Abstract
LONP1 is the principal AAA+ unfoldase and bulk protease in the mitochondrial matrix, so its deletion causes embryonic lethality. The AAA+ unfoldase CLPX and the peptidase CLPP also act in the matrix, especially during stress periods, but their substrates are poorly defined. Mammalian CLPP deletion triggers infertility, deafness, growth retardation, and cGAS-STING-activated cytosolic innate immunity. CLPX mutations impair heme biosynthesis and heavy metal homeostasis. CLPP and CLPX are conserved from bacteria to humans, despite their secondary role in proteolysis. Based on recent proteomic–metabolomic evidence from knockout mice and patient cells, we propose that CLPP acts on phase-separated ribonucleoprotein granules and CLPX on multi-enzyme condensates as first-aid systems near the inner mitochondrial membrane. Trimming within assemblies, CLPP rescues stalled processes in mitoribosomes, mitochondrial RNA granules and nucleoids, and the D-foci-mediated degradation of toxic double-stranded mtRNA/mtDNA. Unfolding multi-enzyme condensates, CLPX maximizes PLP-dependent delta-transamination and rescues malformed nascent peptides. Overall, their actions occur in granules with multivalent or hydrophobic interactions, separated from the aqueous phase. Thus, the role of CLPXP in the matrix is compartment-selective, as other mitochondrial peptidases: MPPs at precursor import pores, m-AAA and i-AAA at either IMM face, PARL within the IMM, and OMA1/HTRA2 in the intermembrane space.
Keywords:
Perrault syndrome type 3 (PRLTS3); iron toxicity; pyridoxal-5′-phosphate; VWA8; GFM1; PNPT1; RNA-G4; ISC; ALAS; OAT 1. CLPP Is a Key Modifier of Growth and Lifespan, but Its Substrates Remain Unclear
LONP1 is the principal proteolysis factor of the mitochondrial matrix, combining an AAA+ ATPase unfoldase domain with a serine peptidase domain within the same protein sequence. LONP1 homo-multimerizes in a ring or barrel shape to maximize its efficiency and plays a crucial role in the turnover of respiratory chain complexes and most other proteins in this compartment [1,2,3]. According to studies on bacteria, CLPXP is perceived as a similar but stress-responsive proteolysis machine, also in the mitochondrial matrix. However, CLPX as an AAA+ ATPase unfoldase component and CLPP as a serine peptidase component are separate proteins. To obtain proteolytic capacity, via assembly in a barrel-like conformation similar to LONP1, they can hetero-multimerize. Nonetheless, in proteolysis, they play a secondary role, becoming prominent only after cellular stress [4]. Both systems have been conserved from bacteria to humans, so each of them has to play very relevant roles in the mitochondrial matrix. Indeed, the loss of LONP1 in a homozygous state causes lethality already during early embryonic development [1]. In contrast, the loss of CLPP was observed to extend the lifespan in the eukaryotic fungus Podospora anserina, and CLPP is constitutively absent from the yeast Saccharomyces cerevisiae [5,6]. This emphasizes a dramatic difference in the functional impact of these two systems, which is at odds with the idea that both act similarly in proteolytic degradation.
Genetic analyses of human diseases recently showed mild LONP1 mutations to be responsible for CODAS syndrome, where craniofacial dysmorphia, cataracts, ptosis, a median nasal groove, delayed tooth eruption, delayed epiphyseal ossification, metaphyseal hip dysplasia, vertebral coronal clefts, short stature, psychomotor and developmental delay, and hearing loss are diagnostic hallmarks [7,8]. In contrast, CLPP mutations cause Perrault syndrome type 3 (PRLTS3) [9,10,11,12,13,14,15]. Perrault syndrome was clinically and genetically defined as the combination of early female infertility due to primary ovarian failure, with the subsequent onset of progressive sensorineural hearing impairment and autosomal recessive inheritance. Later, it was observed that not only deafness but also sensory neuropathy, ataxia, and brain white matter changes can appear as neurodegenerative features [16,17,18,19,20]. Judging by human genetics, the functions of LONP1 and CLPP therefore appear to target different pathways, and their dysfunctions have widely different consequences.
Genetic causes of PRTLS are almost exclusively due to mtDNA/mtRNA or mitoribosome machinery errors [13,21]. The role of mtDNA in Perrault syndrome’s pathogenesis is substantiated by causal mutations in the mitochondrial DNA/RNA helicase TWNK/PEO1 and in the mitochondrial transcription factor TFAM [22,23]. The role of mtRNA processing is corroborated by causal mutations in the mitochondrial rRNA chaperone ERAL1 and the mitochondrial RNase P component PRORP [16,24,25]. The roles of mt-tRNA processing and mitoribosomal translation are evident from causal mutations in the mitochondrial tRNA–amino acid ligases HARS2 and LARS2, as well as the mitoribosome-associated factor RMND1 [13,26,27,28,29,30,31,32,33,34,35]. The detailed correlation of mutant mitochondrial factors with the consequent phenotypes (Figure 4 in [36]) indicates that primary infertility is mostly due to abnormal mtDNA or mt-tRNA processing, whereas hearing impairment is frequently due to abnormal mtRNA processing or mitoribosomal translation. Thus, CLPP appears to selectively modulate mitochondrial RNA processing and translation.
Also in a complete phenotypic contrast, human CLPX mutations were observed to cause erythropoietic protoporphyria 2 (leading to acute skin photosensitivity, mild microcytic anemia, and rarely, severe liver disease) [37,38]. The credibility of these findings is enhanced by observations from yeast to humans that CLPX, independently from CLPP, activates heme biosynthesis [5,37,39].
The recent generation of several independent Clpp-knockout (KO) mouse lines, by means of Gene-Trap random insertion [40] and targeted conditional technology [41], provided authentic genetic animal models of PRLTS3 with the characteristic phenotypes and allowed for the elucidation of the underlying molecular and functional deficits. Independently and in perfect agreement, the biochemical analyses of each research team showed that CLPP homo-multimer rings exist normally without CLPX association in blue-native electrophoresis, where endogenous protein complexes are resolved according to their interactive stability and molecular weight (Figure 2 in [42], Supplementary Figure EV3B in [43]). Thus, in unstressed mammalian cells, CLPP rings do not associate with the energy-providing AAA+ ATPase CLPX and cannot perform the degradation of protein substrates, which would require ATP hydrolysis [4]. Therefore, CLPP functions would normally be limited to act as a peptidase like chymotrypsin [44], trimming proteins or multi-protein assemblies rather than completely eliminating them. This concept is in agreement with a recent review where the role of CLPX and CLPP was seen in the fine-tuning of mitochondrial matrix multi-protein assemblies rather than in proteolysis [44]. CLPX as a monomer or homo-multimer ring would then employ its energy from ATP hydrolysis to unfold proteins or protein complexes without subsequent destruction. Of course, CLPX and CLPP may join forces under conditions of cell stress, e.g., when mitoribosomal translation is stalled and a misfolded nascent polypeptide has to be degraded, or after cell damage to disaggregate and cleave the toxic oligomers of ribonucleoproteins. An illustrated synopsis of this emerging scenario is provided in Figure 1, and the detailed evidence is presented in the subsequent text paragraphs with citations of recent research.
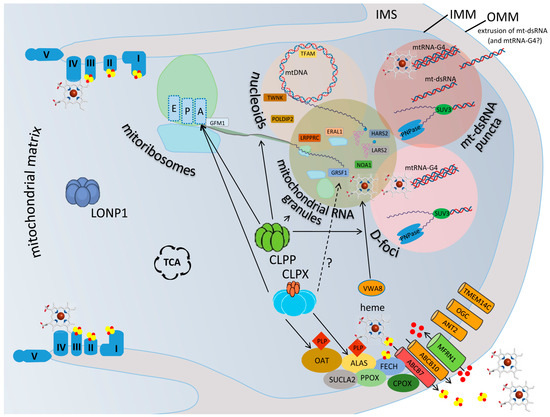
Figure 1.
CLPX and CLPP perform first aid in matrix granules where reaction intermediates are separated from the aqueous phase. A depiction of a mitochondrial matrix compartment between two cristae, where the LONP1 homomultimer is responsible for bulk proteolysis, while the homohexameric ring of the AAA+ unfoldase CLPX and the homoheptameric ring of the peptidase CLPP perform first aid in IMM-associated granular condensates, in cooperation with the AAA+ unfoldase VWA8. CLPX maximizes the flux within the heme-biosynthesis multi-enzyme metabolon, unfolding ALAS and OAT so that they can bind to their cofactor PLP, to perform transaminations at delta-carbon positions. CLPX also associates with the translation elongation factor GFM1 when a nascent peptide is misfolded. Indirectly via heme or directly via GRSF1, CLPX may also influence mtRNA-G4 processing. The heme availability also impacts the respiratory chain and many processes outside the mitochondria. Rather than performing proteolytic degradation, without assistance by the ATPase CLPX, CLPP can only trim short polypeptides from proteins and assemblies, like chymotrypsin. CLPP apparently has access to diverse phase-separated, ribonucleoprotein-containing condensates in the matrix, where the transcription, processing, translation, degradation, and extrusion of mtRNA are decided. The illustration presents the respiratory chain at each crista with its complexes I–V and in association with iron (red dots)–sulfur (yellow dots) clusters, as well as the heme quadrangular molecule. The mitoribosomal large subunit (green globe) and small subunit (light blue) are shown with the sites for aminoacyl binding (A), peptidyl extension (P), and exit to tunnel (E), where the GTPase GFM1 determines the elongation. The various terms and protein symbols are defined in the Abbreviation List below.
To elucidate the exact roles of CLPP serves an urgent unmet medical need, given that the modulation of CLPP activity by drugs is consistently observed to be very efficacious in counteracting solid cancers and infections [45,46,47]. Indeed, the effect of CLPP is strong even under physiological conditions: its dysfunction leads to a short stature in patients [15], and it is unclear if this represents impaired cell growth, reduced proliferation rates, or developmental deficits. In the PRLTS3 mouse models, a reduction in weight of up to 50% was observed, together with an underlying similar decrease in the nitrogen-storing amino acid L-arginine, which is consumed in the maximized biosynthesis of heme instead of fueling growth [48]. This means that nitrogen availability in L-arginine could be the limiting factor for organism growth. In a preliminary meta-analysis of CLPP substrate trapping experiments and of CLPP–null proteome profiling in many organisms from bacteria to humans, an enrichment of mitochondrial ribonucleoproteins was observed, with the unfoldase CLPX, the mitoribosomal translation factor GFM1, and the RNA degradation factor PNPT1 emerging as the proteins that most consistently interact with CLPP and show accumulation upon its loss [36].
2. Novel Evidence on CLPP and CLPX’s Functions from Clpp-KO Mice and PRLTS3 Patients
2.1. Prominent Impact of Absence of CLPP and Excess of CLPX on Mitochondrial Nucleoids
Upon the first generation of two independent Clpp-KO mouse embryonic stem cells by inactivating Gene-Trap insertions in intron 1 and 2 at the Texas Institute of Genomic Medicine (TIGM), the derived mice were shown to serve as authentic models of Perrault syndrome [40]. These homozygous Clpp-KO mice exhibited complete infertility even at an early age, an average weight reduction of up to 70% and length reduction of up to 90% from 12 weeks, impaired locomotor activity by the age of 6 months, sensorineural hearing impairment from 12 to 18 months, and a relative resistance to microbial infections [40]. In contrast to other forms of Perrault syndrome with exclusively female infertility due to primary ovarian insufficiency, CLPP absence according to mouse data also causes male infertility due to azoospermia after diplotene arrest [49]. Further analyses of the lean phenotype of several Clpp-KO mouse lines showed a protection from diet-induced obesity and from insulin resistance but also a deficit to adapt their body’s thermogenesis [50,51].
The molecular analyses of tissues showed the absence of CLPP to cause a >3-fold accumulation of the unfoldase CLPX, together with increased amounts of the mitochondrial protein chaperone mtHSP75 (but not HSPD1) [40,52]. This corresponds partially to previous observations in Caenorhabditis elegans studies on the unfolded protein response of mitochondria (UPRmt), where hsp-6 and hsp-60 were induced [53]. Beyond the expected impairment of proteostasis, careful quantification of mtDNA with qPCR in the testis, ovary, heart, brain, liver, and blood demonstrated a 1.5- to 4-fold increase in the mtDNA copy number [53]. This observation was not reproduced in mice with conditionally targeted Clpp deletion when the full-length mtDNA from the heart muscle was assessed by means of Southern blotting [43], but it was confirmed by an independent team in white adipose tissue from the Gene-Trap Clpp-KO mice with qPCR [51] and also in CLPP-mutant patient skin fibroblasts by means of qPCR [54]. Furthermore, the patient fibroblast analysis by means of microscopy demonstrated an enlargement in the nucleoid area, with an apparent elevation of mtDNA signals [54]. It is important to note that the increased mtDNA copy number was not accompanied by an elevated abundance of TFAM as its primary binding partner. Instead, the proteome profiling of the targeted Clpp-KO mouse heart tissue, Gene-Trap Clpp-KO mouse embryonic fibroblasts, and patient fibroblasts documented prominent accumulation of the nucleoid factor POLDIP2 [52,54,55], a protein that is known to associate with mtSSB [55] and CLPX, which maximizes the activity as well as stability of CLPXP [56]. Mechanistic analyses of the drug ZG36, which acts as a CLPP agonist, showed a converse impact, with a reduction in mtDNA to half [57]. In addition, in Clpp-KO testis at the early stages of spermatogenesis, a consistent accumulation was observed for the Twinky isoform of the mtDNA helicase TWNK/PEO1, which differs from the Twinkle isoform by absent binding to the D-loop [58]. This finding appears to be particularly relevant given that TWNK mutations can cause Perrault syndrome [23,59,60,61,62,63,64,65,66].
These observations are compatible with the concept that the absence of CLPP results in an increased dosage of mtDNA fragments rather than full-length copies, so that their assembly with associated proteins is impaired, and the generation of the polycistronic transcript may be affected.
2.2. Prominent Impact of Absence of CLPP and Excess of CLPX on Mitoribosomes, Mostly on tRNA-/mRNA-Associated and rRNA-Containing mtSSU
Further evidence that CLPP and CLPX target granular components of the mitochondrial matrix was reported for mitoribosomes, initially in the targeted Clpp-KO mouse [43], and then in the Gene-Trap Clpp-KO mouse as well [42].
Regarding ribosomal RNA, the 12S rRNA and MRPS15-MRPS35 protein components of the mitoribosomal 28S small subunit (mtSSU) showed a much more elevated abundance than the 16S rRNA and MRPL12-MRPL37 protein components of the large subunit of mitoribosomes (mtLSU) in Clpp-KO mice [41]. Further analyses of the co-migration of mitochondrial proteins in blue-native gel electrophoresis to define their interaction in assembled complexes confirmed a general accumulation of all the components of the mtSSU in a Clpp-KO testis, brain, and heart in the absence of CLPX co-migration [42].
