Transcriptomic Insights and Cytochrome P450 Gene Analysis in Kadsura coccinea for Lignan Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Transcriptome Library Construction and Sequencing
2.3. Transcriptome Data Assembly and Gene Annotation
2.4. Screening and Phylogenetic Tree Construction of CYP Genes in K. coccinea
2.5. Screening of Candidate CYP Genes Related to Lignan Biosynthesis in K. coccinea
2.6. Quantitative Real-Time PCR Analysis
3. Results
3.1. High-Quality Transcriptome Sequencing and Assembly of K. coccinea
3.2. Annotation of Unigenes in K. coccinea with Multi-Step Bioinformatics Tools
3.3. Elucidating the Molecular Functions and Metabolic Pathways in K. coccinea through Gene Ontology and KEGG Analysis
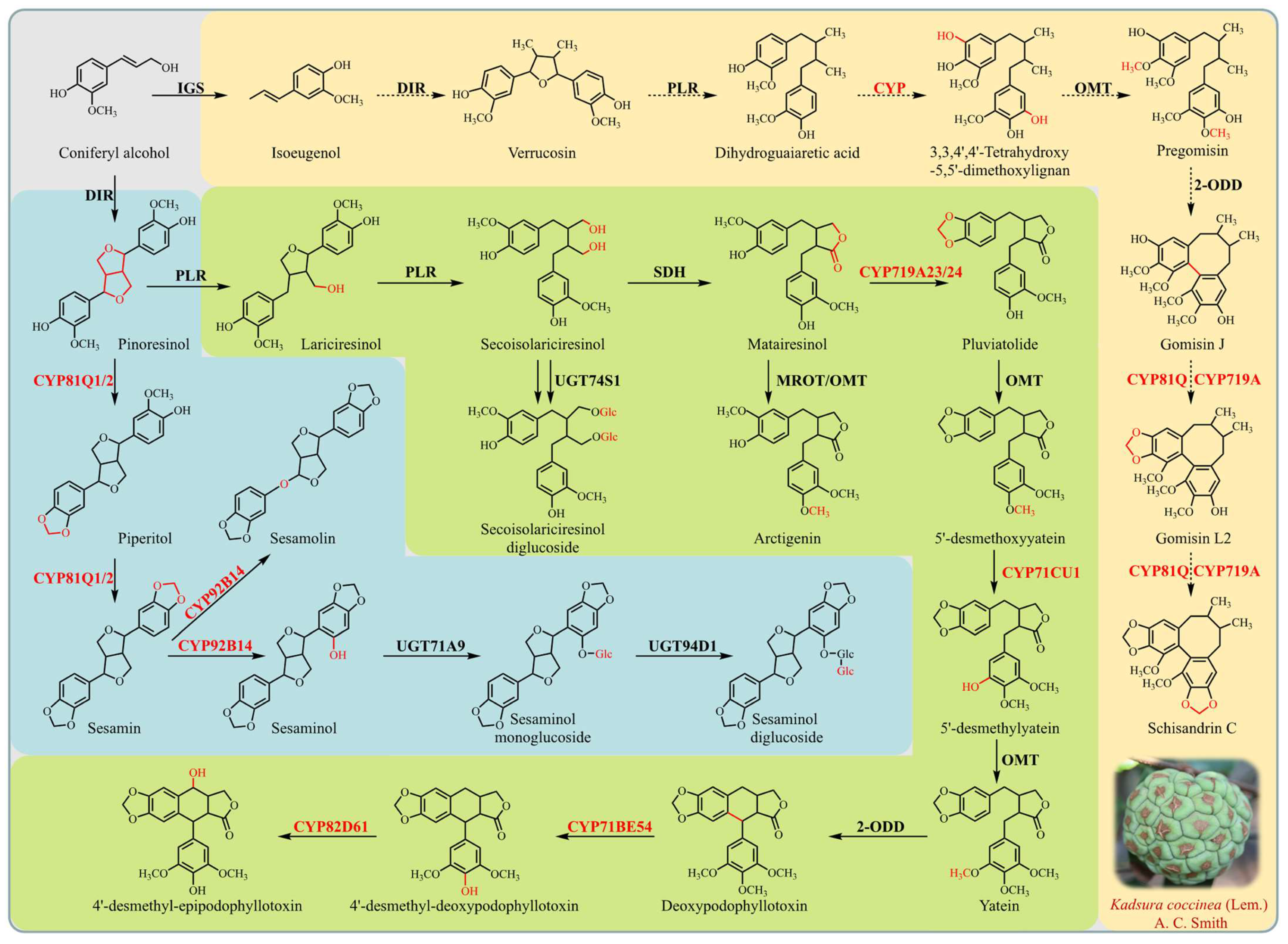
3.4. Elucidating the Role of Cytochrome P450 Genes in Lignan Biosynthesis of K. coccinea
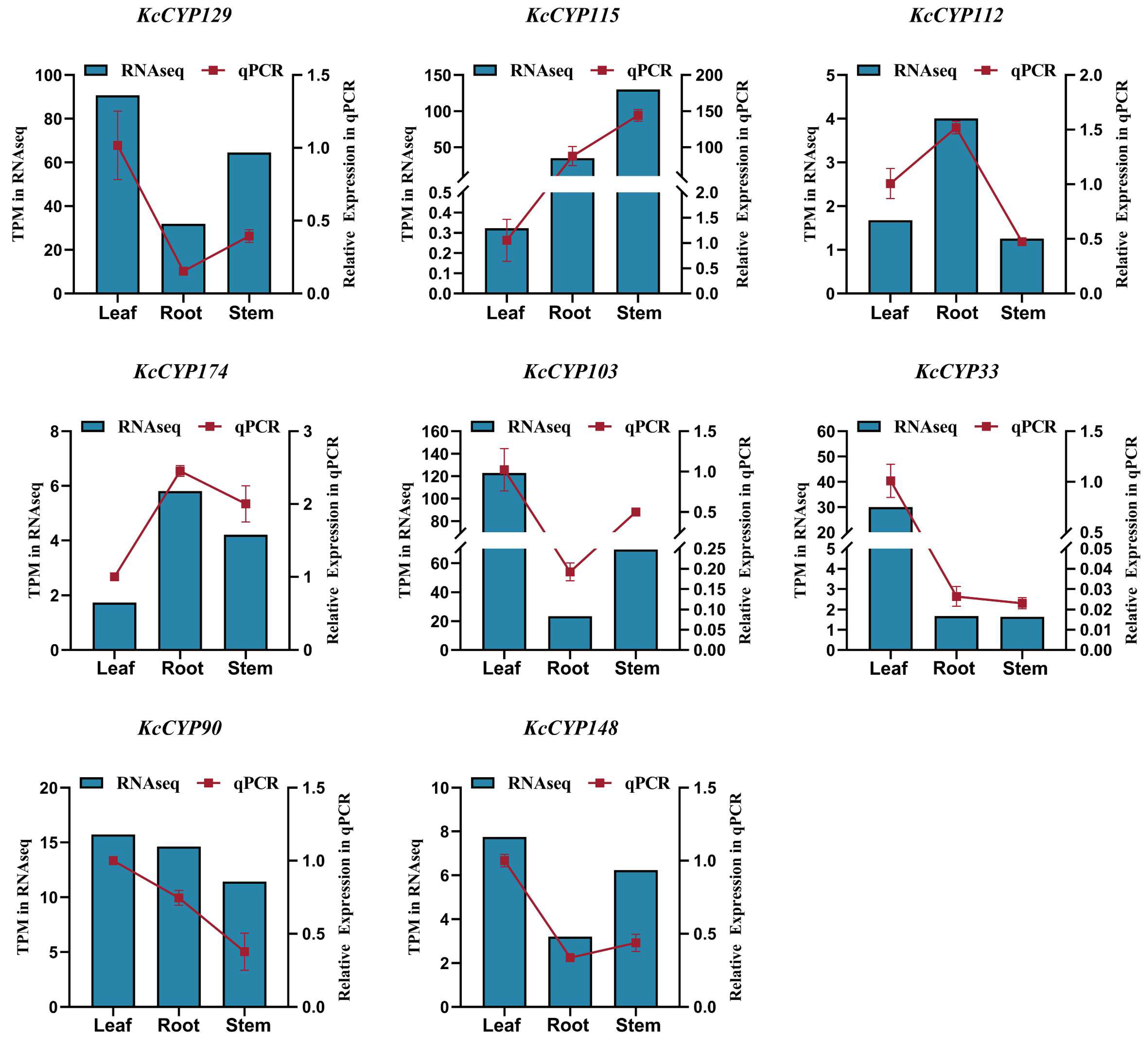
3.5. Validation of RNA-Seq Date by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.Z.; Yang, Y.P.; Cheng, S.W.; Cao, L.; Xie, Q.L.; Wang, M.Y.; Li, B.; Jian, Y.Q.; Liu, B.; Peng, C.Y.; et al. Heilaohuguosus A-S from the fruits of Kadsura coccinea and their hepatoprotective activity. Phytochemistry 2021, 184, 112678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Jian, Y.Q.; Cheng, S.W.; Jia, Y.Z.; Liu, Y.B.; Yu, H.H.; Cao, L.; Li, B.; Peng, C.Y.; Choudhary, M.I. Dibenzocyclooctadiene ligan from Kadsura coccinea alleviate APAP-induced hepatotoxicity via oxidative stress inhibition and activating the Nrf2 pathway in vitro. Bioorg. Chem. 2021, 115, 105277. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Shao, Y.H.; Zhu, Z.P.; Yin, X.X.; Shen, B.Y.; Chen, C.; Xu, Y.F.; Shen, J.J.; Li, H.F.; Li, X.N. Systematically identifying the hepatoprotective ingredients of schisandra lignan extract from pharmacokinetic and pharmacodynamic perspectives. Phytomedicine 2019, 53, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Y.; Xu, Z.X.; Qiao, Z.X.; Wang, X.; Yang, C. Flaxseed lignan alleviates the paracetamol-induced hepatotoxicity associated with regulation of gut microbiota and serum metabolome. Nutrients 2024, 16, 295. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, Z.C.; Sun, J.; Zhuo, S.Y.; Fan, R.; Le, N.; Yao, J.Y.; Long, X. Preliminary study on antibacterial activity of Kadsura coccinea peel in vitro. Lishizhen Med. Mat. Med. Res. 2011, 22, 822–824. [Google Scholar]
