Abstract
Background/Objectives: Late diagnosis and inefficient treatment regimens lead to poor prognosis, with a low 5-year survival rate for both non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). New targeted therapeutic agents can be developed and introduced only by first discovering new driver oncogenes and with a thorough investigation of the known driver genes. The aim of the current study is to investigate the prevalence of alterations in the eight most frequently altered genes in lung cancer—BRAF, EGFR, KRAS, ALK, ROS1, HER2, PD-L1 and PIK3CA. Methods: Real-time polymerase chain reaction (RT-PCR) was used to detect KRAS and EGFR mutations, multiplex PCR and microarray hybridization for KRAS/BRAF/PIK3CA mutations. Immunohistochemical analysis was performed for the detection of ALK, HER2/NEU, ROS-1 and PD-L1 alterations. Results: Overall, 221/603 patients (36.65%) had at least one genetic alteration, of which 22 patients (3.65%) had two genetic alterations and two patients had more than two genetic alterations. Additionally, 50 patients were identified with one or more KRAS mutations (8.29%), 45 patients with EGFR mutations (7.46%), and 1.82% with PIK3CA mutations and 0.66% with BRAF mutations. Furthermore, 50% of the co-occurring alterations were either on KRAS and PIK3CA genes (3/6), on KRAS and BRAF genes (2/6, 33.33%) or on EGFR and PIK3CA genes (1/6, 16.67%), and 10.45% of the patients exhibited PD-L1 overexpression, 5.31% ALK rearrangements, and 2.36% HER2/NEU expression, with no ROS-1 rearrangements detected. Conclusions: Comprehensive testing for somatic alterations in EGFR, BRAF, KRAS, and PIK3CA is significant in guiding therapeutic decisions in lung cancer management. Such testing should be routinely conducted to establish a thorough genetic profile of lung cancers in a manner that is both time-efficient and cost-effective.
1. Introduction
The World Health Organization (WHO)’s International Agency for Research on Cancer (IARC) GLOBOCAN cancer statistics 2022 reports lung cancer as the leading cause of cancer death, with an estimated 1.8 million deaths (18%). It is the most frequently diagnosed cancer worldwide, with 2,480,675 new cases in 2022. The figures are sharply rising in the female population and in emerging countries [1]. In North Macedonia in 2022, lung cancer was both the most commonly diagnosed cancer and the leading cancer death cause, with 1150 new lung cancer cases, representing 15.2% of all new cancer cases diagnosed in 2022 and 1002 deaths [2].
Based on the National Comprehensive Cancer Network (NCCN) Guideline, 85–90% of lung cancers are caused by voluntary or involuntary (secondary) cigarette smoking [3]. Other possible risk factors include disease history (e.g., chronic obstructive pulmonary disease (COPD)), cancer history, family history of lung cancer, and exposure to other carcinogens [4,5], including nickel, silica, fumes, diesel, beryllium, arsenic, cadmium, chromium and asbestos [6,7]. The majority of patients either have metastatic disease at diagnosis or later experience a relapse [8]. In such patients, symptom control, quality of life and prolonged overall survival (OS) are achieved with palliative systemic therapies.
The histological characterization of lung cancer is the cornerstone for selecting systemic therapies. The vast majority of lung cancers (approximately 80%) are non-small-cell lung cancer (NSCLC), including two major types [9]: (1) non-squamous carcinoma, including large-cell lung carcinoma (LCLC), large-cell neuroendocrine carcinoma (LCNEC), adenocarcinoma of lungs and/or bronchii, with or without metastases (e.g., on supraclavicular gland, liver, abdominal, ms ossei c. vertebrae, pleurae, cerebral), and lung cancer not otherwise specified (NOS), and (2) squamous cell (epidermoid) carcinoma of the principal bronchii and/or lungs [10]. The remaining 20% are small-cell lung cancers (SCLCs). Lung cancer classifications are enhanced through the validation of recurring oncogenic mutations, which serve as predictive biomarkers for identifying treatable oncogenic dependencies. Understanding lung cancer morphology is essential due to its diverse spectrum, with many atypical characteristics that complicate accurate diagnosis. In this context, comprehensive genomic analyses are conducted to identify various predictive and prognostic biomarkers, as well as gene expression profiles. The resulting data facilitate the differentiation of cancer subgroups based on molecular phenotypes [11], which is vital for the selection of appropriate treatment strategies.
The primary treatment modalities for patients with NSCLC include surgery, radiotherapy (RT), and systemic therapy. These approaches may be utilized individually or in combination, contingent upon the specific status of the disease. Surgical resection is considered the standard care for patients with stage I or II disease and for single-station non-bulky IIIA disease (N2, metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s)) [12]. For IB (tumor ≥ 4 cm) to IIIA patients, it is usually followed by platinum-doublet chemotherapy, with very few trials showing the efficacy of adjuvant immunotherapy in stage IB to IIIA and several additional ongoing trials evaluating adjuvant oral tyrosine kinase inhibitors (TKIs) or immunotherapy [12]. RT, preferably stereotactic ablative radiotherapy, is recommended for I-IV stage patients in different regimes; the doses and duration depend on the NSCLC stage (e.g., in locally advanced NSCLC; in early-stage NSCLC with contraindications for surgery, e.g., major medical comorbidity, severely limited lung function; or as preoperative or postoperative therapy, as therapy for limited recurrences and oligometastases or as palliative therapy for patients with incurable NSCLC) [13,14,15,16]. The standard systemic therapy for NSCLC typically involves platinum-doublet chemotherapy. This regimen includes the use of cisplatin or carboplatin in combination with pemetrexed for patients with non-squamous metastatic NSCLC. For patients with squamous metastatic NSCLC, cisplatin or carboplatin may be administered alongside either paclitaxel or gemcitabine [12]. The platinum-based combinations have comparable efficacy and low overall response rate (RR), ranging between 17 and 32% [17]. The results are less than satisfactory, as sustainable disease control is infrequently attained due to either intrinsic or acquired drug resistance. For such patients, concurrent or sequential target immunotherapy is crucial. Nonetheless, it has been observed that approximately 85–90% of patients with advanced NSCLC do not exhibit oncogenic alterations that are amenable to targeted therapies, which is associated with a poorer prognosis and an elevated incidence of local relapse [18]. For them, treatment options include chemotherapy or single or combined immunotherapy [12]. Immunotherapeutic agents used in NSCLC are the immune checkpoint inhibitors (ICIs); three PD-1 inhibitors, cemiplimab-rwic, nivolumab and pembrolizumab; and one programmed (cell) death-ligand 1 (PD-L1) antibody, atezolizumab [3]. The survival rates and duration of response tend to improve with higher levels of PD-L1 expression [12].
Currently, there is no established role for additional systemic therapy following adjuvant chemotherapy. However, recent data concerning further adjuvant treatment with immunotherapy and tyrosine kinase inhibitors (TKIs) have raised important questions regarding the existing standard of care [12]. Several molecular alterations, defined as driver mutations, have been targeted with >20 targeted agents approved for the treatment of advanced NSCLC. Such are the alterations in BRAF, epidermal growth factor receptor (EGFR) gene, anaplastic lymphoma kinase (ALK) gene, Kirsten Rat Sarcoma virus (KRAS), repressor of silencing 1 (ROS-1) and PD-L1 for squamous cell carcinoma. Mutations in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and human epidermal growth factor receptor 2 (HER2) have lower frequency, but have emerging therapeutic relevance. Furthermore, the identified key mechanisms of drug resistance in NSCLC indicate that additional mutations in tumor-driving genes may contribute to drug insensitivity and facilitate tumor progression following treatment [18]. Typically, mutations and alterations are observed in a non-overlapping manner; however, between 1% and 3% of NSCLC cases may exhibit concurrent alterations [3].
