Genome-Wide Identification and Analysis of APC E3 Ubiquitin Ligase Genes Family in Triticum aestivum
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of APC Genes Family in Wheat
2.2. Physicochemical Properties of APC Genes in Wheat
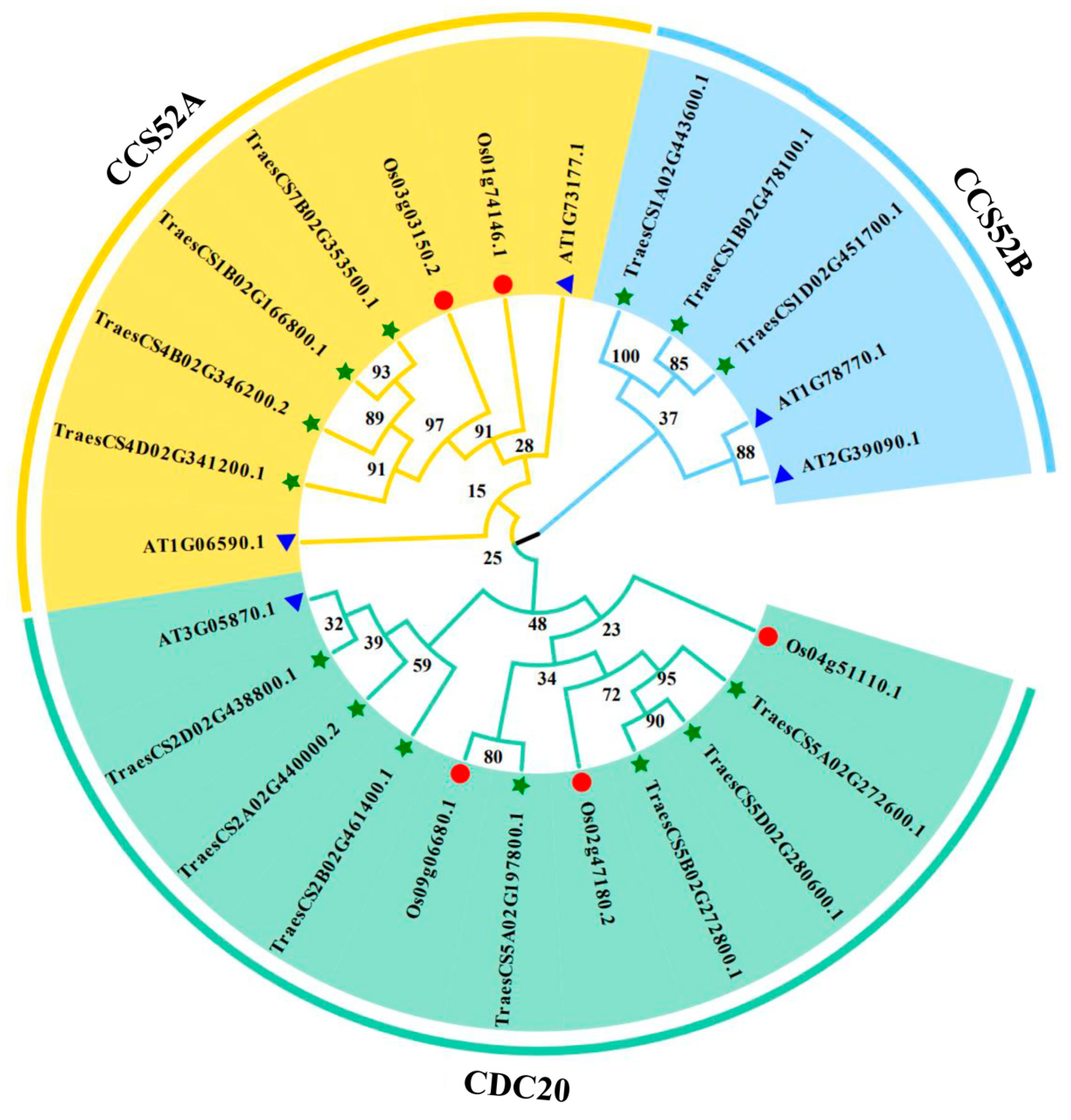
2.3. Multiple Sequence Alignment and Phylogenetic Tree Construction
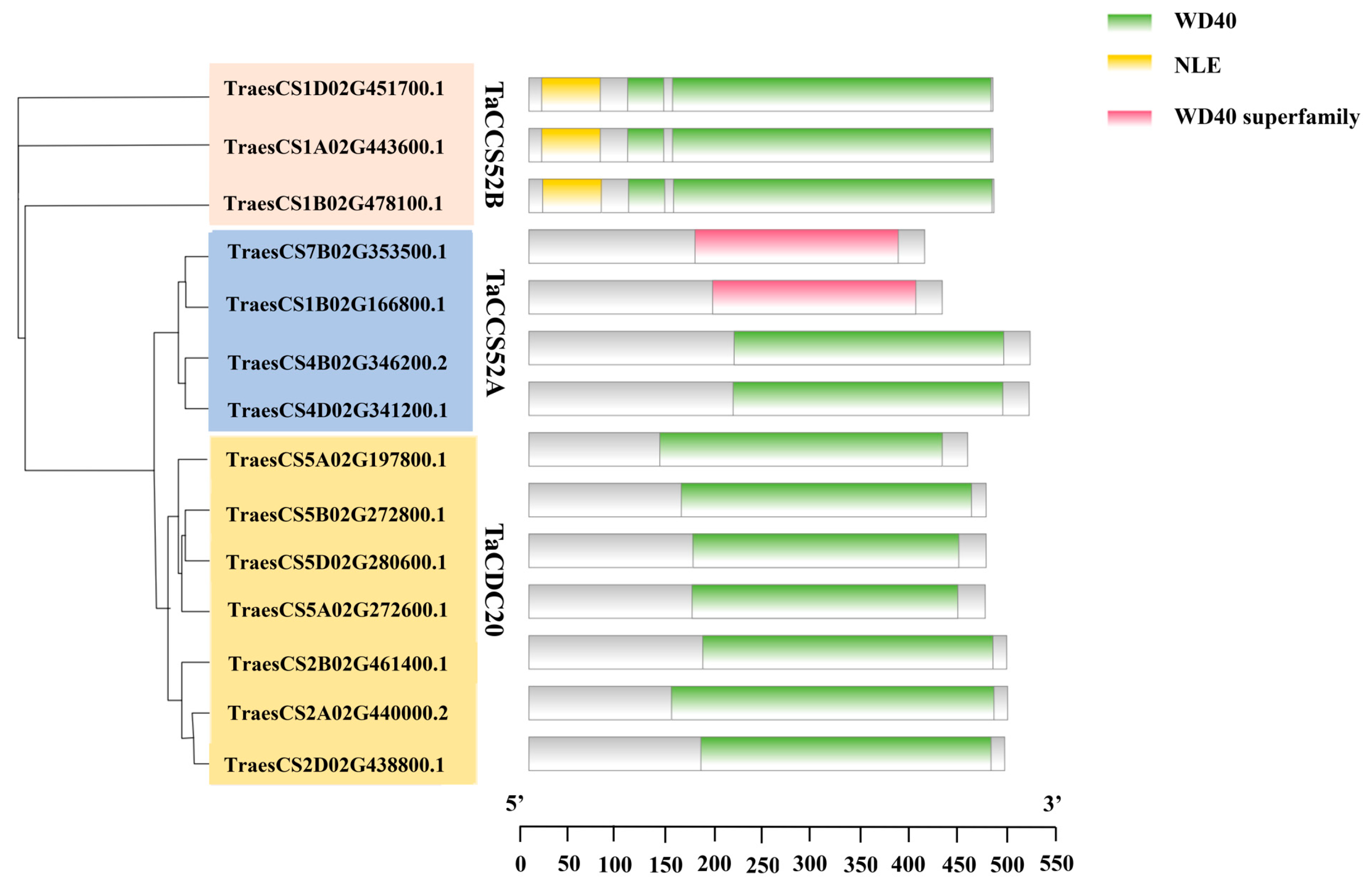
2.4. Gene Structure and Motif Analysis of APC Genes in Wheat
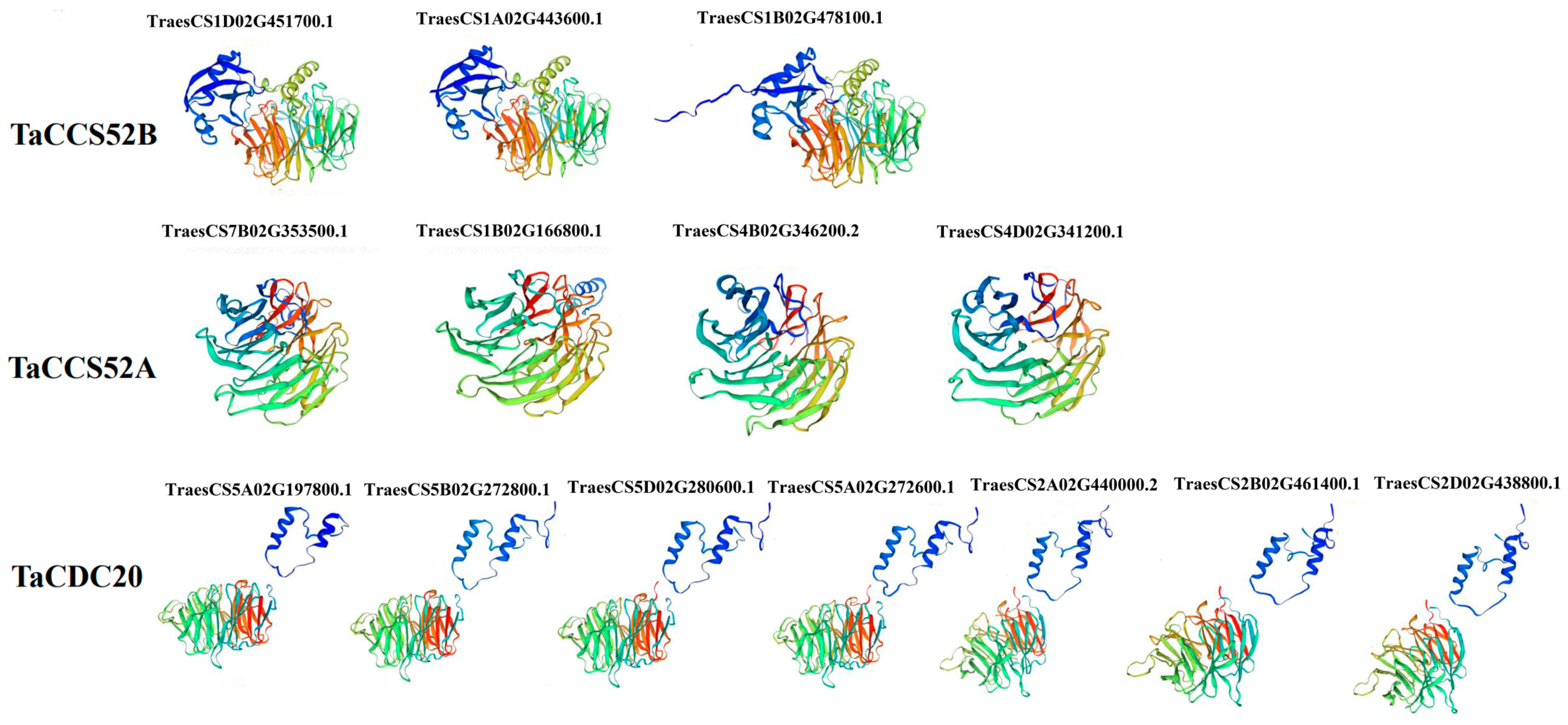
2.5. Predicting Protein Structure of APC Genes in Wheat
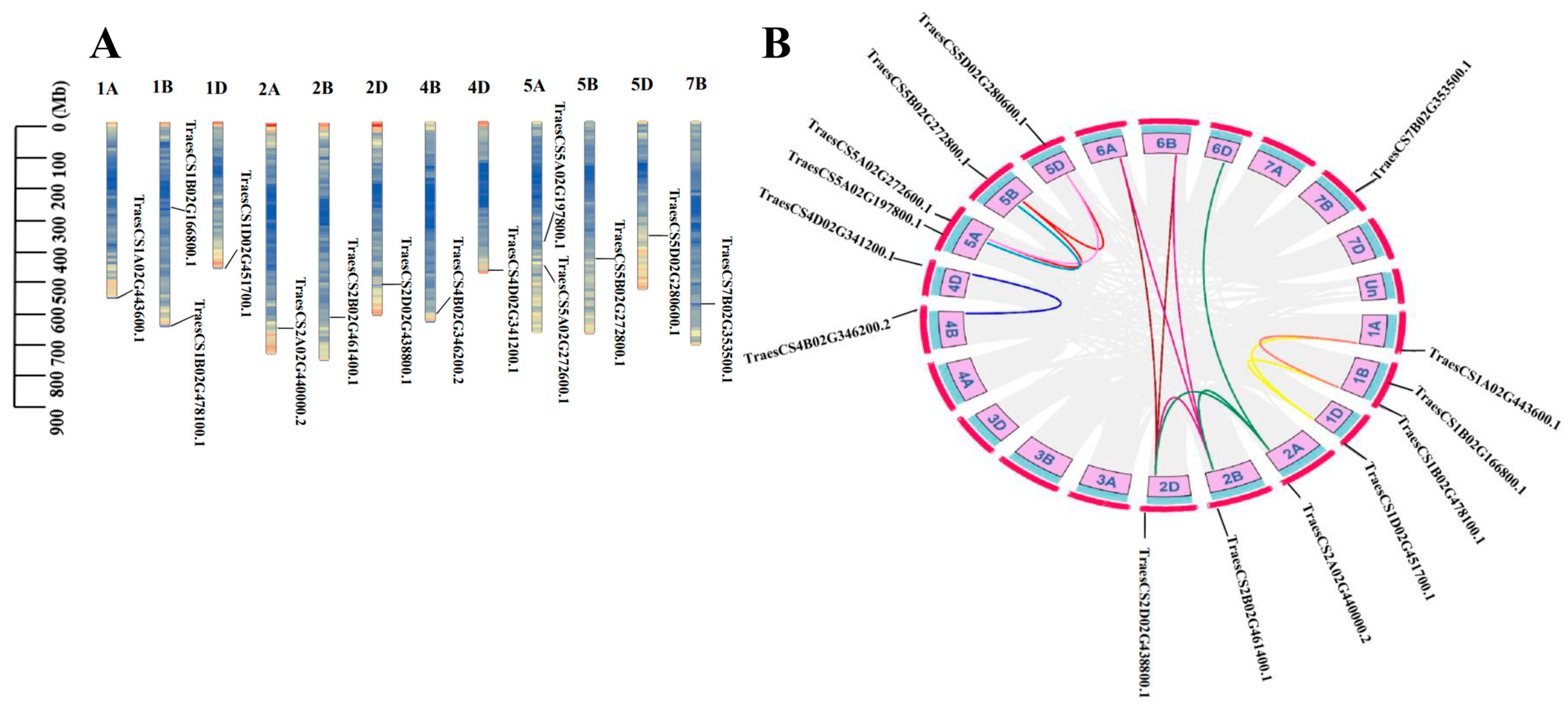
2.6. Chromosome Localization and Gene Duplication
2.7. Prediction of Cis-Acting Elements in the Promoter
2.8. Plant Materials, Growth Conditions, and Viral Inoculation
2.9. RNA Isolation and RT-qPCR Analysis
2.10. Analysis of APC Gene Expression in Wheat under MeJA Treatment
2.11. Expression Profiling of APC Genes in Different Wheat Tissues
2.12. BSMV-Induced Gene Silencing
3. Results
3.1. Identification and Analysis of APC Genes Family in T. aestivum
3.2. Phylogenetic Tree Analysis of APC Genes in Different Species
3.3. Structure of APC Genes and Motif Analysis in Wheat
3.4. Structural Model of APC Proteins in Wheat
3.5. Chromosomal Location and Duplication Events of APC Genes in Wheat
3.6. Prediction and Analysis of Cis-Acting Elements of APC Genes in Wheat
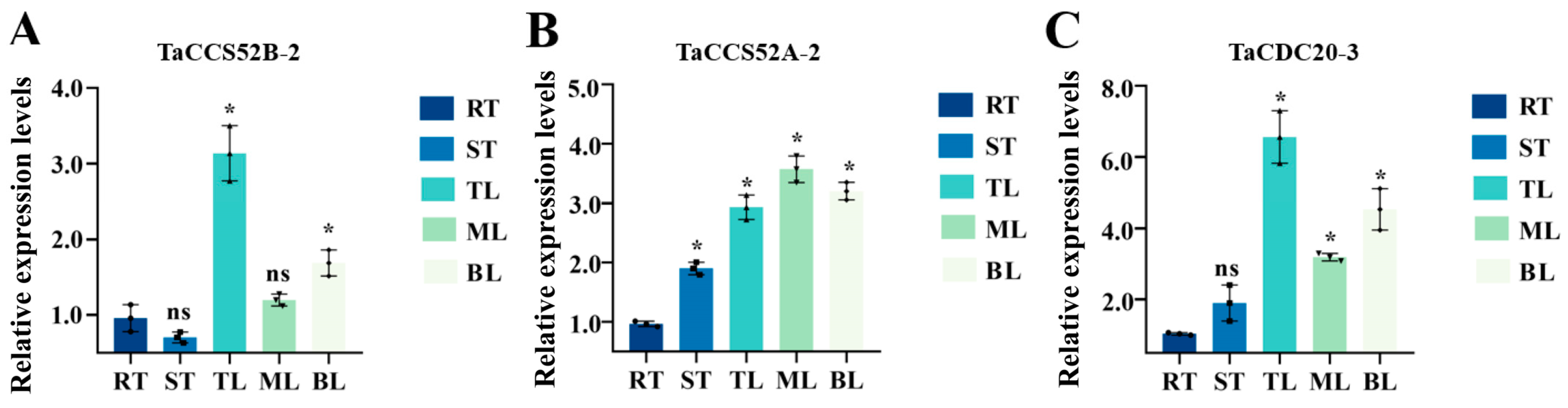
3.7. Tissue Specificity of APC Genes in Wheat
3.8. Expression Levels of APC Genes in Wheat under Different Stresses
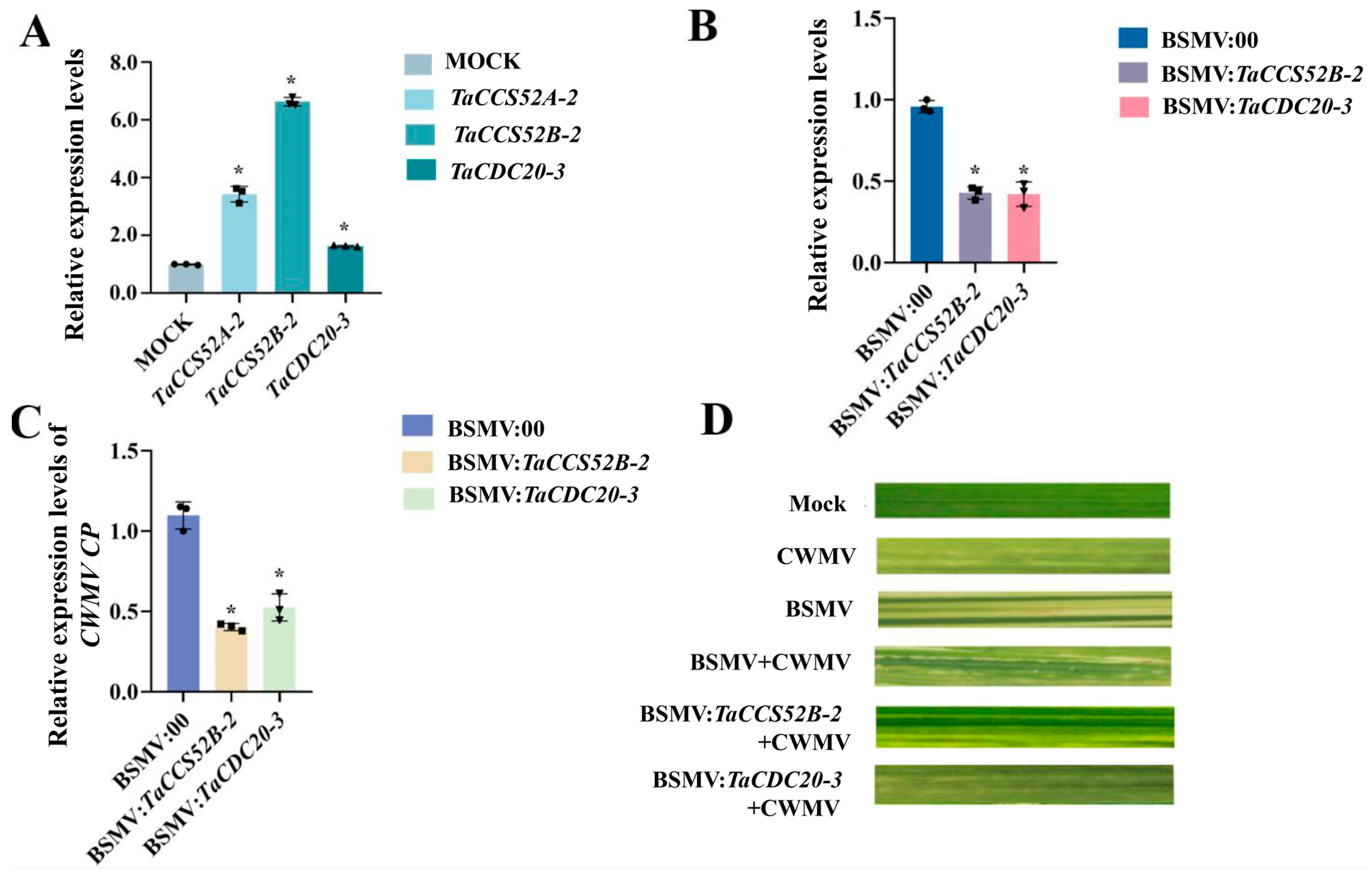
