EDA Mutations Causing X-Linked Recessive Oligodontia with Variable Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrollment of Human Subjects
2.2. Candidate Gene Sequencing
2.3. EDA cDNA Cloning and Mutagenesis
2.4. Cell Culture and Transient Transfection
2.5. Western Blot
2.6. Protein Structure Analysis
2.7. In Silico Prediction of the Mutational Effect
3. Results
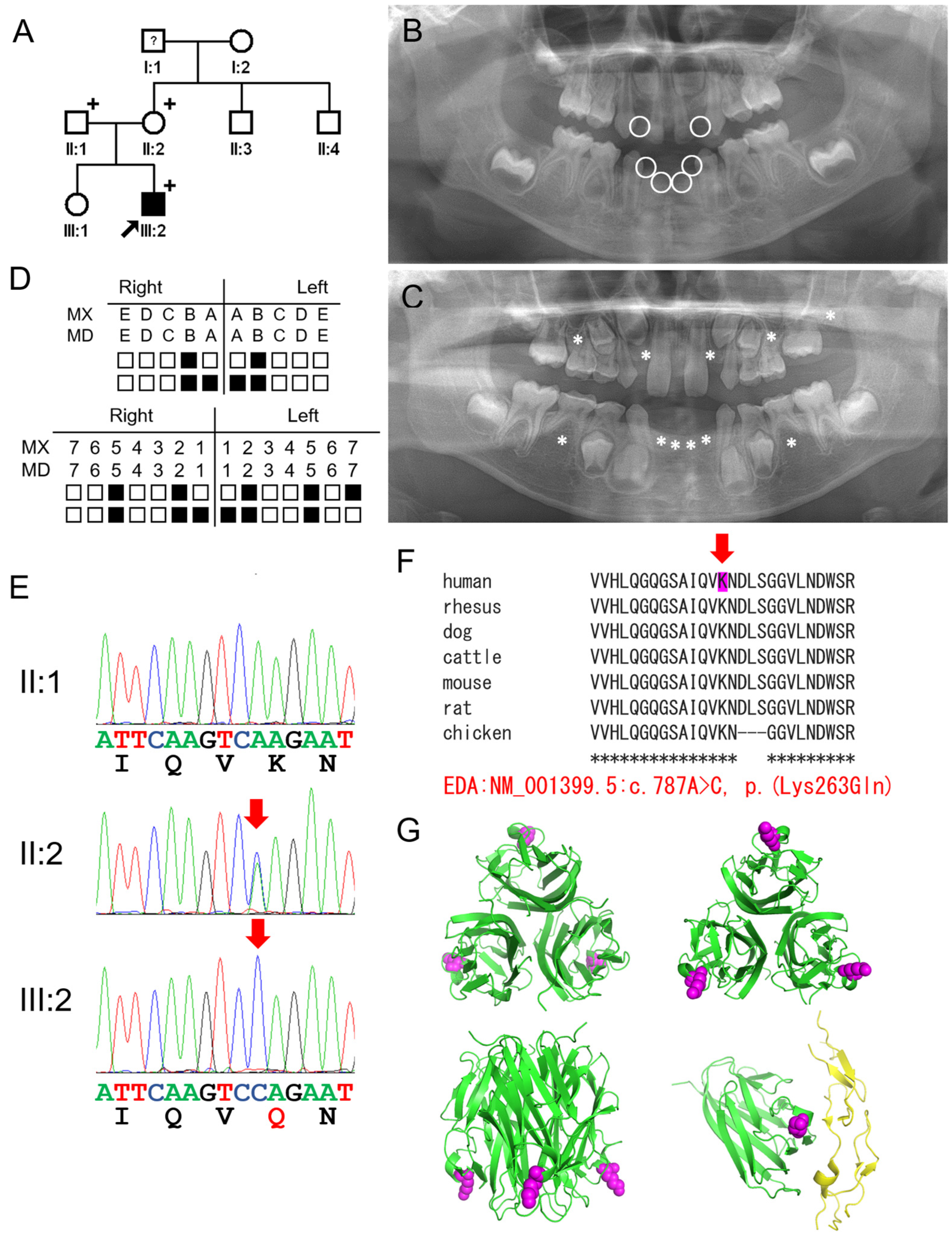
3.1. Family 1
3.2. Family 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, H.; Ye, X.; Shi, L.; Yin, W.; Hua, B.; Song, G.; Shi, B.; Bian, Z. Mutations in the EDA gene are responsible for X-linked hypohidrotic ectodermal dysplasia and hypodontia in Chinese kindreds. Eur. J. Oral Sci. 2008, 116, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Molloy, S.S.; Thomas, L.; Gambee, J.; Bachinger, H.P.; Ferguson, B.; Zonana, J.; Thomas, G.; Morris, N.P. Mutations within a furin consensus sequence block proteolytic release of ectodysplasin-A and cause X-linked hypohidrotic ectodermal dysplasia. Proc. Natl. Acad. Sci. USA 2001, 98, 7218–7223. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Ko, J.; Shin, T.J.; Hyun, H.K.; Lee, S.H.; Kim, J.W. Oligodontia and curly hair occur with ectodysplasin-a mutations. J. Dent. Res. 2014, 93, 371–375. [Google Scholar] [CrossRef]
- Vastardis, H. The genetics of human tooth agenesis: New discoveries for understanding dental anomalies. Am. J. Orthod. Dentofac. Orthop. 2000, 117, 650–656. [Google Scholar] [CrossRef]
- Thesleff, I. Current understanding of the process of tooth formation: Transfer from the laboratory to the clinic. Aust. Dent. J. 2013, 59, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Shen, Y.; Jiang, C.L.; Huang, W.; Wang, F.; Wu, Y.Q. Two novel ectodysplasin A gene mutations and prenatal diagnosis of X-linked hypohidrotic ectodermal dysplasia. Mol. Genet. Genom. Med. 2021, 9, e1824. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Liang, J.L.; Sui, W.G.; Lin, H.; Xue, W.; Chen, J.J.; Zhang, Y.; Gong, W.W.; Dai, Y.; Ou, M.L. EDA mutation as a cause of hypohidrotic ectodermal dysplasia: A case report and review of the literature. Genet. Mol. Res. 2015, 14, 10344–10351. [Google Scholar] [CrossRef]
- Lan, R.; Wu, Y.; Dai, Q.; Wang, F. Gene mutations and chromosomal abnormalities in syndromes with tooth agenesis. Oral Dis. 2023, 29, 2401–2408. [Google Scholar] [CrossRef]