Guidelines for NSCLC advocate for the assessment of driver mutations in all non-squamous tumors. It is important to note that driver mutations are often considered in individuals without a significant smoking history, as well as in those with a minimal smoking history. Furthermore, tumors of squamous histology in non-smokers should be evaluated for testing on an individual basis [12]. The International Association for the Study of Lung Cancer recommends, at a minimum, testing for EGFR, ALK, and ROS1. Recent guidelines suggest the inclusion of additional tests for BRAF, KRAS, MET, ERBB2, and RET [3,19]. It is also advised that ROS1 testing be conducted for all patients diagnosed with adenocarcinoma, and BRAF testing should be considered for patients with metastatic non-squamous NSCLC or NSCLC NOS [3].
The aim of this non-randomized study is to investigated the gene expression and mutation signatures of different subtypes of lung cancers in 603 North Macedonian patients, including NSCLC (adenocarcinoma, squamous-cell carcinoma) and SCLC. Patients were examined for presence of genetic alterations in ≥2 of the following genes: EGFR, BRAF, KRAS, ALK, ROS1, HER2, PD-L1 and PIK3CA.
2. Materials and Methods
2.1. Sample Collection
This non-randomized, open-trial study included 603 patients with different types of lung cancers, treated at the University Clinic of Oncology and Radiology, UCC Skopje, from 17 November 2020 to 23 February 2025. All biopsies are performed at an early treatment stage. Tumor histology and stage were estimated according to WHO and pTNM classification according to the Union of International Cancer Control (UICC) [20]. All included patients had tissue samples taken at the Institute of Pathology at the Faculty of Medicine, University Ss. Cyril and Methodius, Skopje, North Macedonia. All patients gave their written informed consent to participate in the study. The Ethics Committee for research involving people at the Faculty of Medicine, UKIM, in Skopje, North Macedonia, approved investigation with human subjects according to The Code of Ethics of the World Medical Association—Declaration of Helsinki.
2.2. Demographic Properties of the Patients Included in the Study
The age of the lung cancer patients enrolled ranged from 14 to 86 years (mean 62.56 ± 9.34), of which 70.31% (n = 424) were male and 29.68% (n = 179) were female (Table 1). According to the histology, 99.17% (n = 598) patients had NSCLC, and only 0.83% (n = 5) had SCLC. Of the 598 NSCLC patients, 560 (92.87%) had been diagnosed with adenocarcinoma, 27 (4.47%) had been diagnosed with squamous-cell carcinoma and 11 (1.82%) had been diagnosed with LCC. Metastatic NSCLC was diagnosed in 31 patients, 13 of whom had metastases in lymph nodes (mediastinal, supraclavicular) alone, or together with hepatic or pulmonary metastases; brain metastases was present in 4 patients, liver and pleural metastases were present in 3 patients each, 2 patients had either metastases in mediastinum or bone, and 1 patient had abdominal metastasis, 1 pul.bill and bone metastasis and 1 pul.bill and liver metastasis. Additional comprehensive details regarding the patients are presented in Table 1.

Table 1.
Overview of the patient demographics and characteristics included in the study.
2.3. Molecular Testing
Extraction of DNA from formalin-fixed paraffin-embedded (FFPE) samples was performed using Cobas® DNA Sample Preparation Kit (Roche Diagnostics, Basel, Switzerland). The DNA concentration was determined using the ScanDrop2 instrument (Analytik Jenna, Jena, Germany) spectrophotometer and the dilution of isolated DNA was carried out in accordance with the protocol for mutation detection. All samples had appropriate DNA quality/yield.
Detection of EGFR mutations in exons 18–21 (exon 18: G719X (G719A, G719C, and G719S); exon 19: deletions and complex mutations; exon 20: S768I, T790M, and insertions; and exon 21: L858R and L861Q) was performed using Cobas® EGFR Mutation Test V2 (Roche Diagnostics, Basel, Switzerland) on Cobas Z480 IVD real time-polymerase chain reaction (RT-PCR) (Roche Diagnostics, Basel, Switzerland). In order to detect mutations, 2 ng/L isolated DNA was used according to the manufacturer’s protocol. A mutant control and negative control were included in each run to confirm its validity. In FFPE tissue analysis, the Cobas® EGFR Test can detect mutations with a 5% mutation level using a standard input of 50 ng per reaction well.
Mutations in KRAS, BRAF and PIK3CA genes were detected using KRAS/BRAF/PIK3CA Array (Randox Bioscience, Crumlin, UK), using Randox Evidence Investigator System (Randox Bioscience, Crumlin, UK). The examination relies on a combination of multiplex PCR and microarray hybridization for the detection of BRAF V600E point mutation, 20-point mutations in codons 12, 13, 61, and 146 of the KRAS gene, including known resistance mutations (G12A, G12R, G12D, G12C, G12S, G12V, G13D, G13C, G13R, Q61K, Q61L, Q61R, Q61H1, Q61H2, A146T, A146P), and three-point mutations in the PIK3CA gene (E542K, E545K, H1047R). For some samples, the detection of a V600E (1799T>A) mutation of the BRAF gene was performed using a Cobas® 4800 BRAF V600E Mutation IVD Test (Roche Diagnostics, Basel, Switzerland) on Cobas Z480 IVD RT-PCR (Roche Diagnostics, Bssel, Switzerland), with 96.4–99.3% sensitivity and 80.0–99.4% specificity.
2.4. Immunohistochemical (ICH) Analysis
Immunohistochemical staining was conducted following the protocol provided by Ventana Medical Systems (Oro Valley, AR, USA) and Agilent Technologies (Santa Clara, CA, USA). The detection of ALK protein expression via ICH is executed using the primary antibodies against ALK (rabbit, monoclonal, clone D5F3; Cat. No. 06785042001, Ventana Medical Systems, AR, USA), utilizing the Ventana Benchmark ULTRA automated stainer (Ventana Medical Systems, AR, USA). The immunohistochemical detection of PD-L1 protein expression is carried out using the PD-L1 IHC 22C3 pharmDx Kit (Agilent Technologies, CA, USA) along with the EnVision FLEX visualization system on the Autostainer Link 48 (Agilent Technologies, CA, USA). The detection of HER-2 protein expression through immunohistochemistry was performed using the antibodies against HER-2/NEU (rabbit, monoclonal; clone 4B5; Cat. No. 05278368001, Ventana Medical Systems, AR, USA), on the Ventana BenchMark ULTRA automated stainer (Ventana Medical Systems, AR, USA). It is essential to exclude known staining elements, which include light cytoplasmic stippling in alveolar macrophages, cells of neural origin (nerve and ganglion cells), glandular epithelial staining, and cells within lymphocytic infiltrate. Additionally, some background staining may be noted within normal mucosa in NSCLC (including mucin) and in necrotic tumor regions, which were also excluded from the clinical assessment.