3.9. Functional Analysis of APC E3 Ubiquitin Ligases in Wheat during CWMV Infestation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramazi, S.; Allahverdi, A.; Zahiri, J. Evaluation of post-translational modifications in histone proteins: A review on histone modification defects in developmental and neurological disorders. J. Biosci. 2020, 45, 135. [Google Scholar] [CrossRef]
- Yin, J.; Yi, H.; Chen, X.; Wang, J. Post-Translational Modifications of Proteins Have Versatile Roles in Regulating Plant Immune Responses. Int. J. Mol. Sci. 2019, 20, 2807. [Google Scholar] [CrossRef] [PubMed]
- Barbour, H.; Nkwe, N.S.; Estavoyer, B.; Messmer, C.; Gushul-Leclaire, M.; Villot, R.; Uriarte, M.; Boulay, K.; Hlayhel, S.; Farhat, B.; et al. An inventory of crosstalk between ubiquitination and other post-translational modifications in orchestrating cellular processes. IScience 2023, 26, 106276. [Google Scholar] [CrossRef]
- Callis, J. The ubiquitination machinery of the ubiquitin system. TAB 2014, 12, e174. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Tang, D.; Wang, W. The Role of Ubiquitination in Plant Immunity: Fine-Tuning Immune Signaling and Beyond. Plant Cell Physiol. 2022, 63, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Hatakeyama, S. TRIM proteins and diseases. J. Biochem. 2017, 161, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; You, X.; Zhang, C.; Fang, H.; Wang, M.; Zhang, F.; Kang, H.; Xu, X.; Liu, Z.; Wang, J.; et al. An ORFeome of rice E3 ubiquitin ligases for global analysis of the ubiquitination interactome. Genome Biol. 2022, 23, 154. [Google Scholar] [CrossRef]
- Wang, J.; Jennings, A.K.; Kowalski, J.R. The Anaphase-Promoting Complex (APC) ubiquitin ligase affects chemosensory behavior in C. elegans. PeerJ 2016, 4, e2013. [Google Scholar] [CrossRef][Green Version]