Regarding the translation-associated enzymes, the elongation factor GFM1 (also known as EFG1, an ortholog of bacterial fusA-encoded EF-G, see [67]) exhibited an elevated protein abundance in Clpp-KO mice as well, along with abnormal sedimentation in sucrose gradients [41]. Indeed, the co-accumulation of CLPX together with its interactor GFM1 was subsequently confirmed in proteome profiling studies of mouse brains, MEFs, and patient fibroblasts [54]. This is in agreement with the notion that CLPX not only acts in heme biosynthesis but is also able to target the GFM1-associated L7/L12 stalk and central protuberance of mitoribosomal LSU to act in the translation elongation/recycling apparatus [68,69,70,71,72].
These findings identify the molecular details that underlie previous observations that CLPXP is necessary to rescue stalled translation complexes by unfolding the mitoribosome, so that translation elongation via the addition of a CAT tail to the nascent misfolded polypeptide can occur. CLPXP then eliminates this aberrant translation product before its aggregation tendency has toxic effects [73,74,75,76].
2.3. Prominent Impact of Absence of CLPP and Excess of CLPX on Mitochondrial RNA Processing Granules
A third line of evidence on the role of CLPP in mitochondrial matrix granules concerns the RNA processing compartment. It was observed that mtSSU rRNA accumulates in Clpp-KO mice [41]. ERAL1 serves as a mitochondrial rRNA chaperone, while the 12S rRNA associates with ribonucleoproteins to form the mtSSU. Indeed, ERAL1 exhibited not only an elevated protein abundance in Clpp-KO heart mitochondria but also abnormal sedimentation in sucrose gradients [41]. Again, ERAL1 accumulation appears to be particularly relevant, given that ERAL1 mutations can trigger Perrault syndrome [25,77,78,79].
Clpp-KO-triggered accumulation was also documented for a few mitochondrial tRNAs [41]. In particular, the tRNAs for valine (Val) and phenylalanine (Phe) exhibited higher aminoacylation in a Clpp-KO heart [41]. Therefore, it is interesting that a very selective protein accumulation exists for mtLSU components like MRPL18 and MRPL38, which assemble with tRNA-Val/Phe in the central protuberance of mitoribosomes, and that this CLPP-null effect on the central protuberance subunits of the mtLSU is conserved across eukaryotes until the ascomycete fungus P. anserina [42,48]. This selective impact on the mtLSU may also be relevant for mtDNA and the cytosolic stress response: MRPL38 influences the maintenance of the mitochondrial nucleoid, at least in yeast [80]. Furthermore, there is a cytosolic isoform of MRPL18, which modulates the ribosomal translation of molecular chaperones after cell stress [81] and can thus influence the UPR outside of mitochondria.
A key role of a tRNA-associated pathology in CLPP-dependent pathogenesis is also evident from human genetics data. Mutations in the mitochondrial tRNA–aminotransferases for histidine and leucine, HARS2 and LARS2, cause the typical features of Perrault syndrome [21,30,82,83,84,85,86], while mutations in the mitochondrially encoded tRNA sequences trigger different and mostly neurodegenerative phenotypes, including progressive deafness [87]. Mirroring a joint pathogenetic pathway for different variants of Perrault syndrome, Clpp-KO testes from the early stages of spermatogenesis contain elevated amounts of HARS2 [42]. It is furthermore worth noting that the deleterious effects of mutations in DARS2 (mitochondrial tRNA–aspartate aminotransferase) in mice can be partially rescued by an absence of CLPP [41,88], so in conditions of enhanced mitochondrial RNA processing and translation blockade, it can be advantageous to have a CLPP loss-of-function that reduces UPRmt and prolongs the lifespan.
The folding of mitochondrial tRNAs and rRNAs represents another pathway that is affected both by the impact of CLXP on heme and by the impact of CLPP on ribonucleoprotein condensates. Bacterial tRNA and rRNA contain guanine-rich sequences that can adopt quadruplex structures [89]. Also, for mammalian cytosolic ribosomes, the importance of such rRNA quadruplexes for mature conformation has already been documented [90]. When guanine-rich sequences adopt a quadruplex conformation (G-tetrads) with four RNA or DNA strands (RNA-G4 or DNA-G4), their structure can be stabilized by an association with quadrangular porphyrin and heme molecules [90,91,92,93]. This interaction may activate peroxidase- or oxidase-mimicking features in this DNAzyme/RNAzyme complex [92,93], may modify the compaction and processing of DNA/RNA [94], and is crucial in ribosomes for optimal translation efficiency [95]. The high abundance of such rRNA-G4 structures even limits the bioavailability of heme in cells [90]. This pathway seems to be altered in PRLTS3, in view of the selective accumulation of the RNA granule factor GRSF1 (G-Rich Sequence Factor 1) in Clpp-KO tissues [41,54]. Within mitochondria, GRSF1 is responsible for non-coding RNA in the G4 conformation [96,97]. GRSF1 interacts with RNase P to influence the cleavage of polycistronic transcripts [98], and its dysfunction leads to RNA processing defects, the accumulation of mtRNA breakdown products, as well as increased levels of dsRNA and RNA:DNA hybrids [99]. These problems lead to the formation of distinct mitochondrial dsRNA foci [100]. In addition, GRSF1 dysfunction triggers the abnormal loading of mRNAs and lncRNAs on the mitochondrial ribosome and impaired ribosome assembly [101]. GRSF1 also influences the degradation of mtRNA in the degradosome in cooperation with PNPT1 [96,102]. Overall, it is not surprising that GRSF1 is also involved in iron toxicity like CLPX [103] and in lean body phenotypes like CLPP [104,105]. GRSF1 was observed in protein–protein interactions with CLPX [36].
RNA-G4 structures also control the activity of the mitochondrial GTPase NOA1 (also known as C4ORF14) for mitoribosomal assembly [106,107,108]. NOA1 was also identified as a CLPXP target protein [109].
The joint roles of absent CLPP and excess CLPX during the assembly of mitoribosomes are further supported by the selective accumulation of VWA8 in Clpp-KO tissues [42]. The mitochondrial matrix protein VWA8 [110] contains a domain that is related to porphyrin chelatases [42], so it might interact with heme or its precursors. VWA8 also contains an AAA+ unfoldase domain, whose protein targets are undefined in mammals. Its yeast ortholog midasin (also known as Rea1) was clearly shown to be responsible for the maturation of the mitoribosomal LSU [111,112,113].
With excess heme being released from mitochondria in PRLTS3, abnormal G-tetrad processing might also occur in the nucleus, where homologous recombination is known to depend on DNA-G4 structures [114]. Thus, the complete infertility of PRLTS3 patients, with the abortion of nuclear meiosis-I after diplotene arrest [49], might partially be a consequence of defective G4 conformations.
Furthermore, the mitochondrial RNA granule factor LRPPRC undergoes selective accumulation in Clpp-KO tissues [41,54]. LRPPRC is known to modulate the poly(A) tail of mRNAs in mitochondria [115,116,117,118,119], and its dysfunction influences the efficiency of the RNA degradosome together with the accumulation of toxic dsRNA [120].
Jointly, all this evidence indicates that the processing of polycistronic mtRNA, which is transcribed from mtDNA and then cleaved to tRNAs, rRNAs, other non-coding RNAs, and mRNAs, is selectively altered by the absence of CLPP. CLPP could trim components that are stuck within the RNA–protein complexes. CLPX clearly has a function in the disassembly of stalled translation complexes and might play a role in the G4 conformation of rRNA, which is important for the assembly of mitoribosomes.
2.4. Prominent Impact of Absence of CLPP and Excess of CLPX on Mitochondrial D-Foci Where RNA Degradation, Extrusion, and Innate Immunity Activation Are Decided
A fourth indication of the role of CLPP for mitochondrial matrix granules concerns the RNA degradosome in the so-called D-foci [121,122,123]. Its main component, the ribonuclease PNPT1 (which is orthologous to the bacterial polynucleotide phosphorylase/polyadenylase pnp, or PNPase), is associated with CLPP and is dysregulated upon CLPP deletion, exhibiting consistency in hosts ranging from Escherichia coli to mice [36]. Together with the RNA helicase SUPV3L1 (best known as SUV3, see [124]) and the RNA-G4-quadruplex modulating factor GRSF1, PNPT1 eliminates dsRNA, acting as 3′-5′ exonuclease [102,122,125,126,127,128,129], and even its bacterial ortholog pnp is responsible for antiviral immunity [130]. The matrix degradosome in D-foci appears to act not only on the abundant mtRNA, since PNPase and SUV3 show a preference for mtDNA [131,132,133,134,135]. Similarly to mutations in CLPP, mutations in PNPT1 are also the cause of progressive deafness and of a sensory neuropathy with ataxia [136,137,138,139,140,141]. PNPT1 dysfunction causes the accumulation of toxic dsRNAs and their extrusion from mitochondria into the cytosol, where antiviral innate immunity responses are activated [142]. Again, the homozygous absence of CLPP or heterozygous absence of mtDNA-binding TFAM in the mitochondrial matrix triggers cytosolic antiviral innate immunity responses, like the induction of the AAA+ unfoldase RNF213. This unfoldase is also activated by toxic dsRNA mimics such as poly(I:C) administration [143]. In Clpp-KO mouse brains and MEFs, the selective activation of various cytosolic sensors for toxic DNA and RNA was documented [144]. The problems in the packaging of mtDNA and in degrading/extruding toxic nucleic acids from mitochondria in Clpp-KO cells were shown to activate antiviral cytosolic responses via the cGAS/STING pathway [145].
Overall, the resulting steady-state activation of type I interferon signaling explains the marked resistance of CLPP-null mice to bacterial and RNA/DNA virus infections [40,145].
As a preliminary conclusion, the above four paragraphs represent solid evidence that CLPP has a selective impact on matrix granules in which RNA is a component, which mediates the liquid–liquid phase separation (LLPS) around these condensates.
2.5. Prominent Impact of Absence of CLPP via CLPX Accumulation on Heme Biosynthesis and Incorporation into Complex-IV of the Respiratory Chain
The absence of CLPP causes a several-fold accumulation of CLPX, as explained above. CLPX has an important role in the heme metabolism multi-enzyme complex, which is associated with the IMM [146], and serves to separate ferrous iron and reduced porphyrin intermediates from unwanted reactions in the matrix [147,148]. This multi-enzyme chain was previously shown to serve as a metabolon, which is by definition held together by non-covalent interactions, as protein condensate with minimal hydration, to allow for substrate channeling and maximal productivity [149,150,151]. The complex contains ALAS, which is the first enzyme of heme biosynthesis and whose product delta-aminolevulinic acid (deltaALA) is exported from mitochondria into the cytosol. It also contains CPOX-PPOX-FECH on different IMM surfaces as the three terminal enzymes of the biosynthesis chain, whose product, heme, is incorporated into complexes II, III, and IV of the respiratory chain within the IMM [147,152]. ALAS is furthermore associated with SUCLA2 in differentiating erythroid cells [147]. This IMM-associated multi-enzyme complex also serves as a bridge [153,154] between at least three transmembrane proteins. Firstly, MFRN1 (also known as SLC25A37 or mitoferrin-1), which imports iron into the mitochondrial matrix [155]; secondly, ABCB10, which exports biliverdin to the cytosol [156]; and thirdly, ABCB7, which exports glutathione-coordinated iron–sulfur clusters to the cytosol [157], are connected to the IMM-associated heme biosynthesis complex, according to several consistent reports. There is still debate [147] about whether the tight association of IMM transmembrane proteins with this metabolon goes beyond the biliverdin/zinc-mesoporphyrin transporter ABCB10 [158,159,160] to include also the TMEM14C protoporphyrin-IX transporter [161,162,163,164], the protoporphyrin-IX transport modulator ANT2 [165], and the glutathione/succinate transport modulator OGC [166,167]. Enzyme complexes with similar isolations of reaction intermediates from the surrounding matrix have also been observed, e.g., during L-arginine metabolism and bacterial cobalamin metabolism [168,169]. Heme and porphyrins are compounds that need a hydrophobic environment [170,171,172,173]. According to recent human genetics findings, mutations in FECH, ALAS2, and CLPX [174] underlie most cases of the disorder erythropoietic protoporphyria, while mutations in ALAS2 and in the mitochondrial glycine transporter SLC25A38 are the most frequent causes of congenital sideroblastic anemia [175].
CLPX was shown to unfold ALAS, so that its cofactor pyridoxal-5′-phosphate (PLP) can bind and activate it to consume succinate-CoA and glycine for the production of the heme precursor delta-aminolevulinic acid (deltaALA) with optimal efficiency [5,37,38,176,177,178]. CLPXP was claimed to be responsible for ALAS degradation [179]. In the Clpp-KO mouse, the consequent elevation of CLPX abundance will also unfold OAT (ornithine delta-aminotransferase), so that PLP binds to it and triggers the consumption of L-arginine and L-ornithine via a delta-transaminase and delta-aminomutase reaction to produce GSA as a precursor of heme. In parallel, a recruitment of L-glutamate occurs into maximized deltaALA generation via the accumulation of the enzyme ALDH18A1 (also known as delta-1-pyrroline-5-carboxylate synthase) [48,52]. Thus, both ALAS and OAT acquire the ability to perform transaminations at the delta-carbon position, when CLPX unfolds them and enables them to bind to PLP as cofactor [180]. At the same time, iron accumulates in Clpp-KO tissue, together with the heavy metals molybdenum, cobalt, and manganese [42]. Thus, heavy metal toxicity and ferroptosis [181,182] may also be part of PRLTS3 pathogenesis mechanisms. The accumulation of the metal- and heme-binding protein COX15 and the preferential affection of respiratory complex-IV in the IMM of Clpp-KO mice can thus be explained as a consequence of iron/heme dysregulation [42]. Indeed, the expression dysregulation of the heme-binding, mtDNA-encoded Cox1 membrane subunit in complex-IV stood out across the Clpp-KO testis, heart, liver, and brain as the main molecular underpinning of respiratory dysfunction [40]. While heme is a protoporphyrin-IX that is chelated with Fe2+, plant chlorophyll is a protoporphyrin chelated with Mg2+, so both heme and chlorophyll biosynthesis depend on ALAS control by PLP and CLPX. Indeed, the regulation of heme/chlorophyll metabolism by CLPX is conserved from bacteria across phylogenesis to plants [183,184,185,186,187].
Unsurprisingly, the accumulation of CLPX in Clpp-KO tissues not only modulates the binding of PLP to target enzymes but also leads to increased amounts of the PLP storage/transport protein PLPBP in some cell types [42].
Altogether, CLPX appears to fine-tune the biosynthesis and maturation of porphyrins and heme, with marked consequences for iron and heavy metal utilization, as well as respiratory competence, through the continuous modulation of the IMM-associated multi-enzyme complex, which channels hydrophobic reaction intermediates and isolates them from the aqueous phase.