- Fournier, B.P.; Bruneau, M.H.; Toupenay, S.; Kerner, S.; Berdal, A.; Cormier-Daire, V.; Hadj-Rabia, S.; Coudert, A.E.; de La Dure-Molla, M. Patterns of Dental Agenesis Highlight the Nature of the Causative Mutated Genes. J. Dent. Res. 2018, 97, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Qin, C.; Yue, H.; He, H.; Bian, Z. A novel initiation codon mutation of PAX9 in a family with oligodontia. Arch. Oral Biol. 2016, 61, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, X.; Lu, Z. X-linked hypohidrotic ectodermal dysplasia with a deletion in exon 2 of the EDA gene: A case report and literature review. Eur. J. Dermatol. 2024, 34, 310–311. [Google Scholar] [CrossRef]
- Rasool, M.; Schuster, J.; Aslam, M.; Tariq, M.; Ahmad, I.; Ali, A.; Entesarian, M.; Dahl, N.; Baig, S.M. A novel missense mutation in the EDA gene associated with X-linked recessive isolated hypodontia. J. Hum. Genet. 2008, 53, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Krull, F.; Bleyer, M.; Schäfer, J.; Brenig, B. A missense mutation in the highly conserved TNF-like domain of Ectodysplasin A is the candidate causative variant for X-linked hypohidrotic ectodermal dysplasia in Limousin cattle: Clinical, histological, and molecular analyses. PLoS ONE 2024, 19, e0291411. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Lin, B.; Yu, M.; Liu, Y.; Sun, S.; Feng, H.; Liu, H.; Han, D. EDA Variants Are Responsible for Approximately 90% of Deciduous Tooth Agenesis. Int. J. Mol. Sci. 2024, 25, 10451. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lai, L.; Chen, J.; Li, X.; Hou, J. Genotypic and phenotypic correlations in tooth agenesis: Insights from WNT10A and EDA mutations in syndromic and non-syndromic forms. Hum. Genet. 2024, 143, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, W.; Gao, J.; Huang, S. Application of digital virtual simulated design in the prosthodontic rehabilitation of a child with a novel EDA mutation in ectodermal dysplasia: A clinical report. J. Prosthet. Dent. 2024; in press. [Google Scholar] [CrossRef]
- Yu, K.; Sheng, Y.; Wang, F.; Yang, S.; Wan, F.; Lei, M.; Wu, Y. Eight EDA mutations in Chinese patients with tooth agenesis and genotype-phenotype analysis. Oral Dis. 2024, 30, 4598–4607. [Google Scholar] [CrossRef]
- Song, S.; Han, D.; Qu, H.; Gong, Y.; Wu, H.; Zhang, X.; Zhong, N.; Feng, H. EDA gene mutations underlie non-syndromic oligodontia. J. Dent. Res. 2009, 88, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Mues, G.; Tardivel, A.; Willen, L.; Kapadia, H.; Seaman, R.; Frazier-Bowers, S.; Schneider, P.; D’Souza, R.N. Functional analysis of Ectodysplasin-A mutations causing selective tooth agenesis. Eur. J. Hum. Genet. 2010, 18, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Molina, G.; Reyes-Reali, J.; Mendoza-Ramos, M.I.; Villalobos-Molina, R.; Garrido-Guerrero, E.; Méndez-Cruz, A.R. Novel missense mutation in the EDA1 gene identified in a family with hypohidrotic ectodermal dysplasia. Int. J. Dermatol. 2015, 54, 790–794. [Google Scholar] [CrossRef]
