Study of Dandelion (Taraxacum mongolicum Hand.-Mazz.) Salt Response and Caffeic Acid Metabolism under Saline Stress by Transcriptome Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Design
2.2. Phenolic Acid Examination
2.3. MDA, POD, and Pro Measurements
2.4. RNA-Seq Analysis
2.5. Real-Time Quantitative PCR (RT-qPCR) Validation
2.6. Data Processing
3. Results
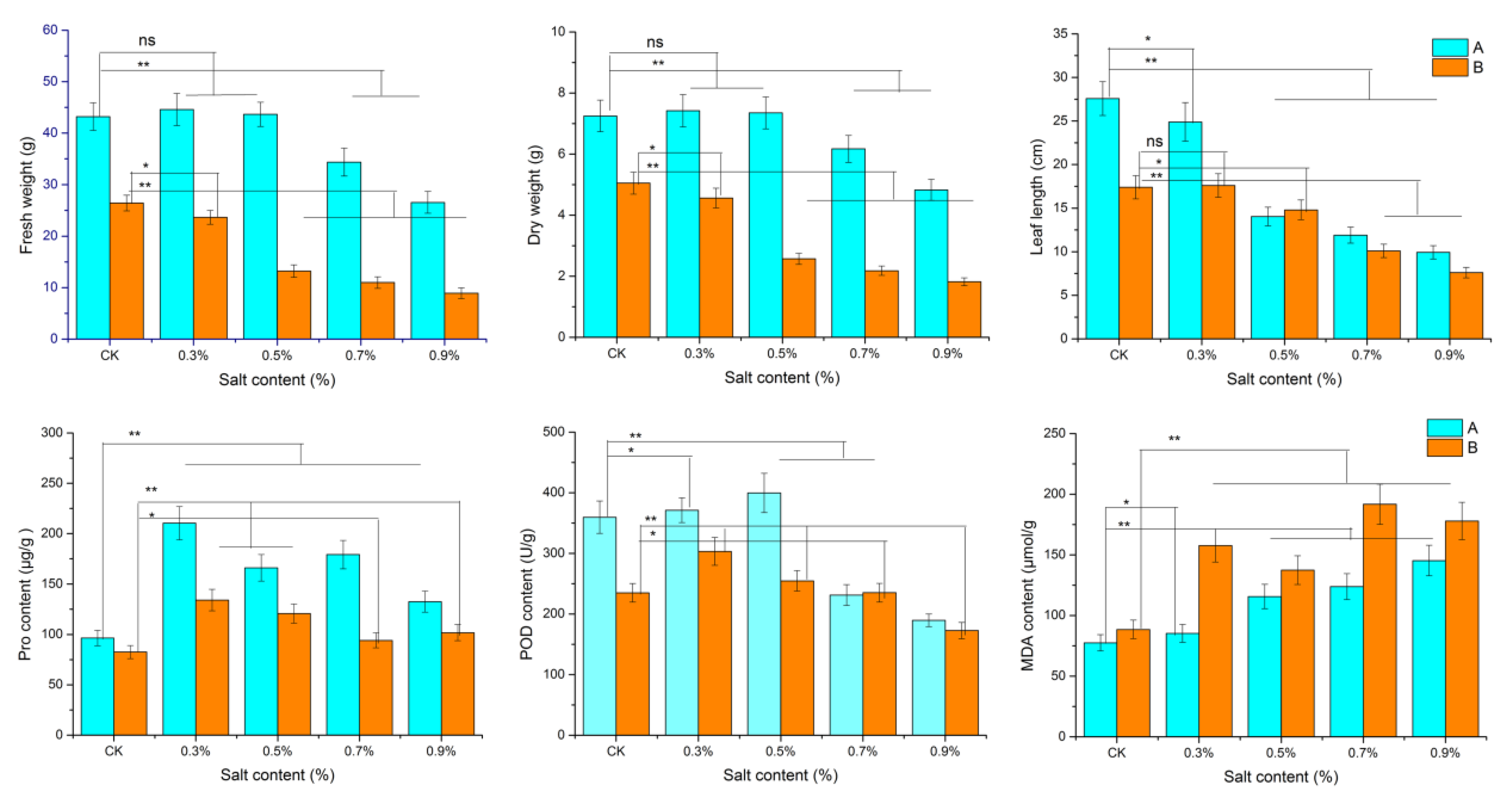
3.1. Dandelion Morphological and Physiological Changes