2.6. Prominent Impact of Absence of CLPP on Fe-S Cluster Containing Peripheral Arm of Respiratory Complex-I
The fine-tuning of multi-protein assemblies, rather than proteolytic degradation, also seems to characterize the selective role of the absence of CLPP and accumulation of CLPX for the respiratory complex-I N-module [188]. Complex-I consists of a membrane arm, embedded in the lipid bilayer of the IMM, and a peripheral arm with the N/Q modules that protrudes into the aqueous phase of the matrix. The two modules serve to surround, isolate, and channel electrons into a tunnel within the IMM and to protect the many embedded iron–sulfur (Fe-S) clusters from oxidation [189,190,191,192,193]. The absence of CLPP only results in a mild reduction in complex-I dependent state 3 respiration in mouse heart mitochondria but not in other tissues, so the mutation-triggered functional deficit is subtle [40,43]. As a molecular underpinning, it was clearly shown that the turnover of the core subunit NDUFV1-NDUFV2-NDUFS2 in the NADH-oxidizing N-module of complex-I has a selective dependence on CLPP in an ongoing exchange process where oxidatively damaged, inactive N-modules are substituted on the tip of the complex-I peripheral arm [194]. In addition, the selective accumulation of SFXN4 in Clpp-KO tissue, as a component of the complex-I assembly machinery that controls metal association, indicates that the biogenesis of complex-I may be altered [42]. It has to be mentioned, however, that none of the established complex-I subunits accumulate in Clpp-KO mouse tissues and that proteome profiling in the CLPP-null fungal eukaryote P. anserina did reveal some accumulated complex-I subunits but not for any specific module [48]. Thus, we assume that complex-I assembly is not a primary and conserved target of CLPP. Our team has observed upregulations of most factors in the iron–sulfur cluster (ISC) biogenesis pathway, most strongly for the 4Fe–4S cluster generating enzymes, in the brain of Clpp-KO mice, but of course, this effect may simply represent a molecular adaptation to maintain sufficient ISC production despite the maximized iron utilization for heme biosynthesis.
Clearly, the functions of CLPP and CLPX seem to consist of the rapid refolding/trimming or substitution of a selected subunit within a complex that keeps working, but not the complete disassembly and disposal of entire respiratory complexes or supercomplexes.
3. Phase-Separated Condensates in Mitochondria and the Cytosol
Research over the past four years showed that nucleoid components, the RNA processing granules, and the RNA degradasome of bacteria and mitochondria assemble in phase separation [100,195,196,197,198,199]. The original concept of LLPS over twenty years ago [200,201] was derived from lipid droplets, where components can move freely within a round compartment that excludes the aqueous phase. In the meantime, it has become clear that such condensates do not need to be liquid but can also assume a gelatinous or even solidified state, particularly in a disease context [202]. Although the mobility of individual components within the condensate may be high, they would certainly move along given structures within the phase-separated condensate, e.g., in the case of nucleolar ribosome biogenesis, mtDNA transcription, or the processing of polycistronic mtRNA. Thus, these condensates may be defined by the multivalent interaction forces that keep long and flexible molecules such as lipids or RNA together [203,204]. They are also defined by the vulnerable reaction intermediates that need protection from the aqueous or membrane phases, such as cleaved unfolded RNA without modifications, unchelated porphyrins that are unassembled with proteins, or pre-fibril oligomers with a propensity to disrupt membranes [205,206,207]. Regarding its multivalent interaction forces and its long flexible structure, RNA was the prime example for understanding LLPS, based on the phase contrast during the microscopic visualization of the nucleolus. Therefore, other RNA-containing granules in the nucleus and cytosol (e.g., paraspeckles, Cajal bodies, U bodies, PcG bodies, Balbiani bodies, stress granules, P-bodies, germ granules, and RNA transport granules) constituted an early focus of LLPS research (Figure 1 in [202]). It was shown that the ribonucleoproteins also contribute to phase separation, with some binding domains deciding the specificity of interactions (known as “stickers”), while the intrinsically disordered regions (IDRs) that often intervene have solvation properties that influence the density transition (known as “spacers”) [203]. Indeed, it was proposed that cells use RNAs and IDR proteins to separate multi-enzyme complexes such as glycolysis into granular compartments that have a different phase than the surrounding cytosol [208]. Other mitochondrial metabolons, such as the TCA cycle, heme biosynthesis, urea cycle, respiratory chain, and breakdown of branched amino acids, also require the efficient channeling of reaction intermediates, which are usually achieved by tight subunit docking and by hydrophobic interactions [209]. Thus, these metabolons might also be separated from the aqueous phase by the multivalent forces of associated non-coding RNA.
In summary, the recently defined targets of CLPP and CLPX are all condensates where phase separation or multi-enzyme assembly protect unstable reaction intermediates from the aqueous phase, and which frequently need a rapid repair of individual subunits while these assemblies keep fulfilling their function. It seems plausible that CLPP and CLPX have the ability to access these condensates and provide the necessary first aid.
4. Proposal
Altogether, it may be impossible to define consistent protein targets of CLPX and CLPP across phylogenesis, given that each organism is adapted to a different environment, has specific metabolic needs, and has to protect other reaction intermediates inside phase-separated multivalent or hydrophobic condensates from the aqueous phase. The polypeptide sequences of degrons that are recognized by CLPX in E. coli might differ from such sequences in mice, and the cleavage pattern of CLPP, while it fine-tunes multi-enzyme complexes, may vary according to steric constraints. There could be no protein that is exclusively the substrate of CLPXP-mediated proteolysis, and all matrix proteins might be finally degraded by LONP1. This is exemplified by CLPX, which is certainly a prominent example of a protein whose abundance depends on CLPP in all organisms studied [36], and yet, its proteolytic destruction was observed to be executed by LONP1 [210]. Instead of eliminating specific proteins in the mitochondrial matrix, CLPX and CLPP seem to have unique access properties to granular compartments, where they can rescue a suboptimal or stalled process, either by unfolding a protein or by the excision of a misassembled component, so that the multi-enzyme complex can improve its performance (see Figure 1). Overall, it is necessary to validate whether the Clpp-KO mouse evidence holds true in other organisms, i.e., that the specific roles of CLPX and CLPP are defined by the granular compartments that they are monitoring. This would be analogous to most other peptidases in mitochondria, where MPP cleaves all precursor proteins at the import pore, m-AAA and i-AAA are responsible for protein quality control at either IMM face, PARL cleaves proteins within the IMM, and OMA1/HTRA2 performs surveillance in the intermembrane space [211,212,213,214]. Our proteome identification of specific factors whose abundance depends on CLPP will also be useful (more so than unspecific mitochondrial–respiratory assays) for comparing the efficacy of drugs that activate or inhibit CLPP. This research area is rapidly advancing and holds great promise for the treatment of cancer and infections. While the authors are no experts in this field, we recommend several innovative reviews and articles produced over the past 10 years on CLPP-modulating drug compounds [215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233] and relevant structure/binding studies [45,234,235,236,237,238,239,240,241,242,243,244] for further reading. A better understanding of CLPXP-dependent UPRmt will also help clarify how extra-mitochondrial signals (such as extruded mt-dsRNA, perhaps mtRNA-G4, and associated ribonucleoproteins) trigger responses of the nucleus and the endoplasmic reticulum UPR, a basic research field where mechanisms are investigated in yeast and nematodes but are poorly defined in mammals at present [41,245,246,247,248,249,250].
Author Contributions
Conceptualization, G.A.; writing—original draft preparation, G.A.; writing—review and editing, J.K., S.G. and G.A.; visualization, J.K. and G.A.; project administration, G.A.; funding acquisition, G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by funds from the Klinikum Goethe Universität Frankfurt/Main.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Hildegard König for help with lab and office infrastructure.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| 4Fe–4S | iron–sulfur clusters consisting of two interleaved 4Fe- and 4S-tetrahedra |
| AAA+ | ATPases Associated with diverse cellular Activities and other ring-shaped P-loop NTPases |
| ABCB7 | ATP Binding Cassette Subfamily B Member 7 |
| ABCB10 | ATP Binding Cassette Subfamily B Member 10 |
| ALAS | delta-Amino-Levulinic Acid Synthase |
| ALAS2 | delta-Amino-Levulinic Acid Synthase 2, erythroid-specific |
| ALDH18A1 | Aldehyde Dehydrogenase 18 family member A1, =P5CS, delta-1-Pyrroline-5-Carboxylate Synthase |
| ANT2 | Adenine Nucleotide Translocator 2, =SLC25A25 |
| ATP | Adenosine Tri-Phosphate |
| ATPase | Adenosine Tri-Phosphatase |
| CAT-tail | C-terminal alanine and threonine tail |
| cGAS | cyclic GMP-AMP Synthase |
| CLPP | Caseinolytic Mitochondrial Matrix Peptidase Proteolytic Subunit |
| CLPX | Caseinolytic Mitochondrial Matrix Peptidase Chaperone Subunit X |
| CODAS | multiple anomalies syndrome with Cerebral, Ocular, Dental, Auricular and Skeletal anomalies |
| Cox1 | mitochondrially encoded Cytochrome C Oxidase I, mRNA |
| COX15 | Cytochrome C Oxidase assembly homolog COX15 |
| CPOX | Copro-Porphyrinogen OXidase |
| D-foci | degradosome granules in mitochondrial matrix |
| D-loop | displacement loop within the mtDNA |
| DARS2 | Aspartyl-tRNA Synthetase 2, mitochondrial |
| deltaALA | delta-aminolevulinic acid |
| DNA | Deoxyribo-Nucleic Acid |
| DNAzyme | catalytically active DNA sequences |
| dsRNA | double-stranded RNA |
| EF-G | Elongation Factor G |
| EFG1 | G Elongation Factor, mitochondrial 1 |
| ERAL1 | Era (E. coli) -Like 12S mitochondrial rRNA chaperone 1 |
| Fe2+ | divalent cation of iron, ferrous iron |
| FECH | Ferrochelatase |
| Fe-S | iron–sulfur |
| G4 | Guanine quadruplex where RNA or DNA acquires four-stranded conformation |
| GFM1 | G elongation Factor Mitochondrial 1 |
| GRSF1 | G-rich RNA Sequence-binding Factor 1 |
| GSA | Glutamate-5-Semi-Aldehyde |
| GTP | Guanosine-5′-triphosphate |
| HARS2 | Histidyl-tRNA Synthetase 2, mitochondrial |
| hsp-6 | heat shock protein family B (small) member 6 |
| hsp-60 | heat shock protein family D (hsp60) member 1, =human HSPD1 |
| HSPD1 | heat shock protein family D (hsp60) member 1, =human HSP60, chaperonin |
| HTRA2 | High-Temperature-Requirement A serine peptidase 2 |
| i-AAA | ATP-dependent zinc metalloprotease YME1 (S. cerevisiae) -Like 1 |
| IDR | Intrinsically Disordered Region |
| IMM | Inner Membrane of Mitochondria |
| ISC | iron–sulfur cluster |
| KO | knockout |
| LARS2 | Leucyl-tRNA Synthetase 2, mitochondrial |
| lncRNA | long non-coding RNA |
| LLPS | liquid–liquid phase separation |
| LONP1 | Lon Peptidase 1, Mitochondrial |
| LRPPRC | Leucine-Rich Pentatrico-Peptide Repeat Containing |
| m-AAA | AFG3-like matrix AAA peptidase subunit 2 and SPG7 matrix AAA peptidase subunit paraplegin |
| MEFs | mouse embryonic fibroblasts |
| MFRN1 | mitoferrin 1, =SLC25A37, Solute Carrier Family 25 Member 37 |
| Mg2+ | divalent cation of magnesium |
| MPP | mitochondrial processing peptidase |
| MRPL12 | Mitochondrial Ribosomal Protein L12 |
| MRPL18 | Mitochondrial Ribosomal Protein L18 |
| MRPL37 | Mitochondrial Ribosomal Protein L37 |
| MRPL38 | Mitochondrial Ribosomal Protein L38 |
| MRPS15 | Mitochondrial Ribosomal Protein S15 |
| MRPS35 | Mitochondrial Ribosomal Protein S35 |
| mtDNA | mitochondrial DNA |
| mtHSP75 | mitochondrial heat shock protein 75, =human TRAP1 |
| mtLSU | mitoribosomal 39S large subunit |
| mtRNA | mitochondrial RNA |
| mtSSB | mitochondrial single-stranded DNA binding protein |
| mtSSU | mitoribosomal 28S small subunit |
| mt-tRNA | mitochondrial transfer RNA |
| NADH | Nicotinamide Adenine Dinucleotide, reduced form |
| NDUFS2 | NADH:Ubiquinone Oxidoreductase Core Subunit S2 |
| NDUFV1 | NADH:Ubiquinone Oxidoreductase Core Subunit V1 |