- He, H.; Han, D.; Feng, H.; Qu, H.; Song, S.; Bai, B.; Zhang, Z. Involvement of and interaction between WNT10A and EDA mutations in tooth agenesis cases in the Chinese population. PLoS ONE 2013, 8, e80393. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Huang, F.; Wang, B.; Tao, Y.; Jiang, Z.; Li, H.; Yao, J.; Xu, P.; Wu, X.; et al. A novel missense mutation in collagenous domain of EDA gene in a Chinese family with X-linked hypohidrotic ectodermal dysplasia. J. Genet. 2015, 94, 115–119. [Google Scholar] [CrossRef]
- Schneider, H.; Hammersen, J.; Preisler-Adams, S.; Huttner, K.; Rascher, W.; Bohring, A. Sweating ability and genotype in individuals with X-linked hypohidrotic ectodermal dysplasia. J. Med. Genet. 2011, 48, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Sadier, A.; Viriot, L.; Pantalacci, S.; Laudet, V. The ectodysplasin pathway: From diseases to adaptations. Trends Genet. 2013, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, X.; Wei, Z.; Long, H.; Lai, W. The EDA/EDAR/NF-κB pathway in non-syndromic tooth agenesis: A genetic perspective. Front. Genet. 2023, 14, 1168538. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, W.H.; Koczorowski, R. Molecular basis of hypohidrotic ectodermal dysplasia: An update. J. Appl. Genet. 2016, 57, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Hymowitz, S.G.; Compaan, D.M.; Yan, M.; Wallweber, H.J.; Dixit, V.M.; Starovasnik, M.A.; de Vos, A.M. The crystal structures of EDA-A1 and EDA-A2: Splice variants with distinct receptor specificity. Structure 2003, 11, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, C.; Wan, F.; Jiang, C.; Chen, J.; Li, X.; Wang, F.; Wu, J.; Lei, M.; Wu, Y. Structural insights into pathogenic mechanism of hypohidrotic ectodermal dysplasia caused by ectodysplasin A variants. Nat. Commun. 2023, 14, 767. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef]
- Zeng, B.; Lu, H.; Xiao, X.; Zhou, L.; Lu, J.; Zhu, L.; Yu, D.; Zhao, W. Novel EDA mutation in X-linked hypohidrotic ectodermal dysplasia and genotype-phenotype correlation. Oral Dis. 2015, 21, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Ruf, S.; Klimas, D.; Honemann, M.; Jabir, S. Genetic background of nonsyndromic oligodontia: A systematic review and meta-analysis. J. Orofac. Orthop. 2013, 74, 295–308. [Google Scholar] [CrossRef]
- Zhang, J.; Han, D.; Song, S.; Wang, Y.; Zhao, H.; Pan, S.; Bai, B.; Feng, H. Correlation between the phenotypes and genotypes of X-linked hypohidrotic ectodermal dysplasia and non-syndromic hypodontia caused by ectodysplasin-A mutations. Eur. J. Med. Genet. 2011, 54, e377–e382. [Google Scholar] [CrossRef]