- Peters, J.M. The anaphase-promoting complex: Proteolysis in mitosis and beyond. Mol. Cell 2002, 9, 931–943. [Google Scholar] [CrossRef]
- Aristarkhov, A.; Eytan, E.; Moghe, A.; Admon, A.; Hershko, A.; Ruderman, J.V. E2-C, a cyclin-selective ubiquitin carrier protein required for the destruction of mitotic cyclins. Proc. Natl. Acad. Sci. USA 1996, 93, 4294–4299. [Google Scholar] [CrossRef]
- Willems, A.; De Veylder, L. The Plant Anaphase-Promoting Complex/Cyclosome. Annu. Rev. Cell Dev. Biol. 2022, 38, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Saleme, M.; Andrade, I.R.; Eloy, N.B. The Role of Anaphase-Promoting Complex/Cyclosome (APC/C) in Plant Reproduction. Front. Plant Sci. 2021, 12, 642934. [Google Scholar] [CrossRef] [PubMed]
- Kimata, Y. APC/C Ubiquitin Ligase: Coupling Cellular Differentiation to G1/G0 Phase in Multicellular Systems. Trends Cell Biol. 2019, 29, 591–603. [Google Scholar] [CrossRef]
- Bao, Z.; Yang, H.; Hua, J. Perturbation of cell cycle regulation triggers plant immune response via activation of disease resistance genes. Proc. Natl. Acad. Sci. USA 2013, 110, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Kudo, C.; Suzuki, T.; Fukuoka, S.; Asai, S.; Suenaga, H.; Sasabe, M.; Takano, Y.; Okuno, T.; Toyoda, K.; Shiraishi, T.; et al. Suppression of Cdc27B expression induces plant defence responses. Mol. Plant Pathol. 2007, 8, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Hua, J. Linking the Cell Cycle with Innate Immunity in Arabidopsis. Mol. Plant 2015, 8, 980–982. [Google Scholar] [CrossRef]
- Sinha, A.; Singh, L.; Rawat, N. Current understanding of atypical resistance against fungal pathogens in wheat. Curr. Opin. Plant Biol. 2022, 68, 102247. [Google Scholar] [CrossRef]