| NDUFV2 | NADH:Ubiquinone Oxidoreductase Core Subunit V2 |
| NOA1 | Nitric Oxide Associated 1 |
| NTPase | Nucleoside-Tri-Phosphatase |
| OAT | Ornithine delta-Amino-Transferase |
| OGC | 2-Oxoglutarate/Malate Carrier protein, mitochondrial |
| OMA1 | Overlapping with the M-AAA protease 1 homolog, zinc metallopeptidase |
| OMM | Outer Membrane of Mitochondria |
| PARL | Presenilin-Associated Rhomboid-Like |
| PcG bodies | polycomb bodies |
| PEO1 | Progressive External Ophthalmoplegia 1 protein = TWNK |
| Phe | phenylalanine |
| PLP | Pyridoxal-5′-Phosphate |
| PLPBP | PLP-binding protein |
| PNPase | Polyribo-Nucleotide Phosphorylase/Nucleotidyl-Transferase 1 = PNPT1 in human |
| PNPT1 | Polyribo-Nucleotide Phosphorylase/Nucleotidyl-Transferase 1 = PNPase |
| POLDIP2 | DNA Polymerase Delta Interacting Protein 2 |
| poly(A) tail | poly(adenine) tail of messenger RNAs |
| poly(I:C) | poly(inosinic:cytidylic) acid |
| PPOX | Proto-Porphyrinogen OXidase |
| PRLTS3 | Perrault Syndrome type 3 |
| PRORP | Protein-Only RNase P catalytic subunit |
| qPCR | quantitative Polymerase Chain Reaction |
| RMND1 | Required for Meiotic Nuclear Division 1 homolog |
| RNA | Ribo-Nucleic Acid |
| RNA-G4 | RNA, guanine-rich, in quadruplex conformation |
| RNase | ribonuclease |
| RNAzyme | catalytically active RNA sequences |
| RNF213 | Ring Finger protein 213 |
| rRNA | ribosomal RNA |
| SFXN4 | Sideroflexin 4 |
| SLC25A37 | Solute Carrier family 25 member 37, Mitoferrin 1 |
| SLC25A38 | Solute Carrier Family 25 Member 38, mitochondrial glycine transporter |
| STING | STimulator of INterferon response cGAMP interactor 1 |
| SUCLA2 | Succinate–CoA Ligase ADP-forming subunit β |
| SUPV3L1 | SUV3, Suv3-like RNA helicase |
| TCA | Tri-Carboxylic Acid cycle, =Krebs cycle |
| TFAM | Transcription Factor A, Mitochondrial |
| TIGM | Texas Institute of Genomic Medicine |
| TMEM14C | transmembrane protein 14C |
| tRNA | transfer RNA |
| TWNK | Twinkle mtDNA helicase, =PEO1 |
| UPR | unfolded protein response |
| UPRmt | unfolded protein response in mitochondria |
| Val | valine |
| VWA8 | von Willebrand Factor A domain containing 8 |
References
- Key, J.; Kohli, A.; Barcena, C.; Lopez-Otin, C.; Heidler, J.; Wittig, I.; Auburger, G. Global Proteome of LonP1(+/−) Mouse Embryonal Fibroblasts Reveals Impact on Respiratory Chain, but No Interdependence between Eral1 and Mitoribosomes. Int. J. Mol. Sci. 2019, 20, 4523. [Google Scholar] [CrossRef]
- Bezawork-Geleta, A.; Brodie, E.J.; Dougan, D.A.; Truscott, K.N. LON is the master protease that protects against protein aggregation in human mitochondria through direct degradation of misfolded proteins. Sci. Rep. 2015, 5, 17397. [Google Scholar] [CrossRef] [PubMed]
- Stahlberg, H.; Kutejova, E.; Suda, K.; Wolpensinger, B.; Lustig, A.; Schatz, G.; Engel, A.; Suzuki, C.K. Mitochondrial Lon of Saccharomyces cerevisiae is a ring-shaped protease with seven flexible subunits. Proc. Natl. Acad. Sci. USA 1999, 96, 6787–6790. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Sauer, R.T. ClpXP, an ATP-powered unfolding and protein-degradation machine. Biochim. Biophys. Acta 2012, 1823, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Kardon, J.R.; Yien, Y.Y.; Huston, N.C.; Branco, D.S.; Hildick-Smith, G.J.; Rhee, K.Y.; Paw, B.H.; Baker, T.A. Mitochondrial ClpX Activates a Key Enzyme for Heme Biosynthesis and Erythropoiesis. Cell 2015, 161, 858–867. [Google Scholar] [CrossRef]
- Fischer, F.; Weil, A.; Hamann, A.; Osiewacz, H.D. Human CLPP reverts the longevity phenotype of a fungal ClpP deletion strain. Nat. Commun. 2013, 4, 1397. [Google Scholar] [CrossRef] [PubMed]
- Dikoglu, E.; Alfaiz, A.; Gorna, M.; Bertola, D.; Chae, J.H.; Cho, T.J.; Derbent, M.; Alanay, Y.; Guran, T.; Kim, O.H.; et al. Mutations in LONP1, a mitochondrial matrix protease, cause CODAS syndrome. Am. J. Med. Genet. A 2015, 167, 1501–1509. [Google Scholar] [CrossRef]
- Strauss, K.A.; Jinks, R.N.; Puffenberger, E.G.; Venkatesh, S.; Singh, K.; Cheng, I.; Mikita, N.; Thilagavathi, J.; Lee, J.; Sarafianos, S.; et al. CODAS syndrome is associated with mutations of LONP1, encoding mitochondrial AAA+ Lon protease. Am. J. Hum. Genet. 2015, 96, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Faridi, R.; Stratton, P.; Salmeri, N.; Morell, R.J.; Khan, A.A.; Usmani, M.A.; Newman, W.G.; Riazuddin, S.; Friedman, T.B. Homozygous novel truncating variant of CLPP associated with severe Perrault syndrome. Clin. Genet. 2024, 105, 584–586. [Google Scholar] [CrossRef]
- Brodie, E.J.; Zhan, H.; Saiyed, T.; Truscott, K.N.; Dougan, D.A. Perrault syndrome type 3 caused by diverse molecular defects in CLPP. Sci. Rep. 2018, 8, 12862. [Google Scholar] [CrossRef]
- Theunissen, T.E.; Szklarczyk, R.; Gerards, M.; Hellebrekers, D.M.; Mulder-Den Hartog, E.N.; Vanoevelen, J.; Kamps, R.; de Koning, B.; Rutledge, S.L.; Schmitt-Mechelke, T.; et al. Specific MRI Abnormalities Reveal Severe Perrault Syndrome due to CLPP Defects. Front. Neurol. 2016, 7, 203. [Google Scholar] [CrossRef]
- Dursun, F.; Mohamoud, H.S.; Karim, N.; Naeem, M.; Jelani, M.; Kirmizibekmez, H. A Novel Missense Mutation in the CLPP Gene Causing Perrault Syndrome Type 3 in a Turkish Family. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Demain, L.A.; Urquhart, J.E.; O’Sullivan, J.; Williams, S.G.; Bhaskar, S.S.; Jenkinson, E.M.; Lourenco, C.M.; Heiberg, A.; Pearce, S.H.; Shalev, S.A.; et al. Expanding the genotypic spectrum of Perrault syndrome. Clin. Genet. 2017, 91, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Jelani, M.; Alrayes, N.; Mohamoud, H.S.; Almramhi, M.M.; Anshasi, W.; Ahmed, N.A.; Wang, J.; Nasir, J.; Al-Aama, J.Y. Exome analysis identified a novel missense mutation in the CLPP gene in a consanguineous Saudi family expanding the clinical spectrum of Perrault Syndrome type-3. J. Neurol. Sci. 2015, 353, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, E.M.; Rehman, A.U.; Walsh, T.; Clayton-Smith, J.; Lee, K.; Morell, R.J.; Drummond, M.C.; Khan, S.N.; Naeem, M.A.; Rauf, B.; et al. Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am. J. Hum. Genet. 2013, 92, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, I.; Demain, L.A.M.; Richer, J.; Thompson, K.; Urquhart, J.E.; Rea, A.; Pagarkar, W.; Rodriguez-Palmero, A.; Schluter, A.; Verdura, E.; et al. Bi-allelic variants in the mitochondrial RNase P subunit PRORP cause mitochondrial tRNA processing defects and pleiotropic multisystem presentations. Am. J. Hum. Genet. 2021, 108, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Newman, W.G.; Friedman, T.B.; Conway, G.S.; Demain, L.A.M. Perrault Syndrome. In GeneReviews((R)); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; GeneReviews®[Internet]: Seattle, WA, USA, 1993. [Google Scholar]
- Kobe, C.; Kracht, L.W.; Timmermann, L.; Bachmann, J.; Schmidt, M.C. Perrault Syndrome with progressive nervous system involvement. Clin. Nucl. Med. 2008, 33, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.E.; Coker, S.B.; Fox, L.A. Neurologic anomalies of Perrault syndrome. Am. J. Med. Genet. 1996, 65, 274–276. [Google Scholar] [CrossRef]
- Linssen, W.H.; Van den Bent, M.J.; Brunner, H.G.; Poels, P.J. Deafness, sensory neuropathy, and ovarian dysgenesis: A new syndrome or a broader spectrum of Perrault syndrome? Am. J. Med. Genet. 1994, 51, 81–82. [Google Scholar] [CrossRef]
- Faridi, R.; Rea, A.; Fenollar-Ferrer, C.; O’Keefe, R.T.; Gu, S.; Munir, Z.; Khan, A.A.; Riazuddin, S.; Hoa, M.; Naz, S.; et al. New insights into Perrault syndrome, a clinically and genetically heterogeneous disorder. Hum. Genet. 2022, 141, 805–819. [Google Scholar] [CrossRef]
- Tucker, E.J.; Rius, R.; Jaillard, S.; Bell, K.; Lamont, P.J.; Travessa, A.; Dupont, J.; Sampaio, L.; Dulon, J.; Vuillaumier-Barrot, S.; et al. Genomic sequencing highlights the diverse molecular causes of Perrault syndrome: A peroxisomal disorder (PEX6), metabolic disorders (CLPP, GGPS1), and mtDNA maintenance/translation disorders (LARS2, TFAM). Hum. Genet. 2020, 139, 1325–1343. [Google Scholar] [CrossRef] [PubMed]
- Gotta, F.; Lamp, M.; Geroldi, A.; Trevisan, L.; Origone, P.; Fugazza, G.; Fabbri, S.; Nesti, C.; Rubegni, A.; Morani, F.; et al. A novel mutation of Twinkle in Perrault syndrome: A not rare diagnosis? Ann. Hum. Genet. 2020, 84, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Rea, A.; Thomas, H.B.; Thompson, K.; Olahova, M.; Maroofian, R.; Zamani, M.; He, L.; Sadeghian, S.; Galehdari, H.; et al. Novel homozygous variants in PRORP expand the genotypic spectrum of combined oxidative phosphorylation deficiency 54. Eur. J. Hum. Genet. 2023, 31, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Chatzispyrou, I.A.; Alders, M.; Guerrero-Castillo, S.; Zapata Perez, R.; Haagmans, M.A.; Mouchiroud, L.; Koster, J.; Ofman, R.; Baas, F.; Waterham, H.R.; et al. A homozygous missense mutation in ERAL1, encoding a mitochondrial rRNA chaperone, causes Perrault syndrome. Hum. Mol. Genet. 2017, 26, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Rioux, A.V.; Bergeron, N.A.; Riopel, J.; Marcoux, N.; Theriault, C.; Gould, P.V.; Garneau, A.P.; Isenring, P. The ever wider clinical spectrum of RMND1-related disorders and limitedness of phenotype-based classifications. J. Mol. Med. 2023, 101, 1229–1236. [Google Scholar] [CrossRef]
- Neyroud, A.S.; Rudinger-Thirion, J.; Frugier, M.; Riley, L.G.; Bidet, M.; Akloul, L.; Simpson, A.; Gilot, D.; Christodoulou, J.; Ravel, C.; et al. LARS2 variants can present as premature ovarian insufficiency in the absence of overt hearing loss. Eur. J. Hum. Genet. 2023, 31, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Ozieblo, D.; Pazik, J.; Stepniak, I.; Skarzynski, H.; Oldak, M. Two Novel Pathogenic Variants Confirm RMND1 Causative Role in Perrault Syndrome with Renal Involvement. Genes 2020, 11, 1060. [Google Scholar] [CrossRef]
- Riley, L.G.; Rudinger-Thirion, J.; Frugier, M.; Wilson, M.; Luig, M.; Alahakoon, T.I.; Nixon, C.Y.; Kirk, E.P.; Roscioli, T.; Lunke, S.; et al. The expanding LARS2 phenotypic spectrum: HLASA, Perrault syndrome with leukodystrophy, and mitochondrial myopathy. Hum. Mutat. 2020, 41, 1425–1434. [Google Scholar] [CrossRef]
- Demain, L.A.M.; Gerkes, E.H.; Smith, R.J.H.; Molina-Ramirez, L.P.; O’Keefe, R.T.; Newman, W.G. A recurrent missense variant in HARS2 results in variable sensorineural hearing loss in three unrelated families. J. Hum. Genet. 2020, 65, 305–311. [Google Scholar] [CrossRef]
- Karstensen, H.G.; Rendtorff, N.D.; Hindbaek, L.S.; Colombo, R.; Stein, A.; Birkebaek, N.H.; Hartmann-Petersen, R.; Lindorff-Larsen, K.; Hojland, A.T.; Petersen, M.B.; et al. Novel HARS2 missense variants identified in individuals with sensorineural hearing impairment and Perrault syndrome. Eur. J. Med. Genet. 2020, 63, 103733. [Google Scholar] [CrossRef]
- Demain, L.A.M.; Antunes, D.; O’Sullivan, J.; Bhaskhar, S.S.; O’Keefe, R.T.; Newman, W.G. A known pathogenic variant in the essential mitochondrial translation gene RMND1 causes a Perrault-like syndrome with renal defects. Clin. Genet. 2018, 94, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, R.; Horikawa, R.; Fujii, E.; Kosaki, K. Biallelic mutations in LARS2 can cause Perrault syndrome type 2 with neurologic symptoms. Am. J. Med. Genet. A 2018, 176, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.B.; Gersak, K.; Michaelson-Cohen, R.; Walsh, T.; Lee, M.K.; Malach, D.; Klevit, R.E.; King, M.C.; Levy-Lahad, E. Mutations in LARS2, encoding mitochondrial leucyl-tRNA synthetase, lead to premature ovarian failure and hearing loss in Perrault syndrome. Am. J. Hum. Genet. 2013, 92, 614–620. [Google Scholar] [CrossRef]
- Pierce, S.B.; Chisholm, K.M.; Lynch, E.D.; Lee, M.K.; Walsh, T.; Opitz, J.M.; Li, W.; Klevit, R.E.; King, M.C. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc. Natl. Acad. Sci. USA 2011, 108, 6543–6548. [Google Scholar] [CrossRef] [PubMed]
- Auburger, G.; Key, J.; Gispert, S. The Bacterial ClpXP-ClpB Family Is Enriched with RNA-Binding Protein Complexes. Cells 2022, 11, 2370. [Google Scholar] [CrossRef] [PubMed]
- Ducamp, S.; Luscieti, S.; Ferrer-Cortes, X.; Nicolas, G.; Manceau, H.; Peoc’h, K.; Yien, Y.Y.; Kannengiesser, C.; Gouya, L.; Puy, H.; et al. A mutation in the iron-responsive element of ALAS2 is a modifier of disease severity in a patient suffering from CLPX associated erythropoietic protoporphyria. Haematologica 2021, 106, 2030–2033. [Google Scholar] [CrossRef] [PubMed]
- Yien, Y.Y.; Ducamp, S.; van der Vorm, L.N.; Kardon, J.R.; Manceau, H.; Kannengiesser, C.; Bergonia, H.A.; Kafina, M.D.; Karim, Z.; Gouya, L.; et al. Mutation in human CLPX elevates levels of delta-aminolevulinate synthase and protoporphyrin IX to promote erythropoietic protoporphyria. Proc. Natl. Acad. Sci. USA 2017, 114, E8045–E8052. [Google Scholar] [CrossRef] [PubMed]
- van der Vorm, L.N.; Paw, B.H. Studying disorders of vertebrate iron and heme metabolism using zebrafish. Methods Cell Biol. 2017, 138, 193–220. [Google Scholar] [CrossRef] [PubMed]
