with poor outcomes and short survival in neonatal sepsis. Sci. Rep. 2021, 11, 13374. [Google Scholar] [CrossRef]
- Sugiyama, K.; Muroi, M.; Kinoshita, M.; Hamada, O.; Minai, Y.; Sugita-Konishi, Y.; Kamata, Y.; Tanamoto, K. NF-κB activation via MyD88-dependent Toll-like receptor signaling is inhibited by trichothecene mycotoxin deoxynivalenol. J. Toxicol. Sci. 2016, 41, 273–279. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Cardona, A.E. The IL-1β phenomena in neuroinflammatory diseases. J. Neural Transm. 2018, 125, 781–795. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wu, H. Ubiquitination-Proteasome System (UPS) and Autophagy Two Main Protein Degradation Machineries in Response to Cell Stress. Cells 2022, 11, 851. [Google Scholar] [CrossRef]
- Lu, X.; Deng, Y.; Yu, D.; Cao, H.; Wang, L.; Liu, L.; Yu, C.; Zhang, Y.; Guo, X.; Yu, G. Histone Acetyltransferase p300 Mediates Histone Acetylation of PS1 and BACE1 in a Cellular Model of Alzheimer’s Disease. PLoS ONE 2014, 9, e103067. [Google Scholar] [CrossRef]
- Matousek, S.B.; Ghosh, S.; Shaftel, S.S.; Kyrkanides, S.; Olschowka, J.A.; O’Banion, M.K. Chronic IL-1β-Mediated Neuroinflammation Mitigates Amyloid Pathology in a Mouse Model of Alzheimer’s Disease without Inducing Overt Neurodegeneration. J. Neuroimmune Pharmacol. 2012, 7, 156–164. [Google Scholar] [CrossRef]
- Ghosh, S.; Wu, M.D.; Shaftel, S.S.; Kyrkanides, S.; LaFerla, F.M.; Olschowka, J.A.; O’Banion, M.K. Sustained Interleukin-1β Overexpression Exacerbates Tau Pathology Despite Reduced Amyloid Burden in an Alzheimer’s Mouse Model. J. Neurosci. 2013, 33, 5053–5064. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Chang, Y.; Yan, S.S.; Shi, H. Hypoxia Inducible Factor-1 as a Target for Neurodegenerative Diseases. Curr. Med. Chem. 2011, 18, 4335–4343. [Google Scholar] [CrossRef]
- Kinger, S.; Jagtap, Y.A.; Kumar, P.; Choudhary, A.; Prasad, A.; Prajapati, V.K.; Kumar, A.; Mehta, G.; Mishra, A. Proteostasis in neurodegenerative diseases. Adv. Clin. Chem. 2024, 121, 270–333. [Google Scholar] [CrossRef]
- Höhn, A.; Tramutola, A.; Cascella, R. Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 5497046. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Huang, J.; Pang, X.; Yang, H.; Gao, C.; Wang, D.; Sun, Y.; Taishi, Y.; Pang, C. Molecular Mechanisms of GFAP and PTPRC in Alzheimer’s Disease: An Analysis of Neuroinflammatory Response and Progression. Curr. Alzheimer Res. 2024, 21, 395–410. [Google Scholar] [CrossRef]
- Ohtake, Y.; Hayat, U.; Li, S. PTEN inhibition and axon regeneration and neural repair. Neural Regen. Res. 2015, 10, 1363. [Google Scholar] [CrossRef]
- Lim, S.M.; Nahm, M.; Kim, S.H. Proteostasis and Ribostasis Impairment as Common Cell Death Mechanisms in Neurodegenerative Diseases. J. Clin. Neurol. 2023, 19, 101. [Google Scholar] [CrossRef]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front Neurol 2023, 14, 1103416. [Google Scholar] [CrossRef]
- Zhang, R.-L.; Wang, W.-M.; Li, J.-Q.; Li, R.-W.; Zhang, J.; Wu, Y.; Liu, Y. The role of miR-155 in cardiovascular diseases: Potential diagnostic and therapeutic targets. Int. J. Cardiol. Cardiovasc. Risk Prev. 2025, 24, 200355. [Google Scholar] [CrossRef]