2.5. Statistical Methods
Descriptive statistics were determined to summarize the mutation frequencies of the genes, along with calculations of the mean age of patients per each specific mutation.
3. Results
The overall results from the genetic profiling of lung cancer patients are given in Table 2 and Figure 1. Overall, 221/603 patients (36.65%) had at least one genetic alteration; 432 patients were tested for the presence of the BRAF V600E mutation, and it was only found in 4 patients (0.93% of tested). Three of them were male and one female; three had adenocarcinoma and one squamous cell carcinoma. Further, 527 patients were tested for EGFR mutations and 45 (8.54%) tested positive, 22 of whom (48.89%) were women and 23 (51.11%) men, with average age of 61.36 years. All 45 patients were diagnosed with adenocarcinoma. More than half of them (n = 24, 53.33%) had exon 19 deletion, and 14 patients (31.11%) had the L858R mutation on exon 21. Exon 20 insertions were detected in two patients, while each of the following mutations was observed in only one patient: exon 19 indel, T790M, L816Q, exon 19 deletion + exon 20 insertion and exon 18 c.2177T>p. (Val726Gly). In addition, 193 patients were tested for KRAS mutations. Of them, 50 (25.91%) had at least one KRAS mutation; 34 (68.00%) were male and 16 (32.00%) were female, with average age of 61.25 years. The majority of patients were diagnosed with adenocarcinoma (n = 44, 88%), two with squamous-cell carcinoma and one with adenosquamous carcinoma and LCLC. The detected mutations included G12C (28%), codon 12/13 mutation (20%), G12D (14%), G12V (12%), G13C (4%), both G12D and G12C (4%), A146T (4%) and Q61H1, Q61L, Q6H2, c.346>T p. (Gly12Cys), and the co-appearance of G12D and G13D, codon 12/13 and G12C and G12D, G13D, Q61R and A146T, each detected in one patient (2%). Furthermore, 142 patients were tested for PIK3CA mutations. Of them, mutations were present only in 11 (7.75%) of the patients; 6 men and 5 women, with an average age of 66.91 years. Nine of the patients were diagnosed with adenocarcinoma, one with planocellular cancer and one with squamous cell cancer. Six of the patients (54.55%) had E545K mutation, E542K was present in three patients (27.27%), and H1047R was present in two of them (18.18%).

Table 2.
Demographics of patients with gene mutations.
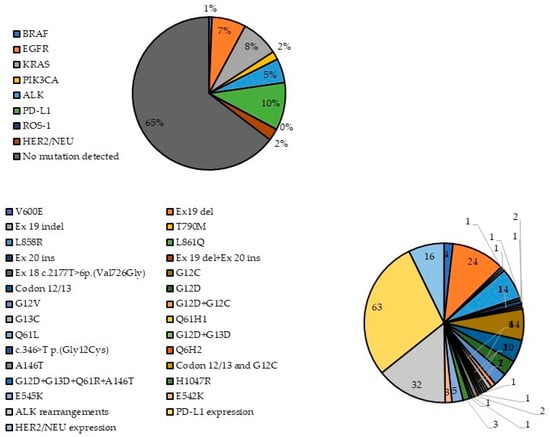
Figure 1.
Frequency of detected gene mutations.
Also, 411 patients were tested for ALK rearrangements; of them, 32 (7.79%) patients tested positive, 22 male and 10 female, with an overall average age 60.94 years. Twenty-six of them had adenocarcinoma, three planocellular cancer, three squamous cell cancer and one patient had LCLC. Additionally, 264 patients were tested for PD-L1 expression; 63 (23.86%) had PD-L1 expressed, and 48 (76.19%) were male and 15 (23.81%) female, with an average age of 63.12 years. SCLC, adenosquamous carcinoma and LCLC were present in one patient each, four patients had squamous cell carcinoma, six had planocellular carcinoma, while most of the patients (50, 79.37%) were diagnosed with adenocarcinoma. Moreover, 117 patients were tested for the presence of ROS-1 rearrangements, but none of them had one. Finally, 144 patients were examined for HER2 rearrangements, which were present in 16 patients (11.11%), 12 of whom were men and 4 women, with an overall mean age of 63.81 years. Nine patients had adenocarcinoma, four squamous cell carcinoma, one patient had planocellular cancer and one had LCLC.
Of the 603 patients, concomitant alterations of up to three different genes were observed in 22 patients (3.65%, n = 603) (Table 3). All patients were diagnosed with adenocarcinoma, 16 men and 7 women with an average age of 63.04 years. PD-L1 rearrangement is the most frequent mutual alteration, and was present in half of the tested patients, and in five cases with ALK rearrangements, which were also frequent (36.36% of the patients with concomitant alterations). In the other four cases, they co-occurred with KRAS mutations (G12V, codon 12/13), and in one case each with PIK3CA (E545K), BRAF (V600E), HER2 and EGFR mutations. Other co-occurring alterations included two patients with KRAS/PIK3CA (G12C, G12D/H1047R and G12C/E542K) and two patients with KRAS (G12C or c.346G>T)/ALK, KRAS (G12C)/HER2, KRAS (G12C)/BRAF, EGFR (L858R)/PIK3CA (H1047R) and ALK/PIK3CA (E545K). Finally, two patients had co-occurrence of three mutations: KRAS codon 12/13, ALK and PD-L1 rearrangements. All other details are given in Table 3.

Table 3.
Demographics of patients with concomitant gene alterations.
4. Discussion
An individualized treatment approach to NSCLC starts with an accurate pathological diagnosis, imaging methods, endoscopic techniques for tissue sampling and staging according to pTNM classification, and the establishment of the correct tumor genotype. Thorough genomic analyses and functional preclinical validation are crucial for broadening the spectrum of potentially actionable genomic aberrations. According to current guidelines, NSCLC patients are screened for treatable oncogenic alterations for the purpose of proper treatment selection. The patients from our study were tested for the following gene expression alterations, in descending order: EGFR (87.07%), BRAF (71.52%), KRAS (31.95%) and PIK3CA (23.51%). Most of the patients had one or more KRAS mutations (8.28%, n = 603), followed by EGFR mutations (5.13%, n = 603), PIK3CA mutations (1.82%, n = 603) and BRAF mutations (0.66%, n = 603). It is anticipated that this proportion will increase as the scope of genotype-specific clinical trials expands at our institution and other centers.
4.1. EGFR Mutations
The identification of activating EGFR mutations, along with the associated increased sensitivity to EGFR TKIs, represents a critical initial step in the implementation of targeted treatment strategies for lung cancer. EGFR is a receptor tyrosine kinase that is typically located on the surface of epithelial cells and is frequently overexpressed in various human malignancies. The NCCN NSCLC Panel advocates for the testing of EGFR mutations and other biomarkers in patients diagnosed with metastatic non-squamous NSCLC or NSCLC NOS, as well as in cases of squamous cell carcinoma [21,22,23,24].