- Gispert, S.; Parganlija, D.; Klinkenberg, M.; Drose, S.; Wittig, I.; Mittelbronn, M.; Grzmil, P.; Koob, S.; Hamann, A.; Walter, M.; et al. Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum. Mol. Genet. 2013, 22, 4871–4887. [Google Scholar] [CrossRef]
- Seiferling, D.; Szczepanowska, K.; Becker, C.; Senft, K.; Hermans, S.; Maiti, P.; Konig, T.; Kukat, A.; Trifunovic, A. Loss of CLPP alleviates mitochondrial cardiomyopathy without affecting the mammalian UPRmt. EMBO Rep. 2016, 17, 953–964. [Google Scholar] [CrossRef]
- Key, J.; Gispert, S.; Koepf, G.; Steinhoff-Wagner, J.; Reichlmeir, M.; Auburger, G. Translation Fidelity and Respiration Deficits in CLPP-Deficient Tissues: Mechanistic Insights from Mitochondrial Complexome Profiling. Int. J. Mol. Sci. 2023, 24, 17503. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowska, K.; Maiti, P.; Kukat, A.; Hofsetz, E.; Nolte, H.; Senft, K.; Becker, C.; Ruzzenente, B.; Hornig-Do, H.T.; Wibom, R.; et al. CLPP coordinates mitoribosomal assembly through the regulation of ERAL1 levels. EMBO J. 2016, 35, 2566–2583. [Google Scholar] [CrossRef] [PubMed]
- Arribas, J.; Castano, J.G. A comparative study of the chymotrypsin-like activity of the rat liver multicatalytic proteinase and the ClpP from Escherichia coli. J. Biol. Chem. 1993, 268, 21165–21171. [Google Scholar] [CrossRef]
- Mabanglo, M.F.; Wong, K.S.; Barghash, M.M.; Leung, E.; Chuang, S.H.W.; Ardalan, A.; Majaesic, E.M.; Wong, C.J.; Zhang, S.; Lang, H.; et al. Potent ClpP agonists with anticancer properties bind with improved structural complementarity and alter the mitochondrial N-terminome. Structure 2023, 31, 185–200.e110. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.V.; Morrow, S.; Rahman Kawakibi, A.; Zhou, L.; Ralff, M.; Ray, J.; Jhaveri, A.; Ferrarini, I.; Lee, Y.; Parker, C.; et al. ONC201 and imipridones: Anti-cancer compounds with clinical efficacy. Neoplasia 2020, 22, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Houry, W.A. Chemical Modulation of Human Mitochondrial ClpP: Potential Application in Cancer Therapeutics. ACS Chem. Biol. 2019, 14, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Gispert, S.; Kandi, A.R.; Heinz, D.; Hamann, A.; Osiewacz, H.D.; Meierhofer, D.; Auburger, G. CLPP-Null Eukaryotes with Excess Heme Biosynthesis Show Reduced L-arginine Levels, Probably via CLPX-Mediated OAT Activation. Biomolecules 2024, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Gispert, S.; Koornneef, L.; Sleddens-Linkels, E.; Kohli, A.; Torres-Odio, S.; Koepf, G.; Amr, S.; Reichlmeir, M.; Harter, P.N.; et al. CLPP Depletion Causes Diplotene Arrest; Underlying Testis Mitochondrial Dysfunction Occurs with Accumulation of Perrault Proteins ERAL1, PEO1, and HARS2. Cells 2022, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Kukat, A.; Szczepanowska, K.; Hermans, S.; Senft, K.; Brandscheid, C.P.; Maiti, P.; Trifunovic, A. CLPP deficiency protects against metabolic syndrome but hinders adaptive thermogenesis. EMBO Rep. 2018, 19, e45126. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Pharaoh, G.; Ranjit, R.; Murphy, A.; Matsuzaki, S.; Nair, B.C.; Forbes, B.; Gispert, S.; Auburger, G.; Humphries, K.M.; et al. Loss of mitochondrial protease ClpP protects mice from diet-induced obesity and insulin resistance. EMBO Rep. 2018, 19, e45009. [Google Scholar] [CrossRef]
- Hofsetz, E.; Demir, F.; Szczepanowska, K.; Kukat, A.; Kizhakkedathu, J.N.; Trifunovic, A.; Huesgen, P.F. The Mouse Heart Mitochondria N Terminome Provides Insights into ClpXP-Mediated Proteolysis. Mol. Cell. Proteom. 2020, 19, 1330–1345. [Google Scholar] [CrossRef]
- Benedetti, C.; Haynes, C.M.; Yang, Y.; Harding, H.P.; Ron, D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 2006, 174, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Torres-Odio, S.; Bach, N.C.; Gispert, S.; Koepf, G.; Reichlmeir, M.; West, A.P.; Prokisch, H.; Freisinger, P.; Newman, W.G.; et al. Inactivity of Peptidase ClpP Causes Primary Accumulation of Mitochondrial Disaggregase ClpX with Its Interacting Nucleoid Proteins, and of mtDNA. Cells 2021, 10, 3354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Kanki, T.; Fukuoh, A.; Ohgaki, K.; Takeya, R.; Aoki, Y.; Hamasaki, N.; Kang, D. PDIP38 associates with proteins constituting the mitochondrial DNA nucleoid. J. Biochem. 2005, 138, 673–678. [Google Scholar] [CrossRef]
- Strack, P.R.; Brodie, E.J.; Zhan, H.; Schuenemann, V.J.; Valente, L.J.; Saiyed, T.; Lowth, B.R.; Angley, L.M.; Perugini, M.A.; Zeth, K.; et al. Polymerase delta-interacting protein 38 (PDIP38) modulates the stability and activity of the mitochondrial AAA+ protease CLPXP. Commun. Biol. 2020, 3, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, P.; Wei, B.; Chen, L.; Song, X.; Pan, Y.; Li, J.; Gan, J.; Zhang, T.; Yang, C.G. Assessment of the structure-activity relationship and antileukemic activity of diacylpyramide compounds as human ClpP agonists. Eur. J. Med. Chem. 2023, 258, 115577. [Google Scholar] [CrossRef]
- Nikali, K.; Suomalainen, A.; Saharinen, J.; Kuokkanen, M.; Spelbrink, J.N.; Lonnqvist, T.; Peltonen, L. Infantile onset spinocerebellar ataxia is caused by recessive mutations in mitochondrial proteins Twinkle and Twinky. Hum. Mol. Genet. 2005, 14, 2981–2990. [Google Scholar] [CrossRef]
- Munson, H.E.; De Simone, L.; Schwaede, A.; Bhatia, A.; Mithal, D.S.; Young, N.; Kuntz, N.; Rao, V.K. Axonal polyneuropathy and ataxia in children: Consider Perrault Syndrome, a case report. BMC Med. Genom. 2023, 16, 278. [Google Scholar] [CrossRef]
- Wei, L.; Hou, L.; Ying, Y.Q.; Luo, X.P. A Novel Missense Mutation in TWNK Gene Causing Perrault Syndrome Type 5 in a Chinese Family and Review of the Literature. Pharmgenom. Pers. Med. 2022, 15, 1–8. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, S.; Li, H.; Xu, X.; Lyu, J. Analysis of TWNK variant in a family affected with Perrault syndrome. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2020, 37, 739–742. [Google Scholar] [CrossRef]
- Kume, K.; Morino, H.; Miyamoto, R.; Matsuda, Y.; Ohsawa, R.; Kanaya, Y.; Tada, Y.; Kurashige, T.; Kawakami, H. Middle-age-onset cerebellar ataxia caused by a homozygous TWNK variant: A case report. BMC Med. Genet. 2020, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Fekete, B.; Pentelenyi, K.; Rudas, G.; Gal, A.; Grosz, Z.; Illes, A.; Idris, J.; Csukly, G.; Domonkos, A.; Molnar, M.J. Broadening the phenotype of the TWNK gene associated Perrault syndrome. BMC Med. Genet. 2019, 20, 198. [Google Scholar] [CrossRef]
- Dominguez-Ruiz, M.; Garcia-Martinez, A.; Corral-Juan, M.; Perez-Alvarez, A.I.; Plasencia, A.M.; Villamar, M.; Moreno-Pelayo, M.A.; Matilla-Duenas, A.; Menendez-Gonzalez, M.; Del Castillo, I. Perrault syndrome with neurological features in a compound heterozygote for two TWNK mutations: Overlap of TWNK-related recessive disorders. J. Transl. Med. 2019, 17, 290. [Google Scholar] [CrossRef] [PubMed]
- Oldak, M.; Ozieblo, D.; Pollak, A.; Stepniak, I.; Lazniewski, M.; Lechowicz, U.; Kochanek, K.; Furmanek, M.; Tacikowska, G.; Plewczynski, D.; et al. Novel neuro-audiological findings and further evidence for TWNK involvement in Perrault syndrome. J. Transl. Med. 2017, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Morino, H.; Pierce, S.B.; Matsuda, Y.; Walsh, T.; Ohsawa, R.; Newby, M.; Hiraki-Kamon, K.; Kuramochi, M.; Lee, M.K.; Klevit, R.E.; et al. Mutations in Twinkle primase-helicase cause Perrault syndrome with neurologic features. Neurology 2014, 83, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Qin, Y.; Achenbach, J.; Li, C.; Kijek, J.; Spahn, C.M.; Nierhaus, K.H. EF-G and EF4: Translocation and back-translocation on the bacterial ribosome. Nat. Rev. Microbiol. 2014, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.E.; Loveland, A.B.; Gamper, H.B., Jr.; Hou, Y.M.; Demo, G.; Korostelev, A.A. Time-resolved cryo-EM visualizes ribosomal translocation with EF-G and GTP. Nat. Commun. 2021, 12, 7236. [Google Scholar] [CrossRef]
- Koripella, R.K.; Sharma, M.R.; Bhargava, K.; Datta, P.P.; Kaushal, P.S.; Keshavan, P.; Spremulli, L.L.; Banavali, N.K.; Agrawal, R.K. Structures of the human mitochondrial ribosome bound to EF-G1 reveal distinct features of mitochondrial translation elongation. Nat. Commun. 2020, 11, 3830. [Google Scholar] [CrossRef] [PubMed]
- Kummer, E.; Ban, N. Structural insights into mammalian mitochondrial translation elongation catalyzed by mtEFG1. EMBO J. 2020, 39, e104820. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, S.; Kumar, V.; Ero, R.; Gao, Y.G. Structure of EF-G-ribosome complex in a pretranslocation state. Nat. Struct. Mol. Biol. 2013, 20, 1077–1084. [Google Scholar] [CrossRef]
- Moraleda, J.M.; Gonzalez, R.; Alegre, A.; Anta, J.P.; San Miguel, J.F. Bone marrow necrosis and treatment with interferon. J. Clin. Pathol. 1986, 39, 1045. [Google Scholar] [CrossRef] [PubMed]
- Sitron, C.S.; Park, J.H.; Giafaglione, J.M.; Brandman, O. Aggregation of CAT tails blocks their degradation and causes proteotoxicity in S. cerevisiae. PLoS ONE 2020, 15, e0227841. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tantray, I.; Lim, J.; Chen, S.; Li, Y.; Davis, Z.; Sitron, C.; Dong, J.; Gispert, S.; Auburger, G.; et al. MISTERMINATE Mechanistically Links Mitochondrial Dysfunction with Proteostasis Failure. Mol. Cell 2019, 75, 835–848.e838. [Google Scholar] [CrossRef] [PubMed]
- Lytvynenko, I.; Paternoga, H.; Thrun, A.; Balke, A.; Muller, T.A.; Chiang, C.H.; Nagler, K.; Tsaprailis, G.; Anders, S.; Bischofs, I.; et al. Alanine Tails Signal Proteolysis in Bacterial Ribosome-Associated Quality Control. Cell 2019, 178, 76–90.e22. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, S.; Roche, E.; Zhou, Y.; Sauer, R.T. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998, 12, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Gruffaz, C.; Smirnov, A. GTPase Era at the heart of ribosome assembly. Front. Mol. Biosci. 2023, 10, 1263433. [Google Scholar] [CrossRef]
- Dennerlein, S.; Rozanska, A.; Wydro, M.; Chrzanowska-Lightowlers, Z.M.; Lightowlers, R.N. Human ERAL1 is a mitochondrial RNA chaperone involved in the assembly of the 28S small mitochondrial ribosomal subunit. Biochem. J. 2010, 430, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, T.; Ohgaki, K.; Yagi, M.; Aoki, Y.; Sakai, A.; Matsumoto, S.; Kang, D. ERAL1 is associated with mitochondrial ribosome and elimination of ERAL1 leads to mitochondrial dysfunction and growth retardation. Nucleic Acids Res. 2010, 38, 5554–5568. [Google Scholar] [CrossRef] [PubMed]
- Accardi, R.; Oxelmark, E.; Jauniaux, N.; de Pinto, V.; Marchini, A.; Tommasino, M. High levels of the mitochondrial large ribosomal subunit protein 40 prevent loss of mitochondrial DNA in null mmf1 Saccharomyces cerevisiae cells. Yeast 2004, 21, 539–548. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, X.; Coots, R.A.; Conn, C.S.; Liu, B.; Qian, S.B. Translational control of the cytosolic stress response by mitochondrial ribosomal protein L18. Nat. Struct. Mol. Biol. 2015, 22, 404–410. [Google Scholar] [CrossRef]
- Xu, P.; Wang, L.; Peng, H.; Liu, H.; Liu, H.; Yuan, Q.; Lin, Y.; Xu, J.; Pang, X.; Wu, H.; et al. Disruption of Hars2 in Cochlear Hair Cells Causes Progressive Mitochondrial Dysfunction and Hearing Loss in Mice. Front. Cell. Neurosci. 2021, 15, 804345. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Wang, X.; Meng, F.; Cui, L.; Yi, Q.; Zhao, Q.; Cang, X.; Cai, Z.; Mo, J.Q.; Liang, Y.; et al. Overexpression of mitochondrial histidyl-tRNA synthetase restores mitochondrial dysfunction caused by a deafness-associated tRNA(His) mutation. J. Biol. Chem. 2020, 295, 940–954. [Google Scholar] [CrossRef] [PubMed]
- van der Knaap, M.S.; Bugiani, M.; Mendes, M.I.; Riley, L.G.; Smith, D.E.C.; Rudinger-Thirion, J.; Frugier, M.; Breur, M.; Crawford, J.; van Gaalen, J.; et al. Biallelic variants in LARS2 and KARS cause deafness and (ovario)leukodystrophy. Neurology 2019, 92, e1225–e1237. [Google Scholar] [CrossRef] [PubMed]
- Perli, E.; Fiorillo, A.; Giordano, C.; Pisano, A.; Montanari, A.; Grazioli, P.; Campese, A.F.; Di Micco, P.; Tuppen, H.A.; Genovese, I.; et al. Short peptides from leucyl-tRNA synthetase rescue disease-causing mitochondrial tRNA point mutations. Hum. Mol. Genet. 2016, 25, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Hornig-Do, H.T.; Montanari, A.; Rozanska, A.; Tuppen, H.A.; Almalki, A.A.; Abg-Kamaludin, D.P.; Frontali, L.; Francisci, S.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M. Human mitochondrial leucyl tRNA synthetase can suppress non cognate pathogenic mt-tRNA mutations. EMBO Mol. Med. 2014, 6, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Levinger, L.; Morl, M.; Florentz, C. Mitochondrial tRNA 3′ end metabolism and human disease. Nucleic Acids Res. 2004, 32, 5430–5441. [Google Scholar] [CrossRef] [PubMed]