- Mu, C.; Gao, M.; Xu, W.; Sun, X.; Chen, T.; Xu, H.; Qiu, H. Mechanisms of microRNA-132 in central neurodegenerative diseases: A comprehensive review. Biomed. Pharmacother. 2024, 170, 116029. [Google Scholar] [CrossRef]
- Maes, O.C.; Chertkow, H.M.; Wang, E.; Schipper, H.M. MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders. Curr. Genom. 2009, 10, 154–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Wang, M.; Li, L.; Huang, J.; Wang, S. Bibliometric analysis of PTEN in neurodevelopment and neurodegeneration. Front. Aging Neurosci. 2024, 16, 1390324. [Google Scholar] [CrossRef]
- Pratt, W.B.; Gestwicki, J.E.; Osawa, Y.; Lieberman, A.P. Targeting Hsp90/Hsp70-Based Protein Quality Control for Treatment of Adult Onset Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 353–371. [Google Scholar] [CrossRef]
- Evans, C.G.; Chang, L.; Gestwicki, J.E. Heat Shock Protein 70 (Hsp70) as an Emerging Drug Target. J. Med. Chem. 2010, 53, 4585–4602. [Google Scholar] [CrossRef]
- Wu, J.; Niu, P.; Zhao, Y.; Cheng, Y.; Chen, W.; Lin, L.; Lu, J.; Cheng, X.; Xu, Z. Impact of miR-223-3p and miR-2909 on inflammatory factors IL-6, IL-1ß, and TNF-α, and the TLR4/TLR2/NF-κB/STAT3 signaling pathway induced by lipopolysaccharide in human adipose stem cells. PLoS ONE 2019, 14, e0212063. [Google Scholar] [CrossRef]
- Naqvi, R.A.; Gupta, M.; George, A.; Naqvi, A.R. MicroRNAs in shaping the resolution phase of inflammation. Semin. Cell Dev. Biol. 2022, 124, 48–62. [Google Scholar] [CrossRef]
- Huang, D.; Cao, L.; Xiao, L.; Song, J.-X.; Zhang, Y.-J.; Zheng, P.; Zheng, S.-G. Hypoxia induces actin cytoskeleton remodeling by regulating the binding of CAPZA1 to F-actin via PIP2 to drive EMT in hepatocellular carcinoma. Cancer Lett. 2019, 448, 117–127. [Google Scholar] [CrossRef]
- Zheng, S.; Qin, F.; Yin, J.; Li, D.; Huang, Y.; Hu, L.; He, L.; Lv, C.; Li, X.; Li, S.; et al. Role and mechanism of actin-related protein 2/3 complex signaling in cancer invasion and metastasis: A review. Medicine 2023, 102, e33158. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xie, G.; Li, X.; Xiong, J. Cytoskeletal Proteins and Alzheimer’s Disease Pathogenesis: Focusing on the Interplay with Tau Pathology. Biomolecules 2025, 15, 831. [Google Scholar] [CrossRef]
- Theunissen, F.; West, P.K.; Brennan, S.; Petrović, B.; Hooshmand, K.; Akkari, P.A.; Keon, M.; Guennewig, B. New perspectives on cytoskeletal dysregulation and mitochondrial mislocalization in amyotrophic lateral sclerosis. Transl. Neurodegener. 2021, 10, 46. [Google Scholar] [CrossRef]
- Campos-Peña, V.; Pichardo-Rojas, P.; Sánchez-Barbosa, T.; Ortíz-Islas, E.; Rodríguez-Pérez, C.E.; Montes, P.; Ramos-Palacios, G.; Sil-va-Adaya, D.; Valencia-Quintana, R.; Cerna-Cortes, J.F.; et al. Amyloid β, Lipid Metabolism, Basal Cholinergic System, and Therapeutics in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12092. [Google Scholar] [CrossRef]