- Tai, B.H.; Yen, P.H.; Hoang, N.; Huong, P.T.T.; Dũng, N.V.; Thanh, B.V.; Cuong, N.T.; Bang, N.A.; Nhiem, N.X.; Kiem, P.V. New dibenzocyclooctadiene lignans from Kadsura induta with their anti-inflammatory activity. RSC Adv. 2022, 12, 25433–25439. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, S.; Yang, Y.; Liu, B.; Choudhary, M.I.; Wang, W. Cytotoxicity of Schisandronic Acid from Kadsura coccinea by Activation of Caspase-3, Cleavage of poly-ADP Ribose Polymerase, and Reduction of Oxidative Stress. Rev. Bras. Farmacogn. 2021, 31, 51–58. [Google Scholar] [CrossRef]
- Liang, C.Q.; Shi, Y.M.; Luo, R.H.; Li, X.Y.; Gao, Z.H.; Li, X.N.; Yang, L.M.; Shang, S.Z.; Li, Y.; Zheng, Y.T.; et al. Kadcoccitones A and B, two new 6/6/5/5-fused tetracyclic triterpenoids from Kadsura coccinea. Org. Lett. 2012, 14, 63626365. [Google Scholar] [CrossRef]
- Li, Y.J.; Huang, G.B.; Quan, H.Y.; Yi, Q.; Tan, M.X. Study on extraction technology and antioxidant activity of total flavonoids from Kadsura coccinea leave. Guihaia 2020, 40, 1381–1388. [Google Scholar]
- Su, H.; He, F.; Wei, G.N.; Nong, Z.H.; Zeng, X.B.; Yang, H.C. Effects of Kadsura coccinea extract on blood clotting time and thrombosis in mice. Pharm. Res. 2017, 36, 565–566. [Google Scholar]
- Yang, Y.P.; Hussain, N.; Zhang, L.; Jia, Y.Z.; Jian, Y.Q.; Li, B.; Choudhary, M.I.; Rahman, A.; Wang, W. Kadsura coccinea: A rich source of structurally diverse and biologically important compounds. Chin. Herb. Med. 2020, 12, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kulik, T.; Buśko, M.; Pszczółkowska, A.; Perkowski, J.; Okorski, A. Plant lignans inhibit growth and trichothecene biosynthesis in Fusarium graminearum. Lett. Appl. Microbiol. 2014, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.L.; Wang, X.N.; Yang, Y.T.; Fang, G.; Pang, Y.Z. Current situation of Zhuang medicine Kadsura coccinea on bioactivity. Chin. J. Clin. Pharm. 2019, 35, 1075–1079. [Google Scholar]
- Lu, J.; Liu, R.R.; Zhao, X.M.; Zhang, X.Y.; Qiu, J.C.; Gong, S.N. Research progress on active components and physiological activities of Kadsura coccinea. Food Res. Devel. 2018, 39, 219–224. [Google Scholar]