- Rumyantseva, A.; Popovic, M.; Trifunovic, A. CLPP deficiency ameliorates neurodegeneration caused by impaired mitochondrial protein synthesis. Brain 2022, 145, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Lyu, B.; Song, Q. The intricate relationship of G-Quadruplexes and bacterial pathogenicity islands. eLife 2024, 12, RP91985. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Fos, S.; Ito, C.; Moore, C.M.; Reddi, A.R.; Williams, L.D. Human ribosomal G-quadruplexes regulate heme bioavailability. J. Biol. Chem. 2020, 295, 14855–14865. [Google Scholar] [CrossRef]
- Chang, T.; Liu, X.; Cheng, X.; Qi, C.; Mei, H.; Shangguan, D. Selective isolation of G-quadruplexes by affinity chromatography. J. Chromatogr. A 2012, 1246, 62–68. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Liu, Z.; Lin, B.; Yi, H.; Xu, F.; Nie, Z.; Yao, S. Insight into G-quadruplex-hemin DNAzyme/RNAzyme: Adjacent adenine as the intramolecular species for remarkable enhancement of enzymatic activity. Nucleic Acids Res. 2016, 44, 7373–7384. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J.C.; Shumayrikh, N.; Sen, D. G-quadruplex structures formed by expanded hexanucleotide repeat RNA and DNA from the neurodegenerative disease-linked C9orf72 gene efficiently sequester and activate heme. PLoS ONE 2014, 9, e106449. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yin, Z.; Xiao, R.; Huang, B.; Cui, Y.; Wang, H.; Xiang, Y.; Wang, L.; Lei, L.; Ye, J.; et al. G-quadruplexes sense natural porphyrin metabolites for regulation of gene transcription and chromatin landscapes. Genome Biol. 2022, 23, 259. [Google Scholar] [CrossRef] [PubMed]
- Varshney, D.; Cuesta, S.M.; Herdy, B.; Abdullah, U.B.; Tannahill, D.; Balasubramanian, S. RNA G-quadruplex structures control ribosomal protein production. Sci. Rep. 2021, 11, 22735. [Google Scholar] [CrossRef] [PubMed]
- Pietras, Z.; Wojcik, M.A.; Borowski, L.S.; Szewczyk, M.; Kulinski, T.M.; Cysewski, D.; Stepien, P.P.; Dziembowski, A.; Szczesny, R.J. Dedicated surveillance mechanism controls G-quadruplex forming non-coding RNAs in human mitochondria. Nat. Commun. 2018, 9, 2558. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Kim, K.M.; Abdelmohsen, K.; Yoon, J.H.; Panda, A.C.; Munk, R.; Kim, J.; Curtis, J.; Moad, C.A.; Wohler, C.M.; et al. HuR and GRSF1 modulate the nuclear export and mitochondrial localization of the lncRNA RMRP. Genes Dev. 2016, 30, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, A.A.; Koppen, M.; Wydro, M.; Rodley, C.D.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.; Martinou, J.C. GRSF1 regulates RNA processing in mitochondrial RNA granules. Cell Metab. 2013, 17, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Hensen, F.; Potter, A.; van Esveld, S.L.; Tarres-Sole, A.; Chakraborty, A.; Sola, M.; Spelbrink, J.N. Mitochondrial RNA granules are critically dependent on mtDNA replication factors Twinkle and mtSSB. Nucleic Acids Res. 2019, 47, 3680–3698. [Google Scholar] [CrossRef] [PubMed]
- Xavier, V.J.; Martinou, J.C. RNA Granules in the Mitochondria and Their Organization under Mitochondrial Stresses. Int. J. Mol. Sci. 2021, 22, 9502. [Google Scholar] [CrossRef]
- Antonicka, H.; Sasarman, F.; Nishimura, T.; Paupe, V.; Shoubridge, E.A. The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab. 2013, 17, 386–398. [Google Scholar] [CrossRef]
- Pietras, Z.; Wojcik, M.A.; Borowski, L.S.; Szewczyk, M.; Kulinski, T.M.; Cysewski, D.; Stepien, P.P.; Dziembowski, A.; Szczesny, R.J. Controlling the mitochondrial antisense—Role of the SUV3-PNPase complex and its co-factor GRSF1 in mitochondrial RNA surveillance. Mol. Cell. Oncol. 2018, 5, e1516452. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Zhu, Y.; Huang, Q.; Gu, Q.; Tian, S.; Ge, J.; Lin, X.; Sha, W. Guanine-rich RNA sequence binding factor 1 regulates neuronal ferroptosis after spinal cord injury in rats via the GPX4 signaling pathway. Brain Res. 2023, 1818, 148497. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, B.; Heydeck, D.; Jahn, D.; Lasse, M.; Sofi, S.; Ufer, C.; Kuhn, H. Male guanine-rich RNA sequence binding factor 1 knockout mice (Grsf1(−/−)) gain less body weight during adolescence and adulthood. Cell Biosci. 2022, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Kim, K.M.; Pandey, P.R.; Noren Hooten, N.; Munk, R.; Kundu, G.; De, S.; Martindale, J.L.; Yang, X.; Evans, M.K.; et al. Loss of RNA-binding protein GRSF1 activates mTOR to elicit a proinflammatory transcriptional program. Nucleic Acids Res. 2019, 47, 2472–2486. [Google Scholar] [CrossRef] [PubMed]
- Al-Furoukh, N.; Goffart, S.; Szibor, M.; Wanrooij, S.; Braun, T. Binding to G-quadruplex RNA activates the mitochondrial GTPase NOA1. Biochim. Biophys. Acta 2013, 1833, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cooper, H.M.; Reyes, A.; Di Re, M.; Kazak, L.; Wood, S.R.; Mao, C.C.; Fearnley, I.M.; Walker, J.E.; Holt, I.J. Human C4orf14 interacts with the mitochondrial nucleoid and is involved in the biogenesis of the small mitochondrial ribosomal subunit. Nucleic Acids Res. 2012, 40, 6097–6108. [Google Scholar] [CrossRef] [PubMed]
- Kolanczyk, M.; Pech, M.; Zemojtel, T.; Yamamoto, H.; Mikula, I.; Calvaruso, M.A.; van den Brand, M.; Richter, R.; Fischer, B.; Ritz, A.; et al. NOA1 is an essential GTPase required for mitochondrial protein synthesis. Mol. Biol. Cell 2011, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Al-Furoukh, N.; Kardon, J.R.; Kruger, M.; Szibor, M.; Baker, T.A.; Braun, T. NOA1, a novel ClpXP substrate, takes an unexpected nuclear detour prior to mitochondrial import. PLoS ONE 2014, 9, e103141. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Ma, W.; Sand, Z.; Finlayson, J.; Wang, T.; Brinton, R.D.; Willis, W.T.; Mandarino, L.J. Von Willebrand factor A domain-containing protein 8 (VWA8) localizes to the matrix side of the inner mitochondrial membrane. Biochem. Biophys. Res. Commun. 2020, 521, 158–163. [Google Scholar] [CrossRef]
- Chen, Z.; Suzuki, H.; Kobayashi, Y.; Wang, A.C.; DiMaio, F.; Kawashima, S.A.; Walz, T.; Kapoor, T.M. Structural Insights into Mdn1, an Essential AAA Protein Required for Ribosome Biogenesis. Cell 2018, 175, 822–834.e818. [Google Scholar] [CrossRef]
- Kawashima, S.A.; Chen, Z.; Aoi, Y.; Patgiri, A.; Kobayashi, Y.; Nurse, P.; Kapoor, T.M. Potent, Reversible, and Specific Chemical Inhibitors of Eukaryotic Ribosome Biogenesis. Cell 2016, 167, 512–524.e514. [Google Scholar] [CrossRef] [PubMed]
- Prattes, M.; Lo, Y.H.; Bergler, H.; Stanley, R.E. Shaping the Nascent Ribosome: AAA-ATPases in Eukaryotic Ribosome Biogenesis. Biomolecules 2019, 9, 715. [Google Scholar] [CrossRef]
- Linke, R.; Limmer, M.; Juranek, S.A.; Heine, A.; Paeschke, K. The Relevance of G-Quadruplexes for DNA Repair. Int. J. Mol. Sci. 2021, 22, 12599. [Google Scholar] [CrossRef]
- McShane, E.; Couvillion, M.; Ietswaart, R.; Prakash, G.; Smalec, B.M.; Soto, I.; Baxter-Koenigs, A.R.; Choquet, K.; Churchman, L.S. A kinetic dichotomy between mitochondrial and nuclear gene expression processes. Mol. Cell 2024, 84, 1541–1555.e11. [Google Scholar] [CrossRef]
- Honarmand, S.; Shoubridge, E.A. Poly (A) tail length of human mitochondrial mRNAs is tissue-specific and a mutation in LRPPRC results in transcript-specific patterns of deadenylation. Mol. Genet. Metab. Rep. 2020, 25, 100687. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.C.; Hornig-Do, H.T.; Bruni, F.; Chang, J.H.; Jourdain, A.A.; Martinou, J.C.; Falkenberg, M.; Spahr, H.; Larsson, N.G.; Lewis, R.J.; et al. A human mitochondrial poly(A) polymerase mutation reveals the complexities of post-transcriptional mitochondrial gene expression. Hum. Mol. Genet. 2014, 23, 6345–6355. [Google Scholar] [CrossRef]
- Chujo, T.; Ohira, T.; Sakaguchi, Y.; Goshima, N.; Nomura, N.; Nagao, A.; Suzuki, T. LRPPRC/SLIRP suppresses PNPase-mediated mRNA decay and promotes polyadenylation in human mitochondria. Nucleic Acids Res. 2012, 40, 8033–8047. [Google Scholar] [CrossRef] [PubMed]
- Ruzzenente, B.; Metodiev, M.D.; Wredenberg, A.; Bratic, A.; Park, C.B.; Camara, Y.; Milenkovic, D.; Zickermann, V.; Wibom, R.; Hultenby, K.; et al. LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J. 2012, 31, 443–456. [Google Scholar] [CrossRef]
- Pajak, A.; Laine, I.; Clemente, P.; El-Fissi, N.; Schober, F.A.; Maffezzini, C.; Calvo-Garrido, J.; Wibom, R.; Filograna, R.; Dhir, A.; et al. Defects of mitochondrial RNA turnover lead to the accumulation of double-stranded RNA in vivo. PLoS Genet. 2019, 15, e1008240. [Google Scholar] [CrossRef]
- Szczesny, R.J.; Wojcik, M.A.; Borowski, L.S.; Szewczyk, M.J.; Skrok, M.M.; Golik, P.; Stepien, P.P. Yeast and human mitochondrial helicases. Biochim. Biophys. Acta 2013, 1829, 842–853. [Google Scholar] [CrossRef]
- Borowski, L.S.; Dziembowski, A.; Hejnowicz, M.S.; Stepien, P.P.; Szczesny, R.J. Human mitochondrial RNA decay mediated by PNPase-hSuv3 complex takes place in distinct foci. Nucleic Acids Res. 2013, 41, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, R.J.; Borowski, L.S.; Malecki, M.; Wojcik, M.A.; Stepien, P.P.; Golik, P. RNA degradation in yeast and human mitochondria. Biochim. Biophys. Acta 2012, 1819, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Clemente, P.; Pajak, A.; Laine, I.; Wibom, R.; Wedell, A.; Freyer, C.; Wredenberg, A. SUV3 helicase is required for correct processing of mitochondrial transcripts. Nucleic Acids Res. 2015, 43, 7398–7413. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Golzarroshan, B.; Lin, C.L.; Agrawal, S.; Tang, W.H.; Wu, C.J.; Yuan, H.S. Dimeric assembly of human Suv3 helicase promotes its RNA unwinding function in mitochondrial RNA degradosome for RNA decay. Protein Sci. 2022, 31, e4312. [Google Scholar] [CrossRef] [PubMed]
- Toompuu, M.; Tuomela, T.; Laine, P.; Paulin, L.; Dufour, E.; Jacobs, H.T. Polyadenylation and degradation of structurally abnormal mitochondrial tRNAs in human cells. Nucleic Acids Res. 2018, 46, 5209–5226. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, R.J.; Borowski, L.S.; Brzezniak, L.K.; Dmochowska, A.; Gewartowski, K.; Bartnik, E.; Stepien, P.P. Human mitochondrial RNA turnover caught in flagranti: Involvement of hSuv3p helicase in RNA surveillance. Nucleic Acids Res. 2010, 38, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Shu, Z.; Lieser, S.A.; Chen, P.L.; Lee, W.H. Human mitochondrial SUV3 and polynucleotide phosphorylase form a 330-kDa heteropentamer to cooperatively degrade double-stranded RNA with a 3′-to-5′ directionality. J. Biol. Chem. 2009, 284, 20812–20821. [Google Scholar] [CrossRef]
- Sarkar, D.; Fisher, P.B. Human polynucleotide phosphorylase (hPNPase old-35): An RNA degradation enzyme with pleiotrophic biological effects. Cell Cycle 2006, 5, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Piazza, F.; Zappone, M.; Sana, M.; Briani, F.; Deho, G. Polynucleotide phosphorylase of Escherichia coli is required for the establishment of bacteriophage P4 immunity. J. Bacteriol. 1996, 178, 5513–5521. [Google Scholar] [CrossRef]
- Chen, P.L. SUV3 Helicase and Mitochondrial Homeostasis. Int. J. Mol. Sci. 2023, 24, 9233. [Google Scholar] [CrossRef]
- Silva, S.; Camino, L.P.; Aguilera, A. Human mitochondrial degradosome prevents harmful mitochondrial R loops and mitochondrial genome instability. Proc. Natl. Acad. Sci. USA 2018, 115, 11024–11029. [Google Scholar] [CrossRef] [PubMed]
- Carzaniga, T.; Sbarufatti, G.; Briani, F.; Deho, G. Polynucleotide phosphorylase is implicated in homologous recombination and DNA repair in Escherichia coli. BMC Microbiol. 2017, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Tarique, M.; Tuteja, R. Rice SUV3 is a bidirectional helicase that binds both DNA and RNA. BMC Plant Biol. 2014, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Minczuk, M.; Piwowarski, J.; Papworth, M.A.; Awiszus, K.; Schalinski, S.; Dziembowski, A.; Dmochowska, A.; Bartnik, E.; Tokatlidis, K.; Stepien, P.P.; et al. Localisation of the human hSuv3p helicase in the mitochondrial matrix and its preferential unwinding of dsDNA. Nucleic Acids Res. 2002, 30, 5074–5086. [Google Scholar] [CrossRef] [PubMed]
- Barbier, M.; Bahlo, M.; Pennisi, A.; Jacoupy, M.; Tankard, R.M.; Ewenczyk, C.; Davies, K.C.; Lino-Coulon, P.; Colace, C.; Rafehi, H.; et al. Heterozygous PNPT1 Variants Cause Spinocerebellar Ataxia Type 25. Ann. Neurol. 2022, 92, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Eaton, A.; Bernier, F.P.; Goedhart, C.; Caluseriu, O.; Lamont, R.E.; Boycott, K.M.; Parboosingh, J.S.; Innes, A.M.; Care4Rare Canada, C. Is PNPT1-related hearing loss ever non-syndromic? Whole exome sequencing of adult siblings expands the natural history of PNPT1-related disorders. Am. J. Med. Genet. A 2018, 176, 2487–2493. [Google Scholar] [CrossRef]
- Matilainen, S.; Carroll, C.J.; Richter, U.; Euro, L.; Pohjanpelto, M.; Paetau, A.; Isohanni, P.; Suomalainen, A. Defective mitochondrial RNA processing due to PNPT1 variants causes Leigh syndrome. Hum. Mol. Genet. 2017, 26, 3352–3361. [Google Scholar] [CrossRef]