The most prevalent EGFR mutations consist of deletions in exon 19, which occur in 45% of patients with EGFR mutations, and the p.L858R point mutations in exon 21, found in 40% of patients with EGFR mutations. These mutations are linked to a favorable response to oral EGFR TKIs [25,26]. Both mutations are observed in 10% of Caucasian patients with NSCLC and up to 50% of Asian patients [27]. The majority of patients exhibiting common EGFR mutations are typically nonsmokers or former light smokers and have been diagnosed with adenocarcinoma histology [28], and are also of younger age and female sex [29], although some studies show no statistically significant association between sex, histologic subtype and racial differences and EGFR mutation status [30,31], or with age, performance status or smoking status [31]. While both mutations are considered comparable in their predictive value for the efficacy of EGFR TKIs, two studies indicate that patients with exon 19 deletions experience a more significant benefit from EGFR TKIs compared to those with exon 21 L858R substitutions. Specifically, the therapeutic effect is found to be 50% greater in the former group, accompanied by an extended overall survival rate [21,32], and response to platinum-based chemotherapy was observed only in patients with exon 19 mutations (RR = 46% versus 0%, p = 0.02) [33].
Other mutations in EGFR exon 20 comprise a diverse array of on-frame duplications and insertions, including insASV, insSVD, and insNPH. These mutations are observed in approximately 2% of patients with NSCLC and in 4–12% of patients with EGFR mutations [34,35,36,37]. These patients have variable low response rates (9–25%) to erlotinib, afatinib or gefitinib [34,38], depending on the specific EGFR exon 20 insertion mutation [34,39,40], although exceptions exist (e.g., p.A763_Y764insFQEA) [35,41,42]. First-line platinum-based chemotherapy, with or without immunotherapy, is also recommended for patients exhibiting EGFR exon 20 mutations, such as carboplatin in combination with either pemetrexed or paclitaxel [43,44]. In this study, the frequency of EGFR mutations was 7.46%; almost equally present in both sexes. Exon 19 deletions and L858R were the two most commonly observed alterations, with only one patient with a rare exon 20 mutation (L816Q), one patient with T790M and one with concomitant exon 19 and 20 EGFR mutation.
4.2. BRAF Mutations
BRAF is a serine/threonine kinase and is an integral component of the canonical MAP/ERK signaling pathway. Activating mutations in BRAF lead to dysregulated signaling within this pathway. The specific BRAF point mutation resulting in an amino acid change at position 600 (p. V600E) has been linked to a favorable response to combined therapeutic approaches utilizing oral inhibitors of BRAF and MEK, such as dabrafenib and trametinib. Additionally, other non-V600E mutations have been identified in NSCLC at a frequency comparable to that of V600E mutations [45]; however, the implications of these mutations for therapy selection remain unclear, and as a result, specific targeted therapies are not currently available [3]. Here, 603 of the patients were assessed only for V600E mutation, with a frequency of 0.66%. Testing for other, non-BRAF V600E mutations, however, should be carried out in larger cohort study in order to explore their impact on guiding personalized treatment regimens.
4.3. KRAS Mutations
KRAS is a G-protein characterized by its intrinsic GTPase activity; mutations that activate KRAS lead to unregulated signaling within the MAP/ERK pathway. Approximately 25% of the patients with adenocarcinoma in a North American population have KRAS mutations [25,30]. The prevalence of KRAS mutations is linked to cigarette smoking, in contrast to many other actionable mutations [46]. Substitutions at KRAS positions G12, G13, and Q61 decrease the enzyme’s ability to hydrolyze GTP, whereas the A146T mutation enhances the formation of KRAS-GTP by increasing nucleotide exchange. This alteration subsequently diminishes the oncogenic potential of this isoform [47]. The presence of a KRAS mutation, particularly at codon 12 (KRAS G12C), is associated with poor survival outcomes and reduced effectiveness of EGFR TKI therapy [25,30,48]. However, these mutations do not serve as reliable predictors of RR, progression-free survival (PFS), or overall survival in patients with lung cancer. Additionally, KRAS mutations exhibit lower sensitivity to chemotherapy compared to wild-type KRAS [47]. It is important to note that KRAS mutations typically do not coexist with genetic variants such as EGFR, ROS1, BRAF, and ALK. Consequently, KRAS testing can help identify patients who may not benefit from additional molecular biomarker assessments [3].
In this study, various KRAS mutations were detected in 50 patients (8.29%), approximately 90% of them on codon 12, which is in accordance with previously published data [47]. The most prevalent KRAS mutations identified were G12C and G12D, representing 20% and 14% of all KRAS mutations, respectively. Additional KRAS mutations included G12V (12%), Q61H (2%), Q61L (2%), Q6H2 (2%), G13C (2%), and c.346G>T (2%). When analyzing KRAS mutations with Cobas® kits, mutations at codons 12 and 13 are specifically highlighted. We observed five instances of co-occurring KRAS mutations, specifically G12D with G12C, G13D with G12C, and a codon 12/13 mutation. Notably, we identified an exceptionally rare case involving four co-occurring KRAS mutations—G12D, G13D, Q61R, and A146T—in a 60-year-old male patient diagnosed with stage IVB adenocarcinoma (T4, N3, M1c).
4.4. PIK3CA Mutations
PIK3CA encodes the lipid kinase PIK3, which plays a crucial role in regulating various cellular functions such as proliferation, cell survival, degranulation, vesicular trafficking, and cell migration. Additionally, PIK3 is a key component of the PIK3/AKT signaling pathway, which is significant in the processes of oncogenesis and the progression of lung cancer [49]. PIK3CA mutations occur infrequently in NSCLC, with a prevalence of 1–4%. The majority of these mutations are missense mutations located in exon 9, specifically c.1624G>A (p.E542K) and c.1633G>A (p.E545K), as well as in exon 20, including c.3140A (p.H1047R), p.M1043I, and p.G1049S. These mutations affect regions that are part of the helical and kinase domains. The relationships between PIK3CA mutations and age, sex, smoking status, histology and lymph node metastasis have been unclear up to now [50]. PIK3CA mutations are correlated with both favorable and unfavorable prognoses of NSCLC patients, as they correlate with worse OS. In particular, for patients undergoing treatment with EGFR TKIs [48], there is evidence of poor PFS and cancer-specific survival [50], as well as a correlation with lymph node metastases [50]. Additionally, it is likely that they are not linked to primary resistance to EGFR TKIs in patients with lung cancer. Furthermore, the occurrence of acquired PIK3CA mutations associated with EGFR TKI treatment is quite uncommon [49].
In our retrospective cohort, PIK3CA mutations were observed in 1.82%; the frequency is consistent with previous studies [48]. Of them, eight were point mutations on exon 9 (E542K and E545K) and two were point mutations on exon 20 (H1047K). PIK3CA mutations were not mutually exclusive with KRAS mutation (G12C alone or with G12D+E542K, E545K and H1047R in three different patients), as it was for EGFR (one patient with H1047R PIK3CA + L858R EGFR), in opposition to previous reports [48].