- Kim, S.J.; Kim, M.R.; Bedgar, D.L.; Moinuddin, S.G.; Cardenas, C.L.; Davin, L.B.; Kang, C.H.; Lewis, N.G. Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.V.; Kim, K.W.; Lee, C.; Costa, M.A.; May, G.D.; Crow, J.A.; Davin, L.B.; Lewis, N.G. Next generation sequencing in predicting gene function in podophyllotoxin biosynthesis. J. Biol. Chem. 2013, 288, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.Q.; Costa, M.A.; Pélissier, H.C.; Davin, L.B.; Lewis, N.G. Secoisolariciresinol dehydrogenase purification, cloning, and functional expression. Implications for human health protection. J. Biol. Chem. 2001, 276, 276. [Google Scholar] [CrossRef]
- Satake, H.; Ono, E.; Murata, J. Recent advances in the metabolic engineering of lignan biosynthesis pathways for the production of transgenic plant: Based foods and supplements. J. Agric. Food Chem. 2013, 61, 11721–11729. [Google Scholar] [CrossRef]
- Behr, M.; Sergeant, K.; Leclercq, C.C.; Planchon, S.; Guignard, C.; Lenouvel, A.; Renaut, J.; Hausman, J.F.; Lutts, S.; Guerriero, G. Insights into the molecular regulation of monolignol-derived product biosynthesis in the growing hemp hypocotyl. BMC Plant Biol. 2018, 18, 1. [Google Scholar] [CrossRef]
- Dar, A.A.; Arumugam, N. Lignans of sesame: Purification methods, biological activities and biosynthesis—A review. Bioorg. Chem. 2013, 50, 1–10. [Google Scholar] [CrossRef]
- Kim, H.J.; Ono, E.; Morimoto, K.; Yamagaki, T.; Okazawa, A.; Kobayashi, A.; Satake, H. Metabolic engineering of lignan biosynthesis in Forsythia cell culture. Plant Cell Physiol. 2009, 50, 2200–2209. [Google Scholar] [CrossRef]
- Satake, H.; Koyama, T.; Bahabadi, S.E.; Matsumoto, E.; Ono, E.; Murata, J. Essences in metabolic engineering of lignan biosynthesis. Metabolites 2015, 5, 270–290. [Google Scholar] [CrossRef] [PubMed]
- Ražná, K.; Harenčár, Ľ.; Kučka, M. The Involvement of microRNAs in Plant Lignan Biosynthesis—Current View. Cells 2022, 11, 2151. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Ohta, D. Diversification of P450 Genes During Land Plant Evolution. Annu. Rev. Plant Biol. 2010, 61, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Ehlting, J.; Hamberger, B.; Million-Rousseau, R.; Werck-Reichhart, D. Cytochromes P450 in phenolic metabolism. Phytoch. Rev. 2006, 5, 239–270. [Google Scholar] [CrossRef]