- Sato, R.; Arai-Ichinoi, N.; Kikuchi, A.; Matsuhashi, T.; Numata-Uematsu, Y.; Uematsu, M.; Fujii, Y.; Murayama, K.; Ohtake, A.; Abe, T.; et al. Novel biallelic mutations in the PNPT1 gene encoding a mitochondrial-RNA-import protein PNPase cause delayed myelination. Clin. Genet. 2018, 93, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Slavotinek, A.M.; Garcia, S.T.; Chandratillake, G.; Bardakjian, T.; Ullah, E.; Wu, D.; Umeda, K.; Lao, R.; Tang, P.L.; Wan, E.; et al. Exome sequencing in 32 patients with anophthalmia/microphthalmia and developmental eye defects. Clin. Genet. 2015, 88, 468–473. [Google Scholar] [CrossRef]
- von Ameln, S.; Wang, G.; Boulouiz, R.; Rutherford, M.A.; Smith, G.M.; Li, Y.; Pogoda, H.M.; Nurnberg, G.; Stiller, B.; Volk, A.E.; et al. A mutation in PNPT1, encoding mitochondrial-RNA-import protein PNPase, causes hereditary hearing loss. Am. J. Hum. Genet. 2012, 91, 919–927. [Google Scholar] [CrossRef]
- Dhir, A.; Dhir, S.; Borowski, L.S.; Jimenez, L.; Teitell, M.; Rotig, A.; Crow, Y.J.; Rice, G.I.; Duffy, D.; Tamby, C.; et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 2018, 560, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Maletzko, A.; Kohli, A.; Gispert, S.; Torres-Odio, S.; Wittig, I.; Heidler, J.; Barcena, C.; Lopez-Otin, C.; Lei, Y.; et al. Loss of mitochondrial ClpP, Lonp1, and Tfam triggers transcriptional induction of Rnf213, a susceptibility factor for moyamoya disease. Neurogenetics 2020, 21, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Maletzko, A.; Key, J.; Wittig, I.; Gispert, S.; Koepf, G.; Canet-Pons, J.; Torres-Odio, S.; West, A.P.; Auburger, G. Increased presence of nuclear DNAJA3 and upregulation of cytosolic STAT1 and of nucleic acid sensors trigger innate immunity in the ClpP-null mouse. Neurogenetics 2021, 22, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Torres-Odio, S.; Lei, Y.; Gispert, S.; Maletzko, A.; Key, J.; Menissy, S.S.; Wittig, I.; Auburger, G.; West, A.P. Loss of Mitochondrial Protease CLPP Activates Type I IFN Responses through the Mitochondrial DNA-cGAS-STING Signaling Axis. J. Immunol. 2021, 206, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.; Druyan, R.; Getz, G.S.; Rabinowitz, M. Intramitochondrial localization of delta-aminolaevulate synthetase and ferrochelatase in rat liver. Biochem. J. 1969, 114, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Medlock, A.E.; Shiferaw, M.T.; Marcero, J.R.; Vashisht, A.A.; Wohlschlegel, J.A.; Phillips, J.D.; Dailey, H.A. Identification of the Mitochondrial Heme Metabolism Complex. PLoS ONE 2015, 10, e0135896. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; Andrew, T.L.; Karr, S.W.; Dailey, H.A. Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J. Biol. Chem. 1988, 263, 3835–3839. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Clark, D.S. Phase-separated biomolecular condensates for biocatalysis. Trends Biotechnol. 2024, 42, 496–509. [Google Scholar] [CrossRef]
- Dahmani, I.; Qin, K.; Zhang, Y.; Fernie, A.R. The formation and function of plant metabolons. Plant J. 2023, 114, 1080–1092. [Google Scholar] [CrossRef]
- Kastritis, P.L.; Gavin, A.C. Enzymatic complexes across scales. Essays Biochem. 2018, 62, 501–514. [Google Scholar] [CrossRef]
- Kim, H.J.; Khalimonchuk, O.; Smith, P.M.; Winge, D.R. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim. Biophys. Acta 2012, 1823, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Maio, N.; Kim, K.S.; Holmes-Hampton, G.; Singh, A.; Rouault, T.A. Dimeric ferrochelatase bridges ABCB7 and ABCB10 homodimers in an architecturally defined molecular complex required for heme biosynthesis. Haematologica 2019, 104, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dailey, H.A.; Paw, B.H. Ferrochelatase forms an oligomeric complex with mitoferrin-1 and Abcb10 for erythroid heme biosynthesis. Blood 2010, 116, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Lane, D.J.; Becker, E.M.; Huang, M.L.; Whitnall, M.; Suryo Rahmanto, Y.; Sheftel, A.D.; Ponka, P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc. Natl. Acad. Sci. USA 2010, 107, 10775–10782. [Google Scholar] [CrossRef] [PubMed]
- Shum, M.; Shintre, C.A.; Althoff, T.; Gutierrez, V.; Segawa, M.; Saxberg, A.D.; Martinez, M.; Adamson, R.; Young, M.R.; Faust, B.; et al. ABCB10 exports mitochondrial biliverdin, driving metabolic maladaptation in obesity. Sci. Transl. Med. 2021, 13, eabd1869. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.A.; Cowan, J.A. Evolution of the human mitochondrial ABCB7 [2Fe-2S](GS)(4) cluster exporter and the molecular mechanism of an E433K disease-causing mutation. Arch. Biochem. Biophys. 2021, 697, 108661. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Fendley, G.A.; Saxberg, A.D.; Zoghbi, M.E. Stimulation of the human mitochondrial transporter ABCB10 by zinc-mesoporphrin. PLoS ONE 2020, 15, e0238754. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Arimura, H.; Fukushige, T.; Minami, K.; Nishizawa, Y.; Tanimoto, A.; Kanekura, T.; Nakagawa, M.; Akiyama, S.; Furukawa, T. Abcb10 role in heme biosynthesis in vivo: Abcb10 knockout in mice causes anemia with protoporphyrin IX and iron accumulation. Mol. Cell. Biol. 2014, 34, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Paradkar, P.N.; Li, L.; Pierce, E.L.; Langer, N.B.; Takahashi-Makise, N.; Hyde, B.B.; Shirihai, O.S.; Ward, D.M.; Kaplan, J.; et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc. Natl. Acad. Sci. USA 2009, 106, 16263–16268. [Google Scholar] [CrossRef]
- Clough, C.A.; Pangallo, J.; Sarchi, M.; Ilagan, J.O.; North, K.; Bergantinos, R.; Stolla, M.C.; Naru, J.; Nugent, P.; Kim, E.; et al. Coordinated missplicing of TMEM14C and ABCB7 causes ring sideroblast formation in SF3B1-mutant myelodysplastic syndrome. Blood 2022, 139, 2038–2049. [Google Scholar] [CrossRef]
- Harding, C.R.; Sidik, S.M.; Petrova, B.; Gnadig, N.F.; Okombo, J.; Herneisen, A.L.; Ward, K.E.; Markus, B.M.; Boydston, E.A.; Fidock, D.A.; et al. Genetic screens reveal a central role for heme metabolism in artemisinin susceptibility. Nat. Commun. 2020, 11, 4813. [Google Scholar] [CrossRef] [PubMed]
- Yien, Y.Y.; Robledo, R.F.; Schultz, I.J.; Takahashi-Makise, N.; Gwynn, B.; Bauer, D.E.; Dass, A.; Yi, G.; Li, L.; Hildick-Smith, G.J.; et al. TMEM14C is required for erythroid mitochondrial heme metabolism. J. Clin. Investig. 2014, 124, 4294–4304. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.; Schultz, I.J.; Pierce, E.L.; Soltis, K.A.; Naranuntarat, A.; Ward, D.M.; Baughman, J.M.; Paradkar, P.N.; Kingsley, P.D.; Culotta, V.C.; et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009, 10, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Kabe, Y.; Kuramori, C.; Kondo, M.; Yamaguchi, Y.; Handa, H. Adenine nucleotide translocator transports haem precursors into mitochondria. PLoS ONE 2008, 3, e3070. [Google Scholar] [CrossRef] [PubMed]
- Kabe, Y.; Ohmori, M.; Shinouchi, K.; Tsuboi, Y.; Hirao, S.; Azuma, M.; Watanabe, H.; Okura, I.; Handa, H. Porphyrin accumulation in mitochondria is mediated by 2-oxoglutarate carrier. J. Biol. Chem. 2006, 281, 31729–31735. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006, 163, 54–67. [Google Scholar] [CrossRef]
- Deery, E.; Schroeder, S.; Lawrence, A.D.; Taylor, S.L.; Seyedarabi, A.; Waterman, J.; Wilson, K.S.; Brown, D.; Geeves, M.A.; Howard, M.J.; et al. An enzyme-trap approach allows isolation of intermediates in cobalamin biosynthesis. Nat. Chem. Biol. 2012, 8, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.W.; Cohen, N.S.; Raijman, L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J. Biol. Chem. 1989, 264, 4038–4044. [Google Scholar] [CrossRef]
- Chen, C.; Hamza, I. Notes from the Underground: Heme Homeostasis in C. elegans. Biomolecules 2023, 13, 1149. [Google Scholar] [CrossRef]
- Chambers, I.G.; Willoughby, M.M.; Hamza, I.; Reddi, A.R. One ring to bring them all and in the darkness bind them: The trafficking of heme without deliverers. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118881. [Google Scholar] [CrossRef]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The Good, the Bad, and the Ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Liou, Y.F.; Charoenkwan, P.; Srinivasulu, Y.; Vasylenko, T.; Lai, S.C.; Lee, H.C.; Chen, Y.H.; Huang, H.L.; Ho, S.Y. SCMHBP: Prediction and analysis of heme binding proteins using propensity scores of dipeptides. BMC Bioinform. 2014, 15 (Suppl. S16), S4. [Google Scholar] [CrossRef]
- Whitman, J.C.; Paw, B.H.; Chung, J. The role of ClpX in erythropoietic protoporphyria. Hematol. Transfus. Cell Ther. 2018, 40, 182–188. [Google Scholar] [CrossRef]
- Fouquet, C.; Le Rouzic, M.A.; Leblanc, T.; Fouyssac, F.; Leverger, G.; Hessissen, L.; Marlin, S.; Bourrat, E.; Fahd, M.; Raffoux, E.; et al. Genotype/phenotype correlations of childhood-onset congenital sideroblastic anaemia in a European cohort. Br. J. Haematol. 2019, 187, 530–542. [Google Scholar] [CrossRef]
- Kardon, J.R.; Moroco, J.A.; Engen, J.R.; Baker, T.A. Mitochondrial ClpX activates an essential biosynthetic enzyme through partial unfolding. eLife 2020, 9, e54387. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.L.; Kardon, J.R.; Sauer, R.T.; Baker, T.A. Structure of the Mitochondrial Aminolevulinic Acid Synthase, a Key Heme Biosynthetic Enzyme. Structure 2018, 26, 580–589.e584. [Google Scholar] [CrossRef]
- Huang, L. An experimental study of the principle of electronic root canal measurement. J. Endod. 1987, 13, 60–64. [Google Scholar] [CrossRef]
- Kubota, Y.; Nomura, K.; Katoh, Y.; Yamashita, R.; Kaneko, K.; Furuyama, K. Novel Mechanisms for Heme-dependent Degradation of ALAS1 Protein as a Component of Negative Feedback Regulation of Heme Biosynthesis. J. Biol. Chem. 2016, 291, 20516–20529. [Google Scholar] [CrossRef] [PubMed]
- Schiroli, D.; Peracchi, A. A subfamily of PLP-dependent enzymes specialized in handling terminal amines. Biochim. Biophys. Acta 2015, 1854, 1200–1211. [Google Scholar] [CrossRef]
- Tarnacka, B.; Jopowicz, A.; Maslinska, M. Copper, Iron, and Manganese Toxicity in Neuropsychiatric Conditions. Int. J. Mol. Sci. 2021, 22, 7820. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Laut, C.L.; Leasure, C.S.; Pi, H.; Carlin, S.M.; Chu, M.L.; Hillebrand, G.H.; Lin, H.K.; Yi, X.I.; Stauff, D.L.; Skaar, E.P. DnaJ and ClpX Are Required for HitRS and HssRS Two-Component System Signaling in Bacillus anthracis. Infect. Immun. 2022, 90, e0056021. [Google Scholar] [CrossRef] [PubMed]
- Farrand, A.J.; Friedman, D.B.; Reniere, M.L.; Ingmer, H.; Frees, D.; Skaar, E.P. Proteomic analyses of iron-responsive, Clp-dependent changes in Staphylococcus aureus. Pathog. Dis. 2015, 73, ftv004. [Google Scholar] [CrossRef] [PubMed]
- Farrand, A.J.; Reniere, M.L.; Ingmer, H.; Frees, D.; Skaar, E.P. Regulation of host hemoglobin binding by the Staphylococcus aureus Clp proteolytic system. J. Bacteriol. 2013, 195, 5041–5050. [Google Scholar] [CrossRef] [PubMed]
- Stanne, T.M.; Sjogren, L.L.; Koussevitzky, S.; Clarke, A.K. Identification of new protein substrates for the chloroplast ATP-dependent Clp protease supports its constitutive role in Arabidopsis. Biochem. J. 2009, 417, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Elliott, M.; Elliott, T. Conditional stability of the HemA protein (glutamyl-tRNA reductase) regulates heme biosynthesis in Salmonella typhimurium. J. Bacteriol. 1999, 181, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowska, K.; Trifunovic, A. Tune instead of destroy: How proteolysis keeps OXPHOS in shape. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148365. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, I.; Sazanov, L.A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 2022, 23, 141–161. [Google Scholar] [CrossRef]
- Giachin, G.; Bouverot, R.; Acajjaoui, S.; Pantalone, S.; Soler-Lopez, M. Dynamics of Human Mitochondrial Complex I Assembly: Implications for Neurodegenerative Diseases. Front. Mol. Biosci. 2016, 3, 43. [Google Scholar] [CrossRef]
- Berrisford, J.M.; Baradaran, R.; Sazanov, L.A. Structure of bacterial respiratory complex I. Biochim. Biophys. Acta 2016, 1857, 892–901. [Google Scholar] [CrossRef]
- Friedrich, T. On the mechanism of respiratory complex I. J. Bioenerg. Biomembr. 2014, 46, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Clason, T.; Ruiz, T.; Schagger, H.; Peng, G.; Zickermann, V.; Brandt, U.; Michel, H.; Radermacher, M. The structure of eukaryotic and prokaryotic complex I. J. Struct. Biol. 2010, 169, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowska, K.; Senft, K.; Heidler, J.; Herholz, M.; Kukat, A.; Hohne, M.N.; Hofsetz, E.; Becker, C.; Kaspar, S.; Giese, H.; et al. A salvage pathway maintains highly functional respiratory complex I. Nat. Commun. 2020, 11, 1643. [Google Scholar] [CrossRef] [PubMed]
- Feric, M.; Demarest, T.G.; Tian, J.; Croteau, D.L.; Bohr, V.A.; Misteli, T. Self-assembly of multi-component mitochondrial nucleoids via phase separation. EMBO J. 2021, 40, e107165. [Google Scholar] [CrossRef] [PubMed]