- Sanfacon, H. Grand Challenge in Plant Virology: Understanding the Impact of Plant Viruses in Model Plants, in Agricultural Crops, and in Complex Ecosystems. Front. Microbiol. 2017, 8, 860. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, S.L.; Zhang, H.M.; Liu, X.-Y.; Li, J.; Li, J.-M.; Chen, J.-P. Analysis of small RNAs derived from Chinese wheat mosaic virus. Arch. Virol. 2014, 159, 3077–3082. [Google Scholar] [CrossRef]
- Xu, M.; Jin, P.; Liu, T.; Gao, S.; Zhang, T.; Zhang, F.; Han, X.; He, L.; Chen, J.; Yang, J. Genome-wide identification and characterization of UBP gene family in wheat (Triticum aestivum L.). PeerJ 2021, 9, e11594. [Google Scholar] [CrossRef]
- Xu, X.; Gao, P.; Zhu, X.; Guo, W.; Ding, J.; Li, C. Estimating the responses of winter wheat yields to moisture variations in the past 35 years in Jiangsu Province of China. PLoS ONE 2018, 13, e191217. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Bolser, D.M.; Kerhornou, A.; Walts, B.; Kersey, P. Triticeae resources in Ensembl Plants. Plant Cell Physiol. 2015, 56, e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; A Yamashita, R.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, A.; Yu, X.; Dong, Z.; Zhao, H.; Xue, H.; Wu, W. Differential expression analysis of the SRB1 gene in fluconazole-resistant and susceptible strains of Candida albicans. J. Antibiot. 2020, 73, 309–313. [Google Scholar] [CrossRef]
- Nystrom, S.L.; Mckay, D.J. Memes: A motif analysis environment in R using tools from the MEME Suite. PLoS Comput. Biol. 2021, 17, e1008991. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Liu, P.; Shi, C.; Liu, S.; Lei, J.; Lu, Q.; Hu, H.; Ren, Y.; Zhang, N.; Sun, C.; Chen, L.; et al. A papain-like cysteine protease-released small signal peptide confers wheat resistance to wheat yellow mosaic virus. Nat. Commun. 2023, 14, 7773. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Han, J.; Wang, E.; Xiao, J.; Hu, R.; Yang, G.; He, G. Genome-Wide Identification and Homoeologous Expression Analysis of PP2C Genes in Wheat (Triticum aestivum L.). Front. Genet. 2019, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wan, L.; Dai, X.; Sun, Y.; Wei, W. Functional characterization of Anaphase Promoting Complex/Cyclosome (APC/C) E3 ubiquitin ligases in tumorigenesis. Biochim. Biophys. Acta 2014, 1845, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, K.; Thomann, A.; Parmentier, Y.; Genschik, P.; Criqui, M.C. The APC/C E3 ligase remains active in most post-mitotic Arabidopsis cells and is required for proper vasculature development and organization. Development 2009, 136, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Gao, S.; Cui, X.; Wang, L.; Yan, S. APC/CCDC20 targets SCFFBL17 to activate replication stress responses in Arabidopsis. Plant Cell 2023, 35, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, Y.; Yu, Y.; Duan, J.; Liao, Z.; Xiong, G.; Meng, X.; Liu, G.; Qian, Q.; Li, J. Degradation of MONOCULM 1 by APC/C(TAD1) regulates rice tillering. Nat. Commun. 2012, 3, 750. [Google Scholar] [CrossRef]
- Lima, M.F.; Eloy, N.B.; Pegoraro, C.; Sagit, R.; Rojas, C.; Bretz, T.; Vargas, L.; Elofsson, A.; de Oliveira, A.C.; Hemerly, A.S. Genomic evolution and complexity of the Anaphase-promoting Complex (APC) in land plants. BMC Plant Biol. 2010, 10, 254. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Zhang, K.; Yu, Q.; Jiang, X.; Cheng, C. Genome-wide identification and expression analysis of anaphase promoting complex/cyclosome (APC/C) in rose. Int. J. Biol. Macromol. 2022, 223, 1604–1618. [Google Scholar] [CrossRef]
