Physical Activity During Pregnancy and Gestational Weight Gain: Implications for Maternal–Fetal Epigenetic Programming and Long-Term Health
Abstract
1. Introduction
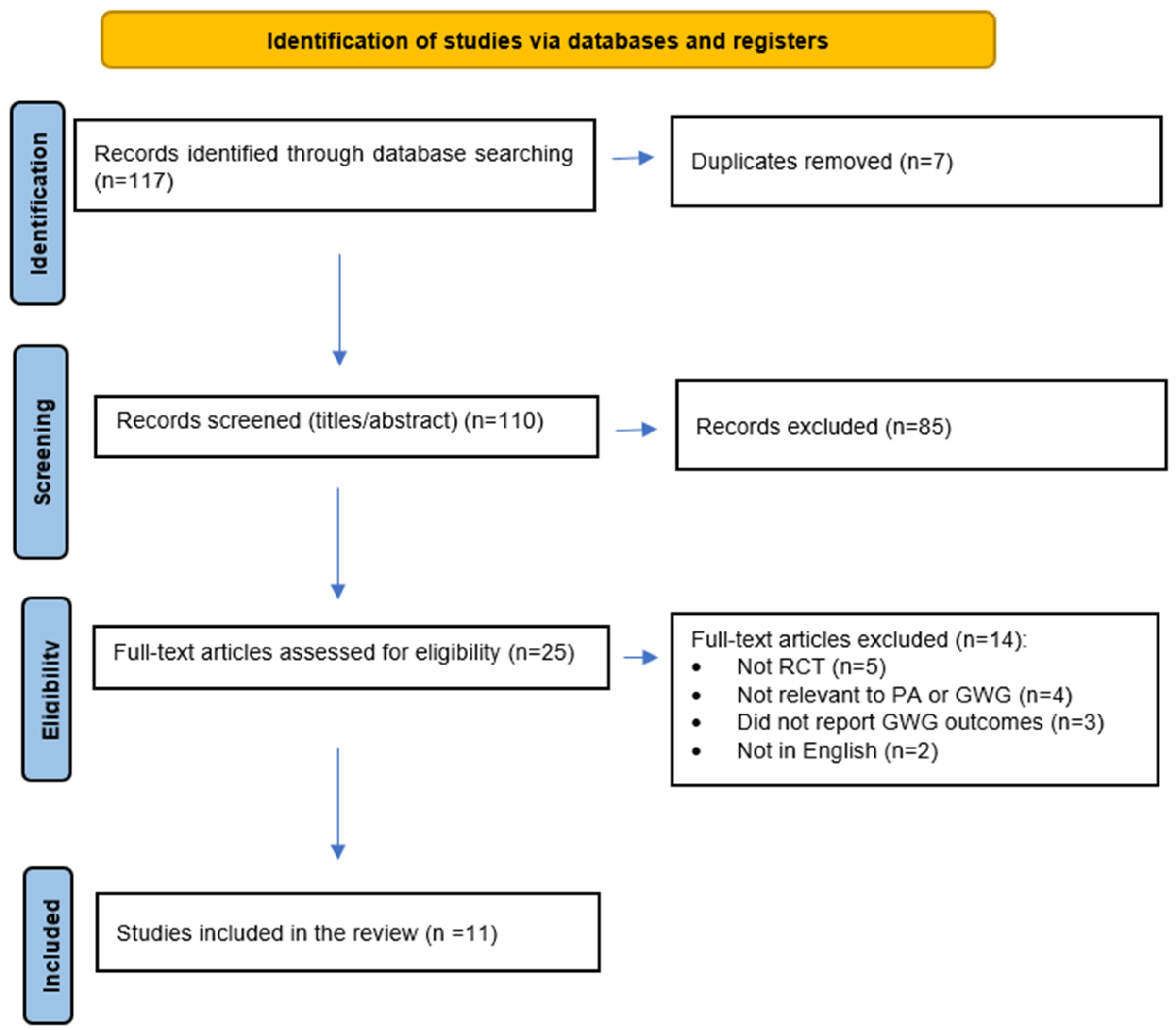
2. Methods
2.1. Literature Search and Study Selection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results
3.1. Effects of Physical Activity on Gestational Weight Gain
3.2. Maternal Metabolic and Physical Health Outcomes
3.3. Fetal and Neonatal Outcomes
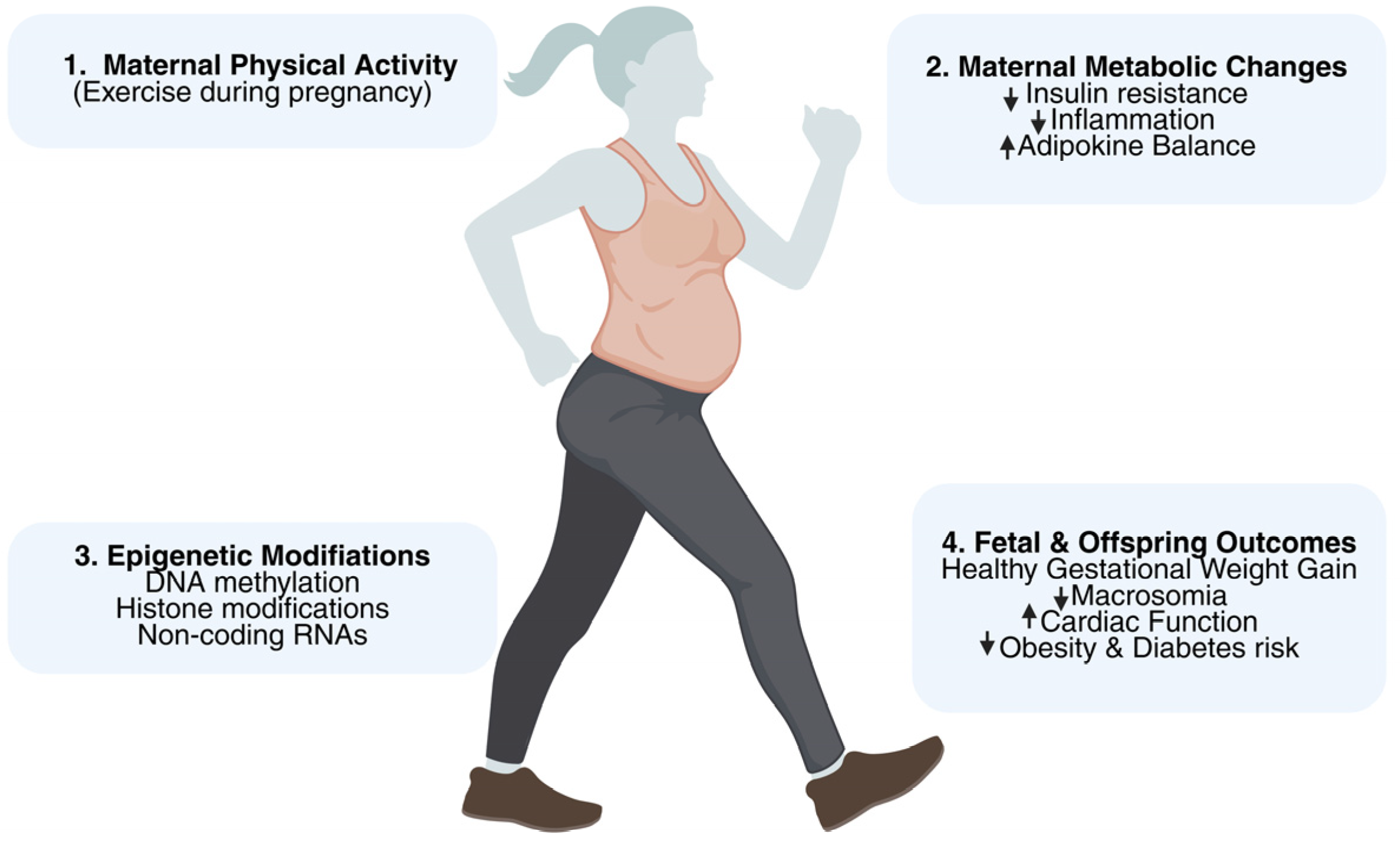
3.4. Epigenetic and Molecular Mechanisms
4. Discussion
4.1. Impact of PA on GWG and Maternal Outcomes
4.2. Variability Among Studies and Role of Adherence
4.3. Broader Maternal and Fetal Benefits
4.4. Practical Recommendations for Clinicians
4.5. Barriers and Equity Considerations
4.6. Epigenetic and Intergenerational Implications
4.7. Future Research and Directions
4.8. Novelty and Contribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GWG | Gestational Weight Gain |
| PA | Physical Activity |
| RCTs | Randomized Control Trials |
| EGWG | Excessive Gestational Weight Gain |
| DOHaD | Developmental Origins of Health and Illness |
References
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics, Committee on Genetics, Society for Maternal-Fetal Medicine. Screening for Fetal Chromosomal Abnormalities: ACOG Practice Bulletin, Number 226. Obstet. Gynecol. 2020, 136, e48–e69. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef] [PubMed]
- ACOG Practice Bulletin, No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207. [Google Scholar] [CrossRef] [PubMed]
- LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group; Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; et al. Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702. [Google Scholar] [CrossRef]
- Obesity in Pregnancy: ACOG Practice Bulletin, Number 230. Obstet. Gynecol. 2021, 137, e128–e144. [CrossRef] [PubMed]
- Goławski, K.; Giermaziak, W.; Ciebiera, M.; Wojtyła, C. Excessive Gestational Weight Gain and Pregnancy Outcomes. J. Clin. Med. 2023, 12, 3211. [Google Scholar] [CrossRef]
- Lackovic, M.; Jankovic, M.; Mihajlovic, S.; Milovanovic, Z.; Rovcanin, M.; Mitic, N.; Nikolic, D. Gestational Weight Gain, Pregnancy Related Complications and the Short-Term Risks for the Offspring. J. Clin. Med. 2024, 13, 445. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Barker, D.J.P. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23 (Suppl 6), 588S–595S. [Google Scholar] [CrossRef]