4.5. ALK and ROS1 Rearrangements
ALK and ROS1 are receptor tyrosine kinases that significantly contribute to the activation of various signaling pathways related to differentiation, proliferation, cell growth, and survival. In NSCLC, these kinases can undergo rearrangements, leading to dysregulation and inappropriate signaling through the ALK/ROS1 kinase domain and the formation of novel fusion genes. This results in the deregulation of kinase activity and the abnormal activation of signaling pathways [51]. Approximately 2–7% of patients with NSCLC demonstrate ALK rearrangements, predominantly in those with adenocarcinoma histology and who are light or never smokers [12]. According to NCCN guidelines, ALK-positive patients are resistant to EGFR TKIs and should be treated with targeted agents such as alectinib, brigatinib, ceritinib, crizotinib and lorlatinib [3], although the latter has the lowest PFS and CNS activity [12]. ROS1 rearrangements occur in 1–2.6% of NSCLC patients [52,53] and are clinically associated with never-smoking history, younger age and adenocarcinoma histological type, frequently with brain metastases [51]. Patients exhibiting ROS1 rearrangements ought to receive treatment with crizotinib, which has a response rate of 72% and a median PFS of 19.2 months [54].
From our cohort, 5.31% of the patients had ALK rearrangements, the frequency being in accordance with the established range. No ROS1 rearrangements were identified in our study, which is in accordance with findings from some previous studies, identifying ROS1 rearrangements and fusions that seldom coincide with modifications in EGFR, KRAS, ALK or other actionable oncogenes in NSCLC [55], although there are studies with contrasting findings (e.g., [56]) indicating rare co-mutations with EGFR (exon 19 deletions, exon 20 insertions, L858R) and extremely rare BRAF, ALK and KRAS co-mutations [55].
4.6. PD-L1 and HER2/NEU Expression
PD-L1 is a co-regulatory molecule that may be present on tumor cells and can suppress T-cell mediated apoptosis. While it is not the ideal choice, PD-L1 expression currently serves as the most effective biomarker for evaluating suitability for immunotherapy [57]. In our study, 10.45% of the patients had PD-L1 overexpression.
HER2, which has been validated as an emerging driver and therapeutic target in both in vitro and in vivo studies for NSCLC, encodes a member of the erbB receptor tyrosine kinase family. The occurrence of HER2 alterations (including mutation, overexpression, and amplification) among NSCLC patients ranges from 2.4% to 38.0%, with a higher frequency observed in adenocarcinomas that exhibit well-differentiated histology. While the diagnostic significance of HER2 alterations remains largely ambiguous, HER2 overexpression is regarded as a negative prognostic indicator, suggesting a lower likelihood of survival [58]. Three recent studies involving 190 [33], 68 [59] and 186 [60] NSCLC patients suggest that HER2 overexpression is not associated with the objective response to chemoradiotherapy, i.e., is not predictive of response to chemotherapy or survival (gefitinib [33], 5-fluorouracil/cisplatin/hyperfractionated RT and docetaxel/cisplatin/RT [59] and cisplatin/vinblastine, followed by RT, with or without concurrent chemotherapy with carboplatin [60]). The influence of HER2 overexpression on sensitivity to EGFR TKIs, and thus RR and PFS and OS in EGFR mutant lung cancers, is highly debatable, with many studies for and against the positive influence on sensitivity and resistance to TKIs, giving controversial results due to small sample sizes and significant heterogeneity [58]. Patients with HER2 overexpression should be treated with ado-trastuzumab emtansine and fam-trastuzumab deruxtecan-nxki [3].
4.7. Co-Occurring Genetic Alterations
The compilation of co-occurring genomic alterations in NSCLC may have a greater influence on tumor heterogeneity than the individual mutations found in oncogenic drivers. Notably, NSCLC adenocarcinomas and squamous cell carcinomas exhibit an average number of somatic mutations that surpasses that observed in numerous other cancer types [61].
In this cohort study, the majority of oncogenic driver mutations tend to occur in a mutually exclusive manner. Despite this, we detected 22 patients (3.65%) with two or more putative driver alterations. PD-L1 alterations were most commonly seen together with ALK rearrangements, KRAS (G12V, codon 12/13), PIK3CA (E545K), BRAF, HER2 and EFR. Other co-occurrences include KRAS/PIK3CA, KRAS/ALK, KRAS/HER2, KRAS/BRAF, EGFR/PIK3CA, and ALK/PIK3CA, and two patients had identical triple alterations in KRAS/ALK/PD-L1. All of the identified co-occurring alterations in our study are well established based on data from previous studies, for example, the co-occurrence of PIK3CA, EGFR and KRAS in lung adenocarcinoma [62]. EGFR and KRAS mutations are known to be mutually exclusive [48], along with mutations in other established drivers, including ERBB2 and BRAF. There are also rearrangements involving ALK, ROS1, and RET, which predominantly do not overlap with KRAS mutations [61]; this was also the case in our study. The clinical significance of most of the co-occurring alterations at the time of primary diagnosis remains to be investigated, although for some co-occurring alterations it is already well established, such as, for example, the co-occurrence of EGFR and PIK3CA mutations, which is observed in 9.0–12.4% of advanced NSCLC adenocarcinomas [61]. In vitro studies indicate that the presence of both EGFR and PIK3CA mutations enhances cellular invasion and migration. Conversely, in vivo research suggests that these mutations may correlate with poorer overall survival in certain studies; however, they do not appear to influence RR or PFS when patients receive first- or second-line EGFR TKI therapy [63].
Unfortunately, in our study, the analysis of genetic alterations by specific histological subtypes was not relevant due to the significantly lower number of patients with squamous cell NSCLC than adenocarcinoma (4.48% vs. 92.87%) and the lower number of squamous-cell cancer patients with genetic mutation (2.82% of all patients) than with adenocarcinoma (32.84% of all patients), not considering co-occurring mutations. Research has established that mutations in EGFR and KRAS, as well as EML4-ALK fusions, represent the most common driver alterations in lung adenocarcinoma. These alterations occur with mutual exclusivity in approximately 35–40% of tumors. Additionally, alterations in HER2 and MAP2K1/MEK1 are also mutually exclusive of mutations in PIK3CA, BRAF, EGFR, and KRAS. In squamous-cell NSCLC, the most significant alterations include amplifications of SOX2, PIK3CA, PDGFRA, and FGFR1, along with mutations in DDR2, AKT1, and NRF2. Furthermore, mutations in TP53, BRAF, PIK3CA and MET are frequently observed in both histological subtypes [64].
5. Conclusions and Limitations
This study reported the frequency of gene mutations in a representative portion of lung cancer patients from North Macedonia for the first time. Reported gene alteration frequencies and patterns correspond to data reported in the existing scientific literature. Nonetheless, it is important to acknowledge several possible limitations of this study that must be taken into account:
- (1)
- The absence of clinical follow-up data results in a lack of correlation between detected mutations, survival outcomes and treatment responses. Consequently, this study offers limited insights into the diagnostic and prognostic significance of multigene testing in the context of lung cancer diagnostics and treatment.
- (2)
- It is possible that genetic changes occurring outside the hotspot regions addressed by the specific assays may have been missed.
- (3)
- Tumor biopsy specimens possess an intrinsic limitation in that they fail to capture inter-metastatic tumor heterogeneity. While driver mutations are regarded as truncal events that are present across all disease sites, other co-occurring genetic alterations may have developed subsequently and could exist at locations distinct from the site where the biopsy was conducted. Liquid biopsies (such as circulating tumor DNA analysis) and advanced sequencing technologies may assist in addressing these limitations.
- (4)
- The use of a retrospective database limits the ability to investigate other sources of potential bias.