- Rey, T.; Zaganelli, S.; Cuillery, E.; Vartholomaiou, E.; Croisier, M.; Martinou, J.C.; Manley, S. Mitochondrial RNA granules are fluid condensates positioned by membrane dynamics. Nat. Cell Biol. 2020, 22, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Guilhas, B.; Walter, J.C.; Rech, J.; David, G.; Walliser, N.O.; Palmeri, J.; Mathieu-Demaziere, C.; Parmeggiani, A.; Bouet, J.Y.; Le Gall, A.; et al. ATP-Driven Separation of Liquid Phase Condensates in Bacteria. Mol. Cell 2020, 79, 293–303.e294. [Google Scholar] [CrossRef] [PubMed]
- Ladouceur, A.M.; Parmar, B.S.; Biedzinski, S.; Wall, J.; Tope, S.G.; Cohn, D.; Kim, A.; Soubry, N.; Reyes-Lamothe, R.; Weber, S.C. Clusters of bacterial RNA polymerase are biomolecular condensates that assemble through liquid-liquid phase separation. Proc. Natl. Acad. Sci. USA 2020, 117, 18540–18549. [Google Scholar] [CrossRef] [PubMed]
- Muthunayake, N.S.; Tomares, D.T.; Childers, W.S.; Schrader, J.M. Phase-separated bacterial ribonucleoprotein bodies organize mRNA decay. Wiley Interdiscip. Rev. RNA 2020, 11, e1599. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Han, T.W.; Xie, S.; Shi, K.; Du, X.; Wu, L.C.; Mirzaei, H.; Goldsmith, E.J.; Longgood, J.; Pei, J.; et al. Cell-free formation of RNA granules: Low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149, 753–767. [Google Scholar] [CrossRef]
- Annunziata, O.; Asherie, N.; Lomakin, A.; Pande, J.; Ogun, O.; Benedek, G.B. Effect of polyethylene glycol on the liquid-liquid phase transition in aqueous protein solutions. Proc. Natl. Acad. Sci. USA 2002, 99, 14165–14170. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Wang, W.; Shi, J.; Stovall, D.B.; Li, D.; Sui, G. Phase-Separated Subcellular Compartmentation and Related Human Diseases. Int. J. Mol. Sci. 2022, 23, 5491. [Google Scholar] [CrossRef] [PubMed]
- Peran, I.; Mittag, T. Molecular structure in biomolecular condensates. Curr. Opin. Struct. Biol. 2020, 60, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Dormann, D. Liquid-Liquid Phase Separation in Disease. Annu. Rev. Genet. 2019, 53, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Sepehri, A.; Lazaridis, T. Putative Pore Structures of Amyloid beta 25-35 in Lipid Bilayers. Biochemistry 2023, 62, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, M.; Fuxreiter, M. Sequence Determinants of the Aggregation of Proteins within Condensates Generated by Liquid-liquid Phase Separation. J. Mol. Biol. 2022, 434, 167201. [Google Scholar] [CrossRef] [PubMed]
- Stockl, M.T.; Zijlstra, N.; Subramaniam, V. alpha-Synuclein oligomers: An amyloid pore? Insights into mechanisms of alpha-synuclein oligomer-lipid interactions. Mol. Neurobiol. 2013, 47, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.G.; Kim, J.K. Compartmentalization and metabolic regulation of glycolysis. J. Cell Sci. 2021, 134, jcs258469. [Google Scholar] [CrossRef]
- Nesterov, S.V.; Ilyinsky, N.S.; Plokhikh, K.S.; Manuylov, V.D.; Chesnokov, Y.M.; Vasilov, R.G.; Kuznetsova, I.M.; Turoverov, K.K.; Gordeliy, V.I.; Fonin, A.V.; et al. Order wrapped in chaos: On the roles of intrinsically disordered proteins and RNAs in the arrangement of the mitochondrial enzymatic machines. Int. J. Biol. Macromol. 2024, 267, 131455. [Google Scholar] [CrossRef]
- Zurita Rendon, O.; Shoubridge, E.A. LONP1 Is Required for Maturation of a Subset of Mitochondrial Proteins, and Its Loss Elicits an Integrated Stress Response. Mol. Cell. Biol. 2018, 38, e00412-17. [Google Scholar] [CrossRef]
- Szczepanowska, K.; Trifunovic, A. Mitochondrial matrix proteases: Quality control and beyond. FEBS J. 2022, 289, 7128–7146. [Google Scholar] [CrossRef]
- Feng, Y.; Nouri, K.; Schimmer, A.D. Mitochondrial ATP-Dependent Proteases-Biological Function and Potential Anti-Cancer Targets. Cancers 2021, 13, 2020. [Google Scholar] [CrossRef]
- Quiros, P.M.; Langer, T.; Lopez-Otin, C. New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 2015, 16, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Bohovych, I.; Chan, S.S.; Khalimonchuk, O. Mitochondrial protein quality control: The mechanisms guarding mitochondrial health. Antioxid. Redox Signal. 2015, 22, 977–994. [Google Scholar] [CrossRef]
- Xiang, X.; Dai, Z.; Luo, B.; Zhao, N.; Liu, S.; Sui, J.; Huang, J.; Zhou, Y.; Gu, J.; Zhang, J.; et al. Rational Design of a Novel Class of Human ClpP Agonists through a Ring-Opening Strategy with Enhanced Antileukemia Activity. J. Med. Chem. 2024, 67, 6769–6792. [Google Scholar] [CrossRef] [PubMed]
- Wedam, R.; Greer, Y.E.; Wisniewski, D.J.; Weltz, S.; Kundu, M.; Voeller, D.; Lipkowitz, S. Targeting Mitochondria with ClpP Agonists as a Novel Therapeutic Opportunity in Breast Cancer. Cancers 2023, 15, 1936. [Google Scholar] [CrossRef] [PubMed]
- Walther, R.; Westermann, L.M.; Carmali, S.; Jackson, S.E.; Brotz-Oesterhelt, H.; Spring, D.R. Identification of macrocyclic peptides which activate bacterial cylindrical proteases. RSC Med. Chem. 2023, 14, 1186–1191. [Google Scholar] [CrossRef]
- Zhou, L.L.; Zhang, T.; Xue, Y.; Yue, C.; Pan, Y.; Wang, P.; Yang, T.; Li, M.; Zhou, H.; Ding, K.; et al. Selective activator of human ClpP triggers cell cycle arrest to inhibit lung squamous cell carcinoma. Nat. Commun. 2023, 14, 7069. [Google Scholar] [CrossRef]
- Greer, Y.E.; Hernandez, L.; Fennell, E.M.J.; Kundu, M.; Voeller, D.; Chari, R.; Gilbert, S.F.; Gilbert, T.S.K.; Ratnayake, S.; Tang, B.; et al. Mitochondrial Matrix Protease ClpP Agonists Inhibit Cancer Stem Cell Function in Breast Cancer Cells by Disrupting Mitochondrial Homeostasis. Cancer Res. Commun. 2022, 2, 1144–1161. [Google Scholar] [CrossRef]
- Cobongela, S.Z.Z.; Makatini, M.M.; Mdluli, P.S.; Sibuyi, N.R.S. Acyldepsipeptide Analogues: A Future Generation Antibiotics for Tuberculosis Treatment. Pharmaceutics 2022, 14, 1956. [Google Scholar] [CrossRef]
- Morreale, F.E.; Kleine, S.; Leodolter, J.; Junker, S.; Hoi, D.M.; Ovchinnikov, S.; Okun, A.; Kley, J.; Kurzbauer, R.; Junk, L.; et al. BacPROTACs mediate targeted protein degradation in bacteria. Cell 2022, 185, 2338–2353.e2318. [Google Scholar] [CrossRef]
- Schwarz, M.; Hubner, I.; Sieber, S.A. Tailored Phenyl Esters Inhibit ClpXP and Attenuate Staphylococcus aureus alpha-Hemolysin Secretion. ChemBioChem 2022, 23, e202200253. [Google Scholar] [CrossRef] [PubMed]
- Brotz-Oesterhelt, H.; Vorbach, A. Reprogramming of the Caseinolytic Protease by ADEP Antibiotics: Molecular Mechanism, Cellular Consequences, Therapeutic Potential. Front. Mol. Biosci. 2021, 8, 690902. [Google Scholar] [CrossRef] [PubMed]
- Bosc, C.; Saland, E.; Bousard, A.; Gadaud, N.; Sabatier, M.; Cognet, G.; Farge, T.; Boet, E.; Gotanegre, M.; Aroua, N.; et al. Mitochondrial inhibitors circumvent adaptive resistance to venetoclax and cytarabine combination therapy in acute myeloid leukemia. Nat. Cancer 2021, 2, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Binepal, G.; Mabanglo, M.F.; Goodreid, J.D.; Leung, E.; Barghash, M.M.; Wong, K.S.; Lin, F.; Cossette, M.; Bansagi, J.; Song, B.; et al. Development of Antibiotics That Dysregulate the Neisserial ClpP Protease. ACS Infect. Dis. 2020, 6, 3224–3236. [Google Scholar] [CrossRef] [PubMed]
- Griffith, E.C.; Zhao, Y.; Singh, A.P.; Conlon, B.P.; Tangallapally, R.; Shadrick, W.R.; Liu, J.; Wallace, M.J.; Yang, L.; Elmore, J.M.; et al. Ureadepsipeptides as ClpP Activators. ACS Infect. Dis. 2019, 5, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Gronauer, T.F.; Mandl, M.M.; Lakemeyer, M.; Hackl, M.W.; Messner, M.; Korotkov, V.S.; Pachmayr, J.; Sieber, S.A. Design and synthesis of tailored human caseinolytic protease P inhibitors. Chem. Commun. 2018, 54, 9833–9836. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Mabanglo, M.F.; Seraphim, T.V.; Mollica, A.; Mao, Y.Q.; Rizzolo, K.; Leung, E.; Moutaoufik, M.T.; Hoell, L.; Phanse, S.; et al. Acyldepsipeptide Analogs Dysregulate Human Mitochondrial ClpP Protease Activity and Cause Apoptotic Cell Death. Cell Chem. Biol. 2018, 25, 1017–1030.e1019. [Google Scholar] [CrossRef] [PubMed]
- Graves, P.R.; Aponte-Collazo, L.J.; Fennell, E.M.J.; Graves, A.C.; Hale, A.E.; Dicheva, N.; Herring, L.E.; Gilbert, T.S.K.; East, M.P.; McDonald, I.M.; et al. Mitochondrial Protease ClpP is a Target for the Anticancer Compounds ONC201 and Related Analogues. ACS Chem. Biol. 2019, 14, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.; Wang, Z.; Coyaud, E.; Voisin, V.; Gronda, M.; Jitkova, Y.; Mattson, R.; Hurren, R.; Babovic, S.; Maclean, N.; et al. Inhibition of the Mitochondrial Protease ClpP as a Therapeutic Strategy for Human Acute Myeloid Leukemia. Cancer Cell 2015, 27, 864–876. [Google Scholar] [CrossRef]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef]
- Nouri, K.; Feng, Y.; Schimmer, A.D. Mitochondrial ClpP serine protease-biological function and emerging target for cancer therapy. Cell Death Dis. 2020, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Maurizi, M.R. Mitochondrial ClpP activity is required for cisplatin resistance in human cells. Biochim. Biophys. Acta 2016, 1862, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Roy, K.K. ClpP Peptidase as a Plausible Target for the Discovery of Novel Antibiotics. Curr. Drug Targets 2024, 25, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.L.; Yang, C.G. Structural insights into the evolutionary simplification of human ClpP activators. Structure 2023, 31, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, L.; Thomy, D.; Lakemeyer, M.; Westermann, L.M.; Ortega, J.; Sieber, S.A.; Sass, P.; Brotz-Oesterhelt, H. Antibiotic Acyldepsipeptides Stimulate the Streptomyces Clp-ATPase/ClpP Complex for Accelerated Proteolysis. mBio 2022, 13, e0141322. [Google Scholar] [CrossRef] [PubMed]
- Culp, E.J.; Sychantha, D.; Hobson, C.; Pawlowski, A.C.; Prehna, G.; Wright, G.D. ClpP inhibitors are produced by a widespread family of bacterial gene clusters. Nat. Microbiol. 2022, 7, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Mabanglo, M.F.; Houry, W.A. Recent structural insights into the mechanism of ClpP protease regulation by AAA+ chaperones and small molecules. J. Biol. Chem. 2022, 298, 101781. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, J.; Zarabi, S.F.; Davis, R.E.; Halgas, O.; Nii, T.; Jitkova, Y.; Zhao, R.; St-Germain, J.; Heese, L.E.; Egan, G.; et al. Mitochondrial ClpP-Mediated Proteolysis Induces Selective Cancer Cell Lethality. Cancer Cell 2019, 35, 721–737.e729. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Wong, K.S.; Zhou, J.L.; Mabanglo, M.F.; Batey, R.A.; Houry, W.A. The Role of ClpP Protease in Bacterial Pathogenesis and Human Diseases. ACS Chem. Biol. 2018, 13, 1413–1425. [Google Scholar] [CrossRef]
- Ripstein, Z.A.; Vahidi, S.; Houry, W.A.; Rubinstein, J.L.; Kay, L.E. A processive rotary mechanism couples substrate unfolding and proteolysis in the ClpXP degradation machinery. eLife 2020, 9, e52158. [Google Scholar] [CrossRef]
- Ghanbarpour, A.; Cohen, S.E.; Fei, X.; Kinman, L.F.; Bell, T.A.; Zhang, J.J.; Baker, T.A.; Davis, J.H.; Sauer, R.T. A closed translocation channel in the substrate-free AAA+ ClpXP protease diminishes rogue degradation. Nat. Commun. 2023, 14, 7281. [Google Scholar] [CrossRef]
- Kim, S.; Fei, X.; Sauer, R.T.; Baker, T.A. AAA+ protease-adaptor structures reveal altered conformations and ring specialization. Nat. Struct. Mol. Biol. 2022, 29, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.T.; Fei, X.; Bell, T.A.; Baker, T.A. Structure and function of ClpXP, a AAA+ proteolytic machine powered by probabilistic ATP hydrolysis. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chaudhary, A.K.; Woytash, J.; Inigo, J.R.; Gokhale, A.A.; Bshara, W.; Attwood, K.; Wang, J.; Spernyak, J.A.; Rath, E.; et al. A mitochondrial unfolded protein response inhibitor suppresses prostate cancer growth in mice via HSP60. J. Clin. Investig. 2022, 132, e149906. [Google Scholar] [CrossRef]
- Kenny, T.C.; Germain, D. From discovery of the CHOP axis and targeting ClpP to the identification of additional axes of the UPRmt driven by the estrogen receptor and SIRT3. J. Bioenerg. Biomembr. 2017, 49, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Al-Furoukh, N.; Ianni, A.; Nolte, H.; Holper, S.; Kruger, M.; Wanrooij, S.; Braun, T. ClpX stimulates the mitochondrial unfolded protein response (UPRmt) in mammalian cells. Biochim. Biophys. Acta 2015, 1853, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.M.; Yang, Y.; Blais, S.P.; Neubert, T.A.; Ron, D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol. Cell 2010, 37, 529–540. [Google Scholar] [CrossRef]
- Dai, C.Y.; Ng, C.C.; Hung, G.C.C.; Kirmes, I.; Hughes, L.A.; Du, Y.; Brosnan, C.A.; Ahier, A.; Hahn, A.; Haynes, C.M.; et al. ATFS-1 counteracts mitochondrial DNA damage by promoting repair over transcription. Nat. Cell Biol. 2023, 25, 1111–1120. [Google Scholar] [CrossRef]
- Haynes, C.M.; Hekimi, S. Mitochondrial dysfunction, aging, and the mitochondrial unfolded protein response in Caenorhabditis elegans. Genetics 2022, 222, iyac160. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).