- Eriksson, J.; Forsén, T.; Osmond, C.; Barker, D. Obesity from cradle to grave. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, 722–727. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef]
- Physical Activity and Exercise During Pregnancy and the Postpartum Period: ACOG Committee Opinion, Number 804. Obstet. Gynecol. 2020, 135, e178–e188. [CrossRef] [PubMed]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.M.; Davies, G.A.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018, 52, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Dumith, S.C.; Domingues, M.R.; Mendoza-Sassi, R.A.; Cesar, J.A. Atividade física durante a gestação e associação com indicadores de saúde materno-infantil. Rev. Saúde. Pública. 2012, 46, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M.; Cordero, Y.; Perales, M.; Lopez, C.; Coteron, J.; Mottola, M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obstet. Gynecol. 2016, 214, 649.e1-8. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Oken, E.; Gillman, M.W. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am. J. Obstet. Gynecol. 2009, 201, 58.e1-8. [Google Scholar] [CrossRef]
- Domingues, M.R.; Barros, A.J.; Matijasevich, A. Leisure time physical activity during pregnancy and preterm birth in Brazil. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2008, 103, 9–15. [Google Scholar] [CrossRef]
- Hamann, V.; Deruelle, P.; Enaux, C.; Deguen, S.; Kihal-Talantikite, W. Physical activity and gestational weight gain: A systematic review of observational studies. BMC Public Health 2022, 22, 1951. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Zhang, C.; van Dam, R.M.; Bowers, K.; Hu, F.B. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: A meta-analysis. Diabetes Care. 2011, 34, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Keating, D.P. Transformative Role of Epigenetics in Child Development Research: Commentary on the Special Section. Child Dev. 2016, 87, 135–142. [Google Scholar] [CrossRef]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2020, 14, 47–50. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Anaclerio, F.; Moscogiuri, L.A.; Konstantinidou, F.; Stuppia, L.; Gatta, V. Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming. Curr. Issues Mol. Biol. 2024, 46, 4358–4378. [Google Scholar] [CrossRef]