3.2. Changes in Caffeic Acid and Its Derivatives
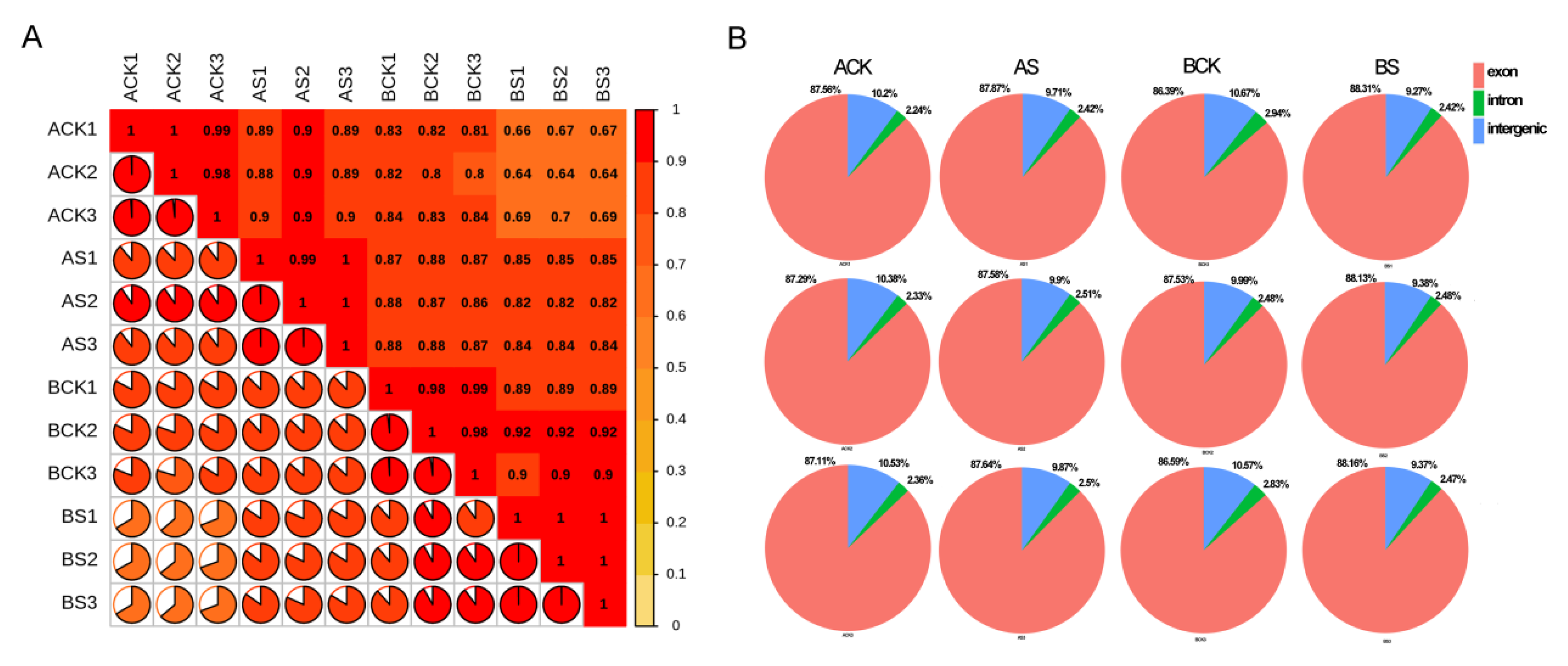
3.3. Transcriptome Sequencing and Alignment
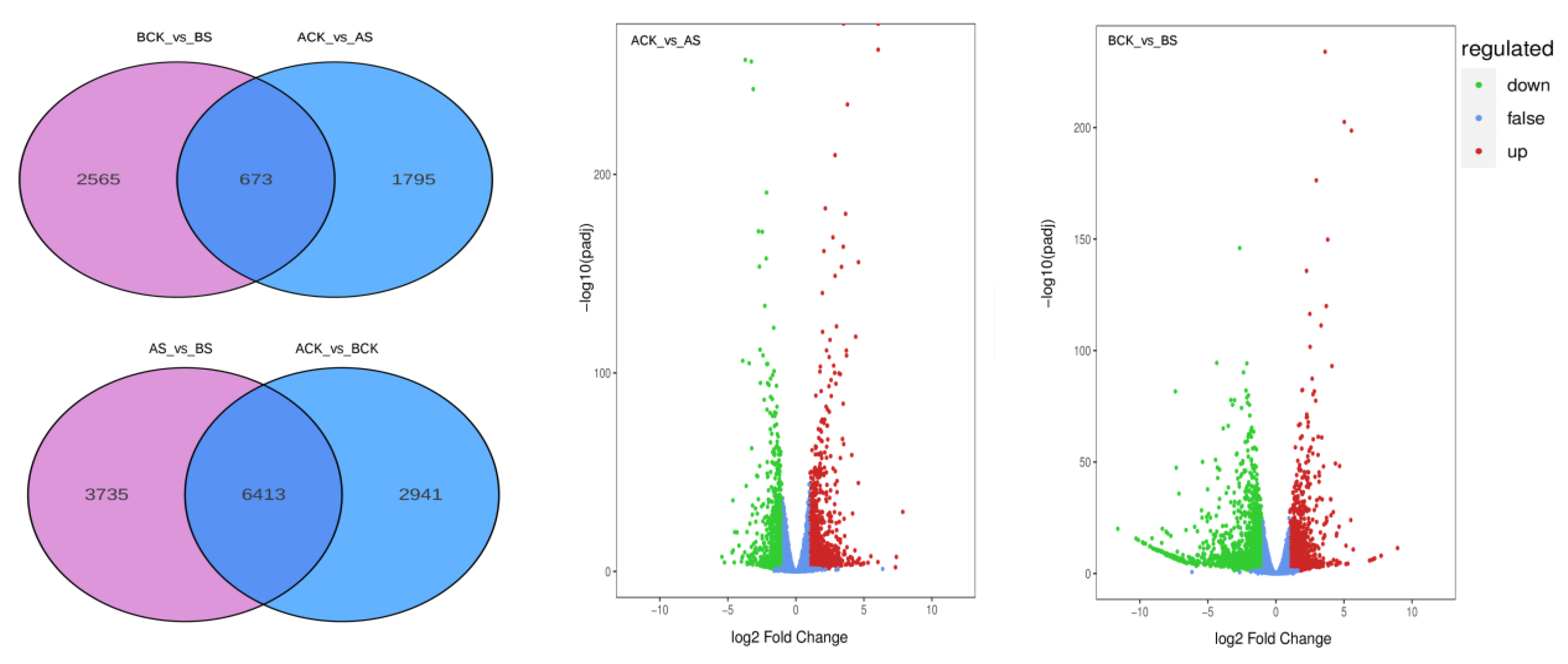