- Lau, W.; Sattely, E. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Nakai, M.; Fukui, Y.; Tomimori, N.; Fukuchi-Mizutani, M.; Saito, M.; Satake, H.; Tanaka, T.; Katsuta, M.; Umezawa, T.; et al. Formation of two methylenedioxy bridges by a Sesamum CYP81Q protein yielding a furofuran lignan, (+)-sesamin. Proc. Natl. Acad. Sci. USA 2006, 103, 10116–10121. [Google Scholar] [CrossRef]
- Murata, J.; Ono, E.; Yoroizuka, S.; Toyonaga, H.; Shiraishi, A.; Mori, S.; Tera, M.; Azuma, T.; Nagano, A.J.; Nakayasu, M.; et al. Oxidative rearrangement of (+)-sesamin by CYP92B14 co-generates twin dietary lignans in sesame. Nat. Commun. 2017, 8, 2155. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T. Diversity in lignan biosynthesis. Phytoch. Rev. 2003, 2, 371–390. [Google Scholar] [CrossRef]
- Liang, Z.; Li, X.; Li, P.; Deng, Y.; Zhong, Y.; Yang, H. Kadsura coccinea Lignan Metabolism Based on Metabolome and Transcriptome Analysis. J. Oncol. 2022, 2022, 3152155. [Google Scholar] [CrossRef]
- Liu, J.S.; Li, L. Kadsulignans L-N, three dibenzocyclooctadiene lignans from Kadsura coccinea. Phytochemistry 1995, 38, 241–245. [Google Scholar] [CrossRef]
- Fang, L.; Xie, C.; Wang, H.; Jin, D.Q.; Xu, J.; Guo, Y.; Ma, Y. Lignans from the roots of Kadsura coccinea and their inhibitory activities on LPS-induced NO production. Phytochem. Lett. 2014, 9, 158–162. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Reuter, K.; Drost, H.G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Chang, H.Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro protein families and domains database: 20 years on. Nucleic. Acids. Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 2020, 36, 2251–2252. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Liu, Y.B.; Yang, Y.B.; Tasneem, S.; Hussain, N.; Daniyal, M.; Yuan, H.W.; Xie, Q.L.; Liu, B.; Sun, J.; Jian, Y.Q.; et al. Lignans from tujia ethnomedicine Heilaohu: Chemical characterization and evaluation of their cytotoxicity and antioxidant activities. Molecules 2018, 23, 2147. [Google Scholar] [CrossRef]
- Zhang, D.D.; Xu, H.N.; Wang, W.; Li, X.Z.; Li, Y.Z.; Jiang, Y.; Song, X.M.; Deng, C. Traditional use, pharmacology and toxicology of the lignans in genus Kadsura: A Systematic review. Rec. Nat. Prod. 2023, 10, 1–52. [Google Scholar] [CrossRef]