- Saftić Martinović, L.; Mladenić, T.; Lovrić, D.; Ostojić, S.; Dević Pavlić, S. Decoding the Epigenetics of Infertility: Mechanisms, Environmental Influences, and Therapeutic Strategies. Epigenomes 2024, 8, 34. [Google Scholar] [CrossRef]
- Virolainen, S.J.; VonHandorf, A.; Viel, K.C.M.F.; Weirauch, M.T.; Kottyan, L.C. Gene-environment interactions and their impact on human health. Genes Immun. 2023, 24, 1–11. [Google Scholar] [CrossRef]
- Langley-Evans, S.C.; Pearce, J.; Ellis, S. Overweight, obesity and excessive weight gain in pregnancy as risk factors for adverse pregnancy outcomes: A narrative review. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2022, 35, 250–264. [Google Scholar] [CrossRef]
- Corson, A.E.; MacDonald, M.; Tzaneva, V.; Edwards, C.M.; Adamo, K.B. Breaking boundaries: A chronology with future directions of women in exercise physiology research, centred on pregnancy. Adv. Exerc. Health Sci. 2024, 1, 67–75. [Google Scholar] [CrossRef]
- Pelaez, M.; Gonzalez-Cerron, S.; Montejo, R.; Barakat, R. Protective Effect of Exercise in Pregnant Women Including Those Who Exceed Weight Gain Recommendations: A Randomized Controlled Trial. Mayo. Clin. Proc. 2019, 94, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Refoyo, I.; Coteron, J.; Franco, E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz. J. Phys. Ther. 2019, 23, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Brik, M.; Fernández--Buhigas, I.; Martin--Arias, A.; Vargas--Terrones, M.; Barakat, R.; Santacruz, B. Does exercise during pregnancy impact on maternal weight gain and fetal cardiac function? A randomized controlled trial. Ultrasound Obstet. Gynecol. 2019, 53, 583–589. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.G.; Hallal, P.C.; Domingues, M.R.; Bertoldi, A.D.; Silveira, M.F.D.; Bassani, D.; da Silva, I.C.M.; da Silva, B.G.C.; Coll, C.V.N.; Evenson, K. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: Results from the PAMELA study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 175. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef]
- Nobles, C.; Marcus, B.H.; Stanek, E.J., 3rd; Braun, B.; Whitcomb, B.W.; Manson, J.E.; Markenson, G.; Chasan-Taber, L. The Effect of an Exercise Intervention on Gestational Weight Gain: The Behaviors Affecting Baby and You (B.A.B.Y.) Study: A Randomized Controlled Trial. Am. J. Health Promot. AJHP. 2018, 32, 736–744. [Google Scholar] [CrossRef]