- (5)
- All participants in the study were Caucasian; therefore, the results obtained may not be relevant to other racial and ethnic groups.
An additional concern to address is the referral bias in the enrollment of patients who are perceived to have a higher likelihood of possessing targetable oncogenic mutations, based on clinical and demographic characteristics. This concern is evidenced by the notably higher incidence of men compared to women in the study population (70.31% vs. 29.68%) included in the study. Nonetheless, our genotyping results align with the documented prevalence of mutations in the tested oncogenes.
In conclusion, our experience with the implementation of systematic prospective genotyping for somatic alterations in genes such as EGFR, BRAF, KRAS, ALK, ROS1, HER2, PD-L1, and PIK3CA illustrates the practicality of this method within clinical workflows. The insights obtained from this approach are designed to enhance diagnostic decision-making and to inform the administration of available targeted therapies, particularly in the context of first-line chemotherapy and personalized treatment plans. As a result, the clinical feasibility of this multigene testing approach is significantly greater than that of single-gene testing. From an economic perspective, multigene testing is increasingly cost-effective in centralized laboratories or high-volume cancer centers. However, in North Macedonia, which operates within a resource-limited setting, the feasibility of multigene testing for lung cancer diagnosis and treatment is somewhat diminished compared to developed nations, primarily due to constraints related to infrastructure and reimbursement policies.
The advent of targeted therapy and immunotherapy has brought about a substantial change in the management of advanced NSCLC, demonstrating superior effectiveness when compared to chemotherapy alone in both first- and second-line treatment scenarios [18]. As a result, the next stage in the accurate diagnosis and treatment of lung cancer will focus on identifying new molecular markers. It is expected that the range of treatable oncogenic changes and prognostic biomarkers will significantly expand [65].
Author Contributions
Conceptualization, A.E., R.J., S.C., M.S. and G.P.; methodology, A.E., S.K.K., S.C., M.S. and G.P.; software, A.E.; validation, A.E., R.J. and G.P.; formal analysis, A.E.; investigation, A.E., R.J., B.K. and M.B.T.; resources, S.K.K.; data curation, A.E.; writing—original draft preparation, A.E.; writing—review and editing, R.J., S.K.K., M.B.T., B.I., P.Z., S.K., B.K., S.C., M.S. and G.P.; visualization, A.E.; supervision, G.P.; project administration, M.B.T. and B.K.; funding acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethics Committee for research involving people at the Faculty of Medicine, University Ss.Cyril and Methodius, in Skopje, North Macedonia, approved investigation with human subjects according to The Code of Ethics of the World Medical Association—Declaration of Helsinki (approval no. 03-5526/1, approval date: 15 September 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ALK | Anaplastic lymphoma kinase |
| COPD | Chronic obstructive pulmonary disease |
| EGFR | Epidermal growth factor receptor |
| FFPE | Formalin-fixed paraffin-embedded |
| HER2 | Human epidermal growth factor receptor 2 |
| IARC | International Agency for Research on Cancer |
| ICI | Immune checkpoint inhibitor |
| KRAS | Kirsten rat sarcoma virus |
| LCLC | Large-cell lung carcinoma |
| LCNEC | Large-cell neuroendocrine carcinoma |
| NCCN | National Comprehensive Cancer Network |
| NOS | Lung cancer not otherwise specified |
| NSCLC | Non-small-cell lung cancer |
| OS | Overall survival |
| PCR | Polymerase-chain reaction |
| PD-L1 | Programmed (cell) death-ligand 1 |
| PFS | Progression-free survival |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| ROS-1 | Repressor of silencing 1 |
| RR | Response rate |
| RT | Radiotherapy |
| SCLC | Small-cell lung cancer |
| TKI | Tyrosine kinase inhibitors |
| UICC | Union of International Cancer Control |
| WHO | World Health Organization |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 9 February 2022).
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr Canc. Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef] [PubMed]
- Fraumeni, J.F. Respiratory Carcinogenesis: An Epidemiologic Appraisal. J. Natl. Cancer Inst. 1975, 55, 1039–1046. [Google Scholar] [CrossRef]
- Janerich, D.T.; Thompson, D.W.; Varela, L.R.; Greenwald, P.; Chorost, S.; Tucci, C.; Zaman, M.; Melamed, M.; Kiely, M.; McKneally, M.F. Lung cancer and exposure to tobacco smoke. N. Engl. J. Med. 1990, 323, 632–636. [Google Scholar] [CrossRef]
- Driscoll, T.; Nelson, D.I.; Steenland, K.; Leigh, J.; Concha-Barrientos, M.; Fingerhut, M.; Prüss-Üstün, A. The global burden of disease due to occupational carcinogens. Am. J. Ind. Med. 2005, 48, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Straif, K.; Benbrahim-Tallaa, L.; Baan, R.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part C: Metals, arsenic, dusts, and fibres. Lancet Oncol. 2009, 10, 453–454. [Google Scholar] [CrossRef]
- Wiesweg, M.; Eberhardt, W.E.E.; Reis, H.; Ting, S.; Savvidou, N.; Skiba, C.; Herold, T.; Christoph, D.C.; Meiler, J.; Worm, K.; et al. High Prevalence of Concomitant Oncogene Mutations in Prospectively Identified Patients with ROS1-Positive Metastatic Lung Cancer. J. Thorac. Oncol. 2017, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Howlander, N.; Noone, A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovic, Z.; Mariotto, A.; Lewis, R.; et al. SEER Cancer Statistics Review (CSR) 1975–2018; National Cancer Institute: Betheseda, MD, USA, 2021. [Google Scholar]
- Tsao, D.-A.; Chang, H.-J.; Lin, C.-Y.; Hsiung, S.-K.; Huang, S.-E.; Ho, S.-Y.; Chang, M.-S.; Chiu, H.-H.; Chen, Y.-F.; Cheng, T.-L.; et al. Gene Expression Profiles for Predicting the Efficacy of the Anticancer Drug 5-Fluorouracil in Breast Cancer. DNA Cell Biol. 2010, 29, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef]