- Pan, D.; Huang, Y.; Jiang, D.; Zhang, Y.; Wu, M.; Han, M.; Jin, X. Discovery of an EP300 Inhibitor using Structure-based Virtual Screening and Bioactivity Evaluation. Curr. Pharm. Des. 2024, 30, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.J.; Margue, C.; Behrmann, I.; Kreis, S. MiRNA-29: A microRNA Family with Tumor-Suppressing and Immune-Modulating Properties. Curr. Mol. Med. 2013, 13, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Shaheen, A.; Osama, M.; Nashwan, A.J.; Bharmauria, V.; Flouty, O. MicroRNAs regulation in Parkinson’s disease, and their potential role as diagnostic and therapeutic targets. NPJ Park. Dis. 2024, 10, 186. [Google Scholar] [CrossRef]
- McGovern, A.J.; González, J.; Ramírez, D.; Barreto, G.E. Identification of HMGCR, PPGARG and prohibitin as potential druggable targets of dihydrotestosterone for treatment against traumatic brain injury using system pharmacology. Int. Immunopharmacol. 2022, 108, 108721. [Google Scholar] [CrossRef]
- Medina, M.W.; Krauss, R.M. The Role of HMGCR Alternative Splicing in Statin Efficacy. Trends Cardiovasc. Med. 2009, 19, 173–177. [Google Scholar] [CrossRef]
- Rosoff, D.B.; Bell, A.S.; Jung, J.; Wagner, J.; Mavromatis, L.A.; Lohoff, F.W. Mendelian Randomization Study of PCSK9 and HMG-CoA Reductase Inhibition and Cognitive Function. J. Am. Coll. Cardiol. 2022, 80, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Deigendesch, N.; Müller, K.; Weishaupt, J.H.; Krannich, A.; Röhle, R.; Meissner, F.; Molawi, K.; Münch, C.; Holm, T.; et al. Interleukin-1 Antagonist Anakinra in Amyotrophic Lateral Sclerosis—A Pilot Study. PLoS ONE 2015, 10, e0139684. [Google Scholar] [CrossRef]
- Retinasamy, T.; Lee, A.L.Y.; Lee, H.S.; Lee, V.L.L.; Shaikh, M.F.; Yeong, K.Y. Repurposing Anakinra for Alzheimer’s Disease: The In Vitro and In Vivo Effects of Anakinra on LPS- and AC-Induced Neuroinflammation. ACS Chem. Neurosci. 2024, 15, 3298–3310. [Google Scholar] [CrossRef]
- Kaidery, N.A.; Ahuja, M.; Sharma, S.M.; Thomas, B. An Emerging Role of miRNAs in Neurodegenerative Diseases: Mechanisms and Perspectives on miR146a. Antioxid. Redox Signal 2021, 35, 580–594. [Google Scholar] [CrossRef]
- Li, S.; Lei, Z.; Sun, T. The role of microRNAs in neurodegenerative diseases: A review. Cell Biol. Toxicol. 2023, 39, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Steinert, J.R.; Marczylo, E.L.; D’Oro, S.; Fiore, R.; Forsythe, I.D.; Schratt, G.; Zoli, M.; Nicotera, P.; Young, K.W. Targeting of the Arpc3 actin nucleation factor by miR-29a/b regulates dendritic spine morphology. J. Cell Biol. 2011, 194, 889–904. [Google Scholar] [CrossRef]
- Banzhaf-Strathmann, J.; Benito, E.; May, S.; Arzberger, T.; Tahirovic, S.; Kretzschmar, H.; Fischer, A.; Edbauer, D. Micro RNA--125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014, 33, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Song, J.; Ouyang, Y.; Han, Q.; Chen, W.; Zhao, X.; Xie, Y.; Chen, Y.; Yuan, W.; Fan, C. Advances in Roles of miR-132 in the Nervous System. Front. Pharmacol. 2017, 8, 770. [Google Scholar] [CrossRef]
- Lagos, D.; Pollara, G.; Henderson, S.; Gratrix, F.; Fabani, M.; Milne, R.S.B.; Gotch, F.; Boshoff, C. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat. Cell Biol. 2010, 12, 513–519. [Google Scholar] [CrossRef]
- Villanueva, B.H.A.; Tsai, P.-W.; Tayo, L.L. Analysis of Gene Expression Networks in Primary Central Nervous System Tumors for Drug Repurposing Prospects. arXiv 2023. [Google Scholar] [CrossRef]