- Jain, B.P.; Pandey, S. WD40 Repeat Proteins: Signalling Scaffold with Diverse Functions. Protein J. 2018, 37, 391–406. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Zhang, X.; Li, Z.; Zhao, Y.; Lohaus, R.; Chang, X.; Dong, W.; Ho, S.Y.W.; Liu, X.; et al. The water lily genome and the early evolution of flowering plants. Nature 2020, 577, 79–84. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, R.; Zhou, Y.; Jiao, Y. Evolutionary strategies drive a balance of the interacting gene products for the CBL and CIPK gene families. New Phytol. 2020, 226, 1506–1516. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, Z.; Wu, F.; Feng, M.; Sun, Y.; Chen, W.; Cheng, Z.; Zhang, X.; Ren, Y.; Lei, C. The APC/C(TE) E3 Ubiquitin Ligase Complex Mediates the Antagonistic Regulation of Root Growth and Tillering by ABA and GA. Plant Cell 2020, 32, 1973–1987. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Gimenez-Ibanez, S.; Boter, M.; Solano, R. Novel players fine-tune plant trade-offs. Essays Biochem. 2015, 58, 83–100. [Google Scholar]
- Zhang, Y.; Pennerman, K.K.; Yang, F.; Yin, G. Maize MeJA-responsive proteins identified by high-resolution 2-DE PAGE. Data Brief. 2015, 5, 129–133. [Google Scholar] [CrossRef]
- Chico, J.M.; Lechner, E.; Fernandez-Barbero, G.; Canibano, E.; García-Casado, G.; Franco-Zorrilla, J.M.; Hammann, P.; Zamarreño, A.M.; García-Mina, J.M.; Rubio, V. CUL3(BPM) E3 ubiquitin ligases regulate MYC2, MYC3, and MYC4 stability and JA responses. Proc. Natl. Acad. Sci. USA 2020, 117, 6205–6215. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, L.; Sun, Y.; Zhang, J.; Chen, Y.; Wang, X.; Liu, L. Transcriptome analysis of brassinolide under low temperature stress in winter wheat. AoB Plants 2023, 15, d5. [Google Scholar] [CrossRef]
- Ji, W.; Hu, X.; Kang, M.; Qiu, X.; Liu, B.; Tang, L.; Zhu, Y.; Cao, W.; Liu, L. Effects of pre-anthesis low-temperature stress on the mineral components in wheat grains. Front. Plant Sci. 2023, 14, 1221466. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pruitt, R.N.; Nurnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Zebell, S.G.; Dong, X. Cell-Cycle Regulators and Cell Death in Immunity. Cell Host Microbe 2015, 18, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Gonzalez, R.; Elena, S.F. Host developmental stages shape the evolution of a plant RNA virus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220005. [Google Scholar] [CrossRef] [PubMed]



| Group | Gene ID | Exons | Gene Location | CDS Length (bp) | Size (aa) | MW (kDa) | PI |
|---|---|---|---|---|---|---|---|
| TaCDC20 | TraesCS2A02G440000.2 | 4 | 2A:690927389-690930130 | 2427 | 490 | 54134.09 | 8.23 |
| TaCDC20 | TraesCS2B02G461400.1 | 5 | 2B:655009903-655012488 | 2169 | 489 | 53906.73 | 8.81 |
| TaCDC20 | TraesCS2D02G438800.1 | 5 | 2D:548080286-548082886 | 2189 | 487 | 53485.13 | 8.80 |
| TaCDC20 | TraesCS5A02G197800.1 | 3 | 5A:401758452-401763369 | 1782 | 449 | 50176.22 | 7.98 |
| TaCDC20 | TraesCS5A02G272600.1 | 5 | 5A:482315663-482317456 | 1404 | 467 | 51151.27 | 7.22 |
| TaCDC20 | TraesCS5B02G272800.1 | 5 | 5B:458385818-458388103 | 1907 | 468 | 51338.56 | 7.23 |
| TaCDC20 | TraesCS5D02G280600.1 | 5 | 5D:381978841-381981151 | 1939 | 468 | 51342.49 | 7.68 |