- Ouyang, D.; Wang, L.C.; Tang, T.; Feng, H. Genomic-Wide Identification and Characterization of the Uridine Diphosphate Glycosyltransferase Family in Eucommia ulmoides Oliver. Plants 2021, 10, 1934. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Xu, Z.; Song, X.; Huang, K.; Li, Y.; Wei, J.; Zhu, X.; Ren, H.; Sun, C. Hybrid Sequencing of Full-Length cDNA Transcripts of the Medicinal Plant Scutellaria baicalensis. Int. J. Mol. Sci. 2019, 20, 4426. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Li, G.; Zhang, A.; Liu, X.; Zhao, M. Transcriptome and metabolome profiling of the medicinal plant Veratrum mengtzeanum reveal key components of the alkaloid biosynthesis. Front Genet. 2023, 14, 1023433. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D. Progress in tracing the evolutionary paths of cytochrome P450. Biochim. Biophys. Acta 2011, 1814, 14–18. [Google Scholar] [CrossRef]
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194211. [Google Scholar] [CrossRef] [PubMed]
- Tian, X. Study on the Catalytic and Regulatory Functions of a Cytochrome P450 in the Gossypol Synthesis Pathway. Ph.D. Thesis, Nanjing University, Nanjing, China, 2018. [Google Scholar]
- Hu, Y.; Di, P.; Chen, J.; Xiao, Y.; Zhang, L.; Chen, W. Isolation and characterization of a gene encoding cinnamoyl-CoA reductase from Isatis indigotica Fort. Mol. Biol. Rep. 2011, 38, 2075–2083. [Google Scholar] [CrossRef]
- Schoch, G.; Goepfert, S.; Morant, M.; Hehn, A.; Meyer, D.; Ullmann, P.; Werck-Reichhart, D. CYP98A3 from Arabidopsis thaliana is a 3′- hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J. Biol. Chem. 2001, 276, 36566–36574. [Google Scholar] [CrossRef]
- Mao, Y.; Ma, Y.; Chen, T.; Ma, X.; Xu, Y.; Bu, J.; Li, Q.; Jin, B.; Wang, Y.; Li, Y.; et al. Functional Integration of Two CYP450 Genes Involved in Biosynthesis of Tanshinones for Improved Diterpenoid Production by Synthetic Biology. ACS Synth. Biol. 2020, 9, 1763–1770. [Google Scholar] [CrossRef]
- Awasthi, P.; Gupta, A.P.; Bedi, Y.S.; Vishwakarma, R.A.; Gandhi, S.G. Mannitol Stress Directs Flavonoid Metabolism toward Synthesis of Flavones via Differential Regulation of Two Cytochrome P450 Monooxygenases in Coleus forskohlii. Front. Plant Sci. 2016, 7, 985. [Google Scholar] [CrossRef]