3.4. Analysis of DEGs
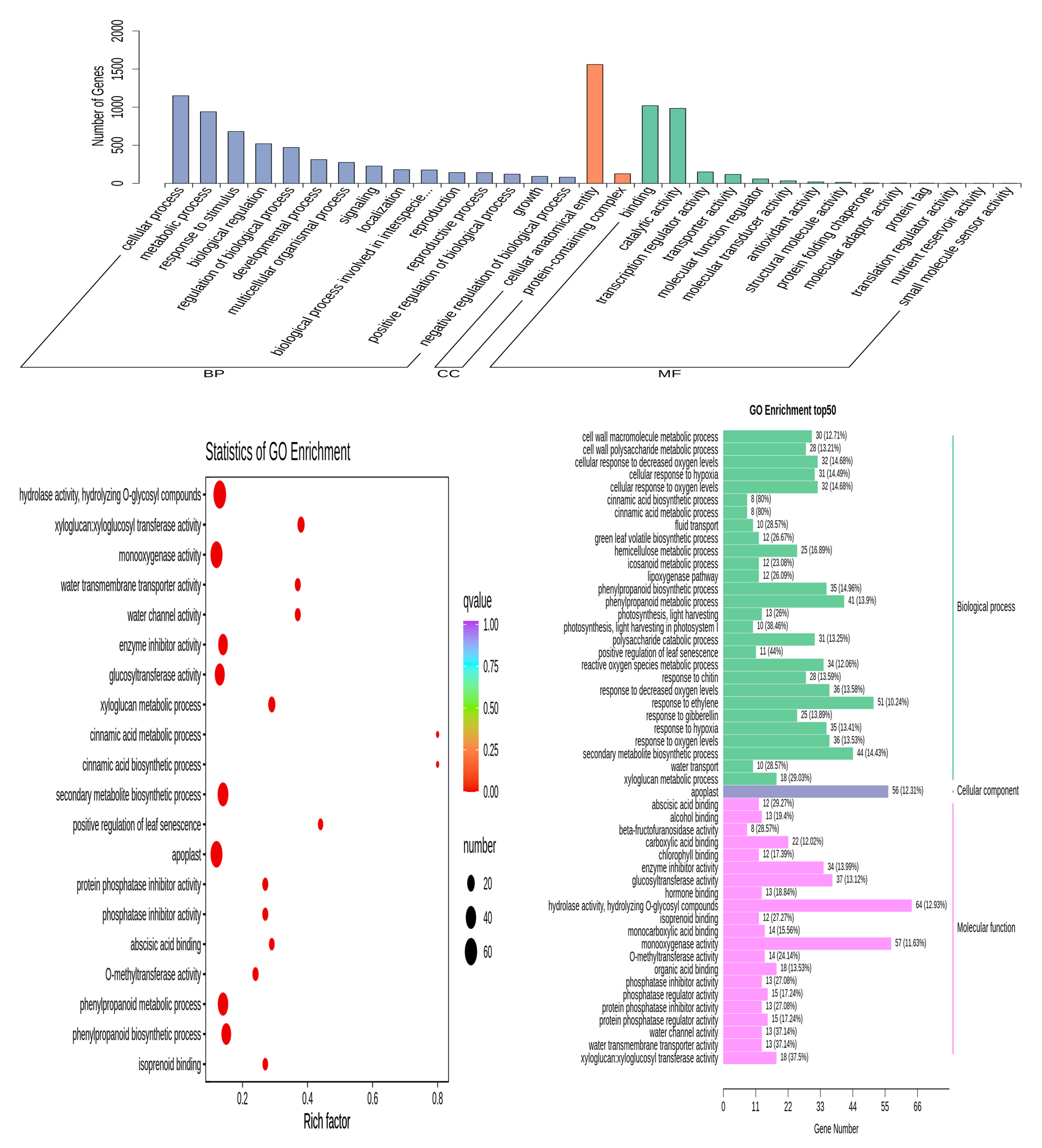
3.5. GO Enrichment Analysis of DEGs
3.6. KEGG Pathways of DEGs
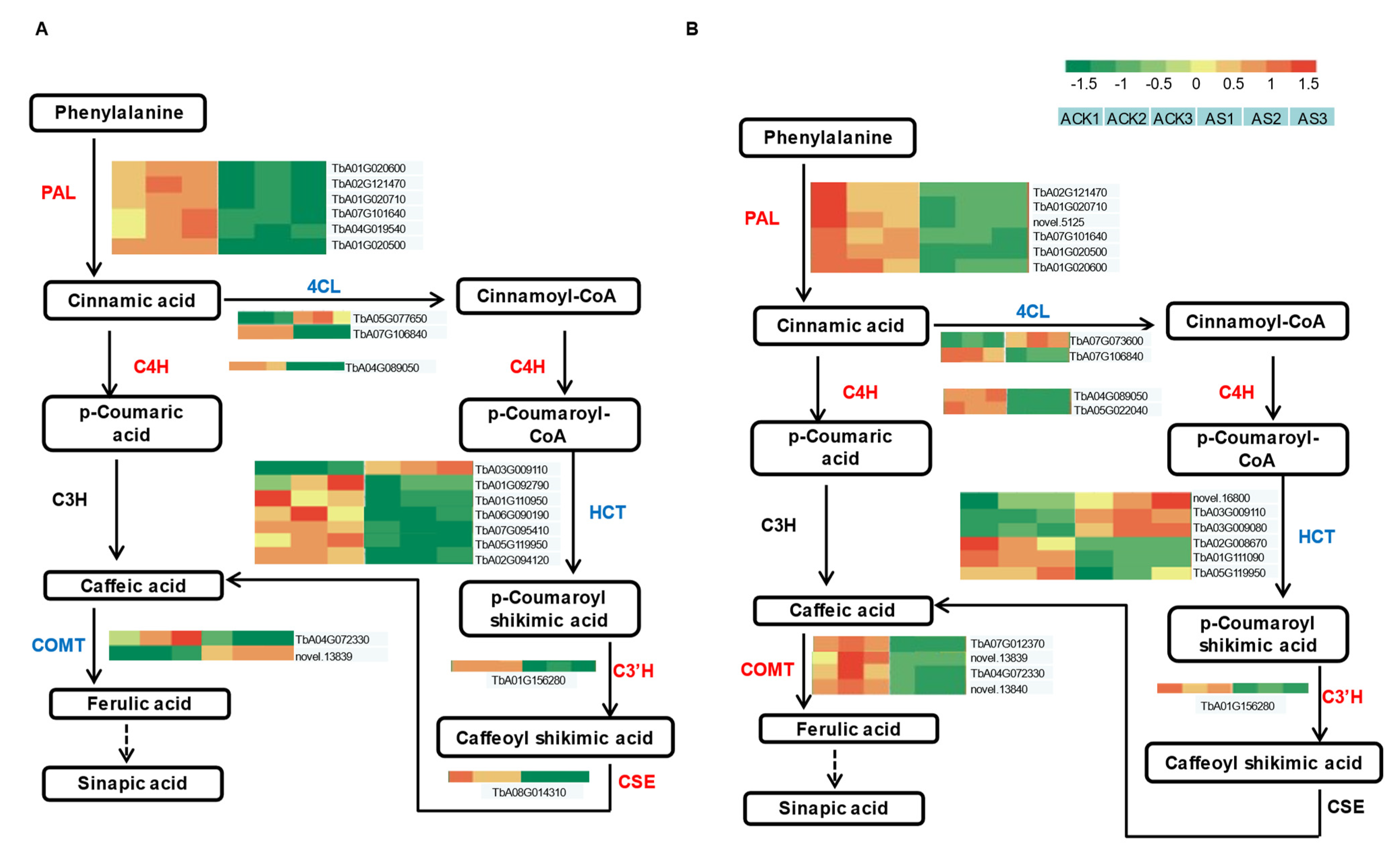