| TaCCS52A | TraesCS4B02G346200.2 | 10 | 4B:640006920-640011111 | 2178 | 513 | 54725.21 | 9.29 |
| TaCCS52A | TraesCS4D02G341200.1 | 10 | 4D:498031682-498035987 | 2304 | 512 | 54685.17 | 9.29 |
| TaCCS52A | TraesCS7B02G353500.1 | 8 | 7B:611443510-611458578 | 1218 | 405 | 43507.27 | 9.24 |
| TaCCS52A | TraesCS1B02G166800.1 | 9 | 1B:294926224-294966206 | 1272 | 423 | 46023.17 | 8.91 |
| TaCCS52B | TraesCS1D02G451700.1 | 12 | 1D:493705055-493710507 | 1799 | 475 | 52278.23 | 8.03 |
| TaCCS52B | TraesCS1A02G443600.1 | 12 | 1A:591948406-591953951 | 2037 | 475 | 52280.25 | 8.29 |
| TaCCS52B | TraesCS1B02G478100.1 | 12 | 1B:686749388-686755409 | 1884 | 476 | 52349.36 | 8.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, T.; Tu, A.; Xie, H.; Hu, H.; Chen, J.; Yang, J. Genome-Wide Identification and Analysis of APC E3 Ubiquitin Ligase Genes Family in Triticum aestivum. Genes 2024, 15, 271. https://doi.org/10.3390/genes15030271
Wang J, Zhang T, Tu A, Xie H, Hu H, Chen J, Yang J. Genome-Wide Identification and Analysis of APC E3 Ubiquitin Ligase Genes Family in Triticum aestivum. Genes. 2024; 15(3):271. https://doi.org/10.3390/genes15030271
Chicago/Turabian StyleWang, Jinnan, Tianye Zhang, Aizhu Tu, Haoxin Xie, Haichao Hu, Jianping Chen, and Jian Yang. 2024. "Genome-Wide Identification and Analysis of APC E3 Ubiquitin Ligase Genes Family in Triticum aestivum" Genes 15, no. 3: 271. https://doi.org/10.3390/genes15030271
APA StyleWang, J., Zhang, T., Tu, A., Xie, H., Hu, H., Chen, J., & Yang, J. (2024). Genome-Wide Identification and Analysis of APC E3 Ubiquitin Ligase Genes Family in Triticum aestivum. Genes, 15(3), 271. https://doi.org/10.3390/genes15030271