- Kochetkov, N.K.; Khorlin, A.; Chizhov, O.S.; Sheichenko, V.I. Schizandrin-lignan of unusual structure. Tetrahedron Lett. 1961, 2, 730–734. [Google Scholar] [CrossRef]
- Guo, X.; Lu, W.; Li, S.Z.; Lin, X.J.; Li, X.B. Analysis and comprehensive evaluation of total lignans in rhizome of Kadsura coccinea from different provenances. Trop. For. 2020, 48, 810. [Google Scholar]
- Chen, C.Y.; Liu, S.Y.; Yan, Y.; Yin, L.; Di, P.; Liu, H.M.; Liu, H.Z. Candidate genes involved in the biosynthesis of lignan in Schisandra chinensis fruit based on transcriptome and metabolomes analysis. Chin. J. Nat. Med. 2020, 18, 684–695. [Google Scholar] [CrossRef]
- Qiang, T.Y.; Liu, J.S.; Dong, Y.Q.; Mu, X.L.; Chen, Y.; Luo, H.M.; Zhang, B.G.; Liu, H.T. Identification, Molecular Cloning, and Functional Characterization of a Coniferyl Alcohol Acyltransferase Involved in the Biosynthesis of Dibenzocyclooctadiene Lignans in Schisandra chinensis. Front. Plant Sci. 2022, 13, 881342. [Google Scholar] [CrossRef]
- Ikezawa, N.; Tanaka, M.; Nagayoshi, M.; Shinkyo, R.; Sakaki, T.; Inouye, K.; Sato, F. Molecular Cloning and Characterization of CYP719, a Methylenedioxy Bridge-forming Enzyme That Belongs to a Novel P450 Family, from cultured Coptis japonica Cells. J. Biol. Chem. 2003, 278, 38557–38565. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Guo, C.; Peng, J.; Shao, F.; Sheng, S.; Wang, S. Transcriptomic Insights and Cytochrome P450 Gene Analysis in Kadsura coccinea for Lignan Biosynthesis. Genes 2024, 15, 270. https://doi.org/10.3390/genes15030270
Fu H, Guo C, Peng J, Shao F, Sheng S, Wang S. Transcriptomic Insights and Cytochrome P450 Gene Analysis in Kadsura coccinea for Lignan Biosynthesis. Genes. 2024; 15(3):270. https://doi.org/10.3390/genes15030270
Chicago/Turabian StyleFu, Hanyu, Chuan Guo, Jiqing Peng, Fengxia Shao, Song Sheng, and Sen Wang. 2024. "Transcriptomic Insights and Cytochrome P450 Gene Analysis in Kadsura coccinea for Lignan Biosynthesis" Genes 15, no. 3: 270. https://doi.org/10.3390/genes15030270
APA StyleFu, H., Guo, C., Peng, J., Shao, F., Sheng, S., & Wang, S. (2024). Transcriptomic Insights and Cytochrome P450 Gene Analysis in Kadsura coccinea for Lignan Biosynthesis. Genes, 15(3), 270. https://doi.org/10.3390/genes15030270