3.7. DEG Analysis of Phenylpropanoid Biosynthesis Pathway
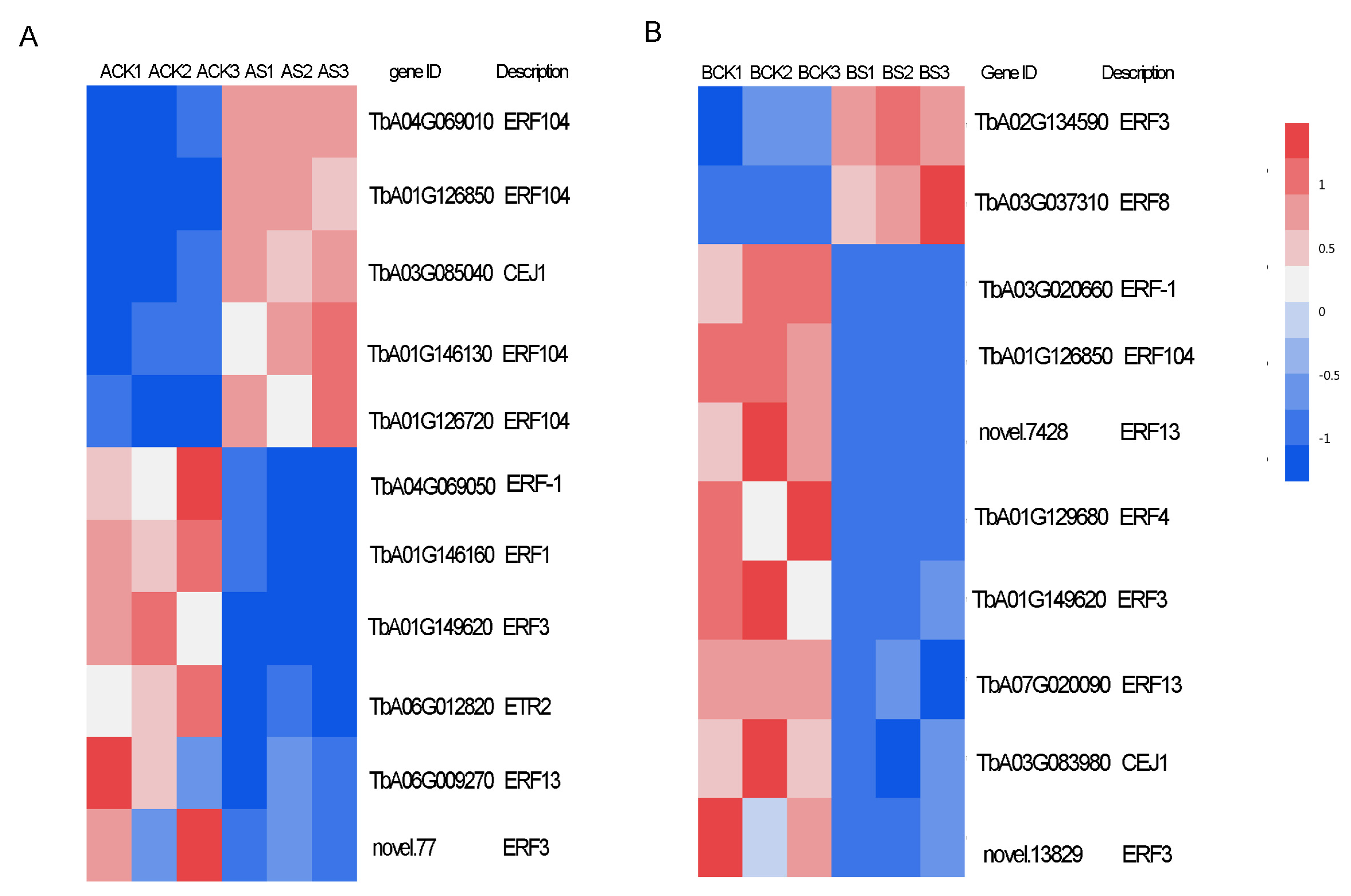
3.8. Transcription Factors (TFs) and Ethylene Response