- Shvetcov, A.; Thomson, S.; Spathos, J.; Cho, A.-N.; Wilkins, H.M.; Andrews, S.J.; Delerue, F.; Couttas, T.A.; Issar, J.K.; Isik, F.; et al. Blood-Based Transcriptomic Biomarkers Are Predictive of Neurodegeneration Rather Than Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 15011. [Google Scholar] [CrossRef]
- Yu, H.; Wang, F.; Wu, J.-J.; Gong, J.; Bi, S.; Mao, Y.; Jia, D.; Chai, G.-S. Integrated transcriptomics reveals the brain and blood biomarkers in Alzheimer’s disease. CNS Neurosci. Ther. 2023, 29, 3943–3951. [Google Scholar] [CrossRef]
- Irmady, K.; Hale, C.R.; Qadri, R.; Fak, J.; Simelane, S.; Carroll, T.; Przedborski, S.; Darnell, R.B. Blood transcriptomic signatures associated with molecular changes in the brain and clinical outcomes in Parkinson’s disease. Nat. Commun. 2023, 14, 3956. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, I.; Gispert, J.D.; Palumbo, E.; Muñoz-Aguirre, M.; Wucher, V.; D’Argenio, V.; Santpere, G.; Navarro, A.; Guigo, R.; Vilor-Tejedor, N. Brain transcriptomic profiling reveals common alterations across neurodegenerative and psychiatric disorders. Comput. Struct. Biotechnol. J. 2022, 20, 4549–4561. [Google Scholar] [CrossRef] [PubMed]

| Accession No. | GSE51799 [30] | GSE234297 [31] | GSE165082 [32] | GSE249477 [33] |
|---|---|---|---|---|
| Condition | Huntington’s Disease | Amyotrophic Lateral Sclerosis (ALS) | Parkinson’s Disease | Alzheimer’s Disease |
| Type | Expression Profiling by High Throughput Sequencing | |||
| Source | Blood Samples | |||
| No. of Samples | 124 | 132 | 26 | 62 |
| Z Summary Score | Interpretation |
|---|---|
| Z summary > 10 | Strong evidence of preservation |
| 2 < Z summary ≤ 10 | Moderate evidence of preservation |
| Z summary ≤ 2 | No Evidence of Preservation |
| Module | Preservation Pattern Across HD, PD, and ALS |
|---|---|
| Red | Preserved across all diseases |
| Turquoise | Preserved in HD, ALS; disrupted in PD |
| Blue | Stable in HD, ALS; weaker in PD |
| Green | Stable in HD, ALS; disrupted in PD |
| Yellow | Variable across diseases |
| Brown | Weak in HD; modest in ALS |
| Gold | Disrupted in PD |
| Pink | Variable across diseases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Cerna, J.L.O.; Talubo, N.D.D.; Villanueva, B.H.A.; Tsai, P.-W.; Tayo, L.L. Conserved Blood Transcriptome Patterns Highlight microRNA and Hub Gene Drivers of Neurodegeneration. Genes 2025, 16, 1178. https://doi.org/10.3390/genes16101178
De La Cerna JLO, Talubo NDD, Villanueva BHA, Tsai P-W, Tayo LL. Conserved Blood Transcriptome Patterns Highlight microRNA and Hub Gene Drivers of Neurodegeneration. Genes. 2025; 16(10):1178. https://doi.org/10.3390/genes16101178
Chicago/Turabian StyleDe La Cerna, Jhyme Lou O., Nicholas Dale D. Talubo, Brian Harvey Avanceña Villanueva, Po-Wei Tsai, and Lemmuel L. Tayo. 2025. "Conserved Blood Transcriptome Patterns Highlight microRNA and Hub Gene Drivers of Neurodegeneration" Genes 16, no. 10: 1178. https://doi.org/10.3390/genes16101178
APA StyleDe La Cerna, J. L. O., Talubo, N. D. D., Villanueva, B. H. A., Tsai, P.-W., & Tayo, L. L. (2025). Conserved Blood Transcriptome Patterns Highlight microRNA and Hub Gene Drivers of Neurodegeneration. Genes, 16(10), 1178. https://doi.org/10.3390/genes16101178