- Simmons, D.; Devlieger, R.; van Assche, A.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Desoye, G.; Harreiter, J.; et al. Effect of physical activity and/or healthy eating on GDM risk: The DALI Lifestyle Study. J. Clin. Endocrinol. Metab. 2016, 102, 903–913. [Google Scholar] [CrossRef]
- Barakat, R.; Cordero, Y.; Coteron, J.; Luaces, M.; Montejo, R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: A randomised controlled trial. Br. J. Sports Med. 2012, 46, 656–661. [Google Scholar] [CrossRef]
- Dekker Nitert, M.; Barrett, H.L.; Denny, K.J.; McIntyre, H.D.; Callaway, L.K.; the BAMBINO group. Exercise in pregnancy does not alter gestational weight gain, MCP --1 or leptin in obese women. Aust. N Z J Obstet. Gynaecol. 2015, 55, 27–33. [Google Scholar] [CrossRef]
- Ruchat, S.M.; Davenport, M.H.; Giroux, I.; Hillier, M.; Batada, A.; Sopper, M.M.; Hammond, J.M.; Mottola, M.F. Nutrition and exercise reduce excessive weight gain in normal-weight pregnant women. Med. Sc.i Sports Exerc. 2012, 44, 1419–1426. [Google Scholar] [CrossRef]
- McDonald, S.M.; Isler, C.; Haven, K.; Newton, E.; Kuehn, D.; Kelley, G.; Chasan-Taber, L.; May, L.E. Moderate intensity aerobic exercise during pregnancy and 1-month infant Morphometry. Birth. Defects Res. 2021, 113, 238–247. [Google Scholar] [CrossRef]
- Atkinson, S.A.; Maran, A.; Dempsey, K.; Perreault, M.; Vanniyasingam, T.; Phillips, S.M.; Hutton, E.K.; Mottola, M.F.; Wahoush, O.; Xie, F.; et al. Be Healthy in Pregnancy (BHIP): A Randomized Controlled Trial of Nutrition and Exercise Intervention from Early Pregnancy to Achieve Recommended Gestational Weight Gain. Nutrients 2022, 14, 810. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.G.; Ricardo, L.I.; Evenson, K.R.; Hallal, P.C. Leisure-Time Physical Activity in Pregnancy and Maternal-Child Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Cohort Studies. Sports Med. Auckl. NZ 2017, 47, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Hébert, J.R.; Frongillo, E.A.; Adams, S.A.; Turner-McGrievy, G.M.; Hurley, T.G.; Miller, D.R.; Ockene, I.S. Perspective: Randomized Controlled Trials Are Not a Panacea for Diet-Related Research. Adv. Nutr. Bethesda. Md. 2016, 7, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rodriguez, R.; Price, L.L.; Somogie, J.; Hauguel-de Mouzon, S.; Kalhan, S.C.; Catalano, P.M. Maternal Lipid Metabolism Is Associated with Neonatal Adiposity: A Longitudinal Study. J. Clin. Endocrinol. Metab. 2022, 107, e3759–e3768. [Google Scholar] [CrossRef]