- Keller, S.M.; Komaki, R.; Johnson, D.H. A Randomized Trial of Postoperative Adjuvant Therapy in Patients with Completely Resected Stage II or IIIa Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2000, 343, 1217–1222. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Graham, M.V.; Ettinger, D.S.; Johnstone, D.W.; Pilepich, M.V.; Machtay, M.; Komaki, R.; Atkins, J.; Curran, W.J. Phase II Trial of Postoperative Adjuvant Paclitaxel/Carboplatin and Thoracic Radiotherapy in Resected Stage II and IIIA Non-Small-Cell Lung Cancer Promising Long-Term Results of the Radiation Therapy Oncology Group-RTOG 9705. J. Clin. Oncol. 2005, 23, 3480–3487. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-M.; Pan, C.; Eisbruch, A.; Haken, R.K.T. Physical Models and Simpler Dosimetric Descriptors of Radiation Late Toxicity. Semin. Radiat. Oncol. 2007, 17, 108–120. [Google Scholar] [CrossRef]
- Sonett, J.R.; Suntharalingam, M.; Edelman, M.J.; Patel, A.B.; Gamliel, Z.; Doyle, A.; Hausner, P.; Krasna, M. Pulmonary Resection After Curative Intent Radiotherapy (>59 Gy) and Concurrent Chemotherapy in Non–Small-Cell Lung Cancer. Ann. Thorac. Surg. 2004, 78, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, L.; Favaretto, A.; Rosell, R. Platinum Drugs and DNA Repair Mechanisms in Lung Cancer. Anticancer Res. 2014, 34, 493–501. [Google Scholar] [PubMed]
- Leonetti, A.; Wever, B.; Mazzaschi, G.; Assaraf, Y.G.; Rolfo, C.; Quaini, F.; Tiseo, M.; Giovannetti, E. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist. Updates 2019, 46, 100644. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch. Pathol. Lab. Med. 2018, 142, 321–346. [Google Scholar] [CrossRef]
- Sobin, L.H.; Gospodarowicz, M.K.; Witterkind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016; Volume 1, ISBN 978-1-119-26357-9. [Google Scholar]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.A.; Riely, G.J.; Zakowski, M.F.; Li, A.R.; Patel, J.D.; Heelan, R.T.; Kris, M.G.; Sandler, A.B.; Carbone, D.P.; Tsao, A.; et al. Molecular Characteristics of Bronchioloalveolar Carcinoma and Adenocarcinoma, Bronchioloalveolar Carcinoma Subtype, Predict Response to Erlotinib. J. Clin. Oncol. 2008, 26, 1472–1478. [Google Scholar] [CrossRef]
- Sequist, L.V.; Martins, R.G.; Spigel, D.; Grunberg, S.M.; Spira, A.; Jänne, P.A.; Joshi, V.A.; McCollum, D.; Evans, T.L.; Muzikansky, A.; et al. First-Line Gefitinib in Patients with Advanced Non–Small-Cell Lung Cancer Harboring Somatic EGFR Mutations. J. Clin. Oncol. 2008, 26, 2442–2449. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Bunn, P.A. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009, 10, 432–433. [Google Scholar] [CrossRef]
- Paik, P.K.; Varghese, A.M.; Sima, C.S.; Moreira, A.L.; Ladanyi, M.; Kris, M.G.; Rekhtman, N. Response to Erlotinib in Patients with EGFR Mutant Advanced Non-Small Cell Lung Cancers with a Squamous or Squamous-like Component. Mol. Cancer Ther. 2012, 11, 2535–2540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shigematsu, H.; Lin, L.; Takahashi, T.; Nomura, M.; Suzuki, M.; Wistuba, I.I.; Fong, K.M.; Lee, H.; Toyooka, S.; Shimizu, N.; et al. Clinical and Biological Features Associated with Epidermal Growth Factor Receptor Gene Mutations in Lung Cancers. J. Natl. Cancer Inst. 2005, 97, 339–346. [Google Scholar] [CrossRef]
- Eberhard, D.A.; Johnson, B.E.; Amler, L.C.; Goddard, A.D.; Heldens, S.L.; Herbst, R.S.; Ince, W.L.; Jänne, P.A.; Januario, T.; Johnson, D.H.; et al. Mutations in the Epidermal Growth Factor Receptor and in KRAS Are Predictive and Prognostic Indicators in Patients with Non–Small-Cell Lung Cancer Treated with Chemotherapy Alone and in Combination with Erlotinib. J. Clin. Oncol. 2005, 23, 5900–5909. [Google Scholar] [CrossRef]
- Lee, C.K.; Wu, Y.-L.; Ding, P.N.; Lord, S.J.; Inoue, A.; Zhou, C.; Mitsudomi, T.; Rosell, R.; Pavlakis, N.; Links, M.; et al. Impact of Specific Epidermal Growth Factor Receptor (EGFR) Mutations and Clinical Characteristics on Outcomes After Treatment with EGFR Tyrosine Kinase Inhibitors Versus Chemotherapy in EGFR -Mutant Lung Cancer: A Meta-Analysis. J. Clin. Oncol. 2015, 33, 1958–1965. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Zhou, C.; Liam, C.-K.; Wu, G.; Liu, X.; Zhong, Z.; Lu, S.; Cheng, Y.; Han, B.; Chen, L.; et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE study. Ann. Oncol. 2015, 26, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Cappuzzo, F.; Ligorio, C.; Toschi, L.; Rossi, E.; Trisolini, R.; Paioli, D.; Magrini, E.; Finocchiaro, G.; Bartolini, S.; Cancellieri, A.; et al. EGFR and HER2 Gene Copy Number and Response to First-Line Chemotherapy in Patients with Advanced Non-small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. 2007, 2, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ramalingam, S.S.; Kim, T.M.; Kim, S.-W.; Yang, J.C.-H.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Garcia-Campelo, M.R.; Felip, E.; et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients with EGFR Exon 20 Insertion–Positive Metastatic Non–Small Cell Lung Cancer. JAMA Oncol. 2021, 7, e214761. [Google Scholar] [CrossRef]
- Yasuda, H.; Park, E.; Yun, C.-H.; Sng, N.J.; Lucena-Araujo, A.R.; Yeo, W.-L.; Huberman, M.S.; Cohen, D.W.; Nakayama, S.; Ishioka, K.; et al. Structural, biochemical and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci. Transl. Med. 2013, 5, 216ra177. [Google Scholar] [CrossRef]
- Riess, J.W.; Gandara, D.R.; Frampton, G.M.; Madison, R.; Peled, N.; Bufill, J.A.; Dy, G.; Ou, S.-H.I.; Stephens, P.J.; McPherson, J.; et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1560–1568. [Google Scholar] [CrossRef]
- Riely, G.J.; Neal, J.W.; Camidge, D.R.; Spira, A.I.; Piotrowska, Z.; Costa, D.B.; Tsao, A.S.; Patel, J.D.; Gadgeel, S.M.; Bazhenova, L.; et al. Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non–Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations From a Phase 1/2 Trial. Cancer Discov. 2021, 11, 1688–1699. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion–Mutated Non–Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, H.; Matsumoto, S.; Ohe, Y.; Satouchi, M.; Furuya, N.; Kim, Y.H.; Seto, T.; Soejima, K.; Hayakawa, D.; Kato, T.; et al. OA07.03 Clinical Outcome of Non-Small Cell Lung Cancer with EGFR/HER2 Exon 20 Insertions Identified in the LC-SCRUM-Japan. J. Thorac. Oncol. 2019, 14, S224. [Google Scholar] [CrossRef]
- Ou, S.-H.I.; Lin, H.; Hong, J.-L. Real-world response and outcomes in NSCLC patients with EGFR exon 20 insertion mutations. J. Clin. Oncol. 2021, 39, 9098. [Google Scholar] [CrossRef]