- Ruiz-Heiland, G.; Jabir, S.; Wende, W.; Blecher, S.; Bock, N.; Ruf, S. Novel missense mutation in the EDA gene in a family affected by oligodontia. J. Orofac. Orthop. 2016, 77, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.; Wang, L.; Li, C.; Archacki, S.; Huang, C.; Liu, J.Y.; Wang, Q.; Liu, M.; Tang, Z. Mutation p.Leu354Pro in EDA causes severe hypohidrotic ectodermal dysplasia in a Chinese family. Gene 2012, 491, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, T.; Li, T.; Ye, Y.; Feng, C.; Wang, H.; Zhang, X. A novel EDAR variant identified in non-syndromic tooth agenesis: Insights from molecular dynamics. Arch. Oral Biol. 2023, 146, 105600. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhang, R.; Li, M.; Zou, Y.; Jiang, S.; Zhang, Y.; Liu, S.; Yu, B. A Novel Ectodysplasin a Gene mutation of X-Linked Hypohidrotic Ectodermal Dysplasia. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1505–1517. [Google Scholar] [CrossRef]


| Forward Primer | Reverse Primer | |
|---|---|---|
| c.457C>T p.(Arg153Cys) | 5′-GAAGAAAGTAGGTGTGTTCGCCGC-3′ | 5′-GCGGCGAACACACCTACTTTCTTC-3′ |
| c.787A>C p.(Lys263Gln) | 5′-GCAATTCAAGTCCAGAATGATCTTTC-3′ | 5′-GAAAGATCATTCTGGACTTGAATTGC-3′ |
| Patient ID | EDA Mutations | Missing Teeth (FDI Notation) | Diagnosis |
|---|---|---|---|
| II:1 family 1 | c.457C>T p.(Arg153Cys) | #52, 62, 71, 72, 73, 81, 82, 83 12, 13, (15), (17), 22, 23, (25), (27), 31, 32, 33, (34), (35), (37), 41, 42, 43, (44), (45), (47) | X-linked ED |
| III:2 family 4 | c.787A>C p.(Lys263Gln) | #52, 62, 71, 72, 81, 82 12, 15, 22, 25, 27, 31, 32, 35, 41, 42, 45 | Oligodontia |
| PolyPhen-2 | Mutation Taster | CADD 1.7 | |
|---|---|---|---|
| c.457C>T p.(Arg153Cys) | Possibly damaging (score: 0.853) | Disease-causing (prob: 0.889) | 23.1 |
| c.787A>C p.(Lys263Gln) | Probably damaging (score: 0.992) | Disease-causing (prob: 0.994) | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Kim, Y.J.; Chae, W.; Kim, S.H.; Kim, J.-W. EDA Mutations Causing X-Linked Recessive Oligodontia with Variable Expression. Genes 2025, 16, 12. https://doi.org/10.3390/genes16010012
Lee YJ, Kim YJ, Chae W, Kim SH, Kim J-W. EDA Mutations Causing X-Linked Recessive Oligodontia with Variable Expression. Genes. 2025; 16(1):12. https://doi.org/10.3390/genes16010012
Chicago/Turabian StyleLee, Ye Ji, Youn Jung Kim, Wonseon Chae, Seon Hee Kim, and Jung-Wook Kim. 2025. "EDA Mutations Causing X-Linked Recessive Oligodontia with Variable Expression" Genes 16, no. 1: 12. https://doi.org/10.3390/genes16010012
APA StyleLee, Y. J., Kim, Y. J., Chae, W., Kim, S. H., & Kim, J.-W. (2025). EDA Mutations Causing X-Linked Recessive Oligodontia with Variable Expression. Genes, 16(1), 12. https://doi.org/10.3390/genes16010012