- Gómez-Vilarrubla, A.; Mas-Parés, B.; Carreras-Badosa, G.; Bonmatí-Santané, A.; Martínez-Calcerrada, J.-M.; Niubó-Pallàs, M.; de Zegher, F.; Ibáñez, L.; López-Bermejo, A.; Bassols, J. DNA Methylation Signatures in Paired Placenta and Umbilical Cord Samples: Relationship with Maternal Pregestational Body Mass Index and Offspring Metabolic Outcomes. Biomedicines 2024, 12, 301. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Kakongoma, N.; Hua, W.; Xu, J.; Wang, Y.; He, S.; Gu, H.; Shi, J.; Hu, W. DNA methylation and expression profiles of placenta and umbilical cord blood reveal the characteristics of gestational diabetes mellitus patients and offspring. Clin. Epigenetics 2022, 14, 69. [Google Scholar] [CrossRef]
- Deaton, A.; Cartwright, N. Understanding and misunderstanding randomized controlled trials. Soc. Sci. Med. 2018, 210, 2–21. [Google Scholar] [CrossRef]
- Mahmoud, A.M. An Overview of Epigenetics in Obesity: The Role of Lifestyle and Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 1341. [Google Scholar] [CrossRef]
- Banik, A.; Kandilya, D.; Ramya, S.; Stünkel, W.; Chong, Y.S.; Dheen, S.T. Maternal Factors that Induce Epigenetic Changes Contribute to Neurological Disorders in Offspring. Genes 2017, 8, 150. [Google Scholar] [CrossRef]
- Mittal, R.; Prasad, K.; Lemos, J.R.N.; Arevalo, G.; Hirani, K. Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management. Int. J. Mol. Sci. 2025, 26, 2320. [Google Scholar] [CrossRef]
- Cai, S.; Quan, S.; Yang, G.; Chen, M.; Ye, Q.; Wang, G.; Yu, H.; Wang, Y.; Qiao, S.; Zeng, X. Nutritional Status Impacts Epigenetic Regulation in Early Embryo Development: A Scoping Review. Adv. Nutr. 2021, 12, 1877–1892. [Google Scholar] [CrossRef]
| First Author (Year) | Country | Sample Size | Intervention (Frequency/Timing) | Population Characteristics | Primary/Secondary Outcomes | Key Findings |
|---|---|---|---|---|---|---|
| Pelaez et al. (2019) [31] | Spain | 345 | Moderate–vigorous exercise 3×/week from 12–38 weeks | BMI stratified; avg age ~30 | GWG, adherence, GDM | ↓EGWG (esp. BMI ≥ 25), improved fitness |
| Barakat et al. (2019) [32] | Spain | 594 | Moderate exercise 3×/week 8–39 weeks | Healthy singleton pregnancies | GWG, GDM incidence | ↓Total GWG, ↓GDM |
| Brik et al. (2019) [33] | Spain | 120 | 60-min sessions 3×/week | Low-risk pregnancies | GWG, fetal cardiac function | No GWG reduction, improved fetal cardiac parameters |