- Vasconcelos, P.E.N.S.; Gergis, C.; Viray, H.; Varkaris, A.; Fujii, M.; Rangachari, D.; VanderLaan, P.A.; Kobayashi, I.S.; Kobayashi, S.S.; Costa, D.B. EGFR-A763_Y764insFQEA Is a Unique Exon 20 Insertion Mutation That Displays Sensitivity to Approved and In-Development Lung Cancer EGFR Tyrosine Kinase Inhibitors. JTO Clin. Res. Rep. 2020, 1, 100051. [Google Scholar] [CrossRef]
- Yasuda, H.; Kobayashi, S.; Costa, D.B. EGFR exon 20 insertion mutations in non-small-cell lung cancer: Preclinical data and clinical implications. Lancet Oncol. 2012, 13, e23–e31. [Google Scholar] [CrossRef]
- Chelabi, S.; Mignard, X.; Leroy, K.; Monnet, I.; Brosseau, S.; Theou-Anton, N.; Massiani, M.-A.; Friard, S.; Duchemann, B.; Fabre, E.; et al. EGFR Exon 20 Insertion in Metastatic Non-Small-Cell Lung Cancer: Survival and Clinical Efficacy of EGFR Tyrosine-Kinase Inhibitor and Chemotherapy. Cancers 2021, 13, 5132. [Google Scholar] [CrossRef]
- Byeon, S.; Kim, Y.; Lim, S.W.; Cho, J.H.; Park, S.; Lee, J.; Sun, J.-M.; Choi, Y.-L.; Lee, S.-H.; Ahn, J.S.; et al. Clinical Outcomes of EGFR Exon 20 Insertion Mutations in Advanced Non-small Cell Lung Cancer in Korea. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2019, 51, 623–631. [Google Scholar] [CrossRef]
- Perrone, F.; Mazzaschi, G.; Minari, R.; Verzè, M.; Azzoni, C.; Bottarelli, L.; Nizzoli, R.; Pluchino, M.; Altimari, A.; Gruppioni, E.; et al. Multicenter Observational Study on Metastatic Non-Small Cell Lung Cancer Harboring BRAF Mutations: Focus on Clinical Characteristics and Treatment Outcome of V600E and Non-V600E Subgroups. Cancers 2022, 14, 2019. [Google Scholar] [CrossRef]
- Slebos, R.J.C.; Hruban, R.H.; Dalesio, O.; Mooi, W.J.; Offerhaus, G.J.A.; Rodenhuis, S. Relationship Between K-ras Oncogene Activation and Smoking in Adenocarcinoma of the Human Lung. J. Natl. Cancer Inst. 1991, 83, 1024–1027. [Google Scholar] [CrossRef]
- Shen, M.; Qi, R.; Ren, J.; Lv, D.; Yang, H. Characterization with KRAS Mutant Is a Critical Determinant in Immunotherapy and Other Multiple Therapies for Non-Small Cell Lung Cancer. Front. Oncol. 2022, 11, 17. [Google Scholar] [CrossRef]
- Ludovini, V. Phosphoinositide-3-Kinase Catalytic Alpha and KRAS Mutations are Important Predictors of Resistance to Therapy with Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients with Advanced Non-small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 9. [Google Scholar] [CrossRef]
- Wu, S.-G.; Chang, Y.-L.; Yu, C.-J.; Yang, P.-C.; Shih, J.-Y. The Role of PIK3CA Mutations among Lung Adenocarcinoma Patients with Primary and Acquired Resistance to EGFR Tyrosine Kinase Inhibition. Sci. Rep. 2016, 6, 35249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Li, J.; Li, J.; Che, G. Clinical Significance of PIK3CA Gene in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 3608241. [Google Scholar] [CrossRef] [PubMed]
- Gendarme, S.; Bylicki, O.; Chouaid, C.; Guisier, F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Curr. Oncol. 2022, 29, 641–658. [Google Scholar] [CrossRef]
- Dugay, F.; Llamas-Gutierrez, F.; Gournay, M.; Medane, S.; Mazet, F.; Chiforeanu, D.C.; Becker, E.; Lamy, R.; Léna, H.; Rioux-Leclercq, N.; et al. Clinicopathological characteristics of ROS1- and RET-rearranged NSCLC in caucasian patients: Data from a cohort of 713 non-squamous NSCLC lacking KRAS/EGFR/HER2/BRAF/PIK3CA/ALK alterations. Oncotarget 2017, 8, 53336–53351. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lim, S.M.; Kim, H.J.; Hwang, S.K.; Park, J.K.; Shin, E.; Bae, M.K.; Ou, S.-H.I.; Wang, J.; Jewell, S.S.; et al. The frequency and impact of ROS1 rearrangement on clinical outcomes in never smokers with lung adenocarcinoma. Ann. Oncol. 2013, 24, 2364–2370. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1 -Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef]
- Lin, J.J.; Ritterhouse, L.L.; Ali, S.M.; Bailey, M.; Schrock, A.B.; Gainor, J.F.; Ferris, L.A.; Mino-Kenudson, M.; Miller, V.A.; Iafrate, A.J.; et al. ROS1 Fusions Rarely Overlap with Other Oncogenic Drivers in Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Lambros, L.; Guibourg, B.; Uguen, A. ROS1-rearranged Non–Small Cell Lung Cancers with Concomitant Oncogenic Driver Alterations: About Some Rare Therapeutic Dilemmas. Clin. Lung Cancer 2018, 19, e73–e74. [Google Scholar] [CrossRef]
- Kerr, K.M.; Hirsch, F.R. Programmed Death Ligand-1 Immunohistochemistry: Friend or Foe? Arch. Pathol. Lab. Med. 2016, 140, 326–331. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, Y. Targeting HER2 Alterations in Non–Small-Cell Lung Cancer: A Comprehensive Review. JCO Precis. Oncol. 2020, 4, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Kuyama, S.; Hotta, K.; Tabata, M.; Segawa, Y.; Fujiwara, Y.; Takigawa, N.; Kiura, K.; Ueoka, H.; Eguchi, K.; Tanimoto, M. Impact of HER2 Gene and Protein Status on the Treatment Outcome of Cisplatin-Based Chemoradiotherapy for Locally Advanced Non-small Cell Lung Cancer. J. Thorac. Oncol. 2008, 3, 477–482. [Google Scholar] [CrossRef]
- Graziano, S.L.; Tatum, A.; Herndon, J.E.; Box, J.; Memoli, V.; Green, M.R.; Kern, J.A. Use of neuroendocrine markers, p53, and HER2 to predict response to chemotherapy in patients with stage III non-small cell lung cancer: A Cancer and Leukemia Group B study. Lung Cancer 2001, 33, 115–123. [Google Scholar] [CrossRef]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Sholl, L.M.; Aisner, D.L.; Varella-Garcia, M.; Berry, L.D.; Dias-Santagata, D.; Wistuba, I.I.; Chen, H.; Fujimoto, J.; Kugler, K.; Franklin, W.A.; et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: The Lung Cancer Mutation Consortium experience. J. Thorac. Oncol. 2015, 10, 768–777. [Google Scholar] [CrossRef]
- Eng, J.; Woo, K.M.; Sima, C.S.; Plodkowski, A.; Hellmann, M.D.; Chaft, J.; Kris, M.G.; Arcila, M.E.; Ladanyi, M.; Drilon, A. Impact of concurrent PIK3CA mutations on response to EGFR tyrosine kinase inhibition in EGFR-mutant lung cancers and on prognosis in oncogene-driven lung adenocarcinomas. J. Thorac. Oncol. 2015, 10, 1713–1719. [Google Scholar] [CrossRef]
- Pikor, L.A.; Ramnarine, V.R.; Lam, S.; Lam, W.L. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013, 82, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rabe, K.F. Precision Diagnosis and Treatment for Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 849–861. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).