| da Silva et al. (2017) [34] | Brazil | 639 | Exercise 3×/week 16–36 weeks | Mixed-risk population | GWG, preterm birth, GDM | NS |
| Wang et al. (2017) [35] | China | 300 | Cycling 3×/week < 13–37 weeks | Overweight/obese women | GWG, GDM, insulin resistance | ↓GWG, ↓GDM, ↓birth weight |
| Nobles et al. (2018) [36] | USA | 241 | 12-week structured program | Ethnically diverse | GWG (primary), adherence | 30% ↓odds EGWG (NS) |
| Simmons et al. (2016) [37] | Europe | 436 | HE, PA, HE + PA interventions | Obese women | GDM risk, GWG | HE + PA ↓GWG; metabolic outcomes NS |
| Barakat et al. (2012) [38] | Spain | 200 | Moderate exercise entire pregnancy | Healthy nulliparas | GWG, maternal/fetal outcomes | ↓EGWG, safe intervention |
| Dekker Nitert et al. (2015) [39] | Australia | 35 | Individualized plan | Obese women | GWG, metabolic markers | NS |
| Ruchat et al. (2012) [40] | Canada | 94 | Walking + nutrition control | Normal BMI women | GWG, postpartum weight | ↑IOM compliance, ↓postpartum weight |
| McDonald et al. (2021) [41] | USA | 128 | Moderate exercise < 16 wks–delivery | Healthy pregnancies | Neonatal morphometry, GWG | ↓neonatal adiposity; GWG slightly ↑ (NS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagorianakou, N.; Makrydimas, S.; Moustakli, E.; Mitrogiannis, I.; Vitale, E.; Makrydimas, G. Physical Activity During Pregnancy and Gestational Weight Gain: Implications for Maternal–Fetal Epigenetic Programming and Long-Term Health. Genes 2025, 16, 1173. https://doi.org/10.3390/genes16101173
Zagorianakou N, Makrydimas S, Moustakli E, Mitrogiannis I, Vitale E, Makrydimas G. Physical Activity During Pregnancy and Gestational Weight Gain: Implications for Maternal–Fetal Epigenetic Programming and Long-Term Health. Genes. 2025; 16(10):1173. https://doi.org/10.3390/genes16101173
Chicago/Turabian StyleZagorianakou, Nektaria, Stylianos Makrydimas, Efthalia Moustakli, Ioannis Mitrogiannis, Ermanno Vitale, and George Makrydimas. 2025. "Physical Activity During Pregnancy and Gestational Weight Gain: Implications for Maternal–Fetal Epigenetic Programming and Long-Term Health" Genes 16, no. 10: 1173. https://doi.org/10.3390/genes16101173
APA StyleZagorianakou, N., Makrydimas, S., Moustakli, E., Mitrogiannis, I., Vitale, E., & Makrydimas, G. (2025). Physical Activity During Pregnancy and Gestational Weight Gain: Implications for Maternal–Fetal Epigenetic Programming and Long-Term Health. Genes, 16(10), 1173. https://doi.org/10.3390/genes16101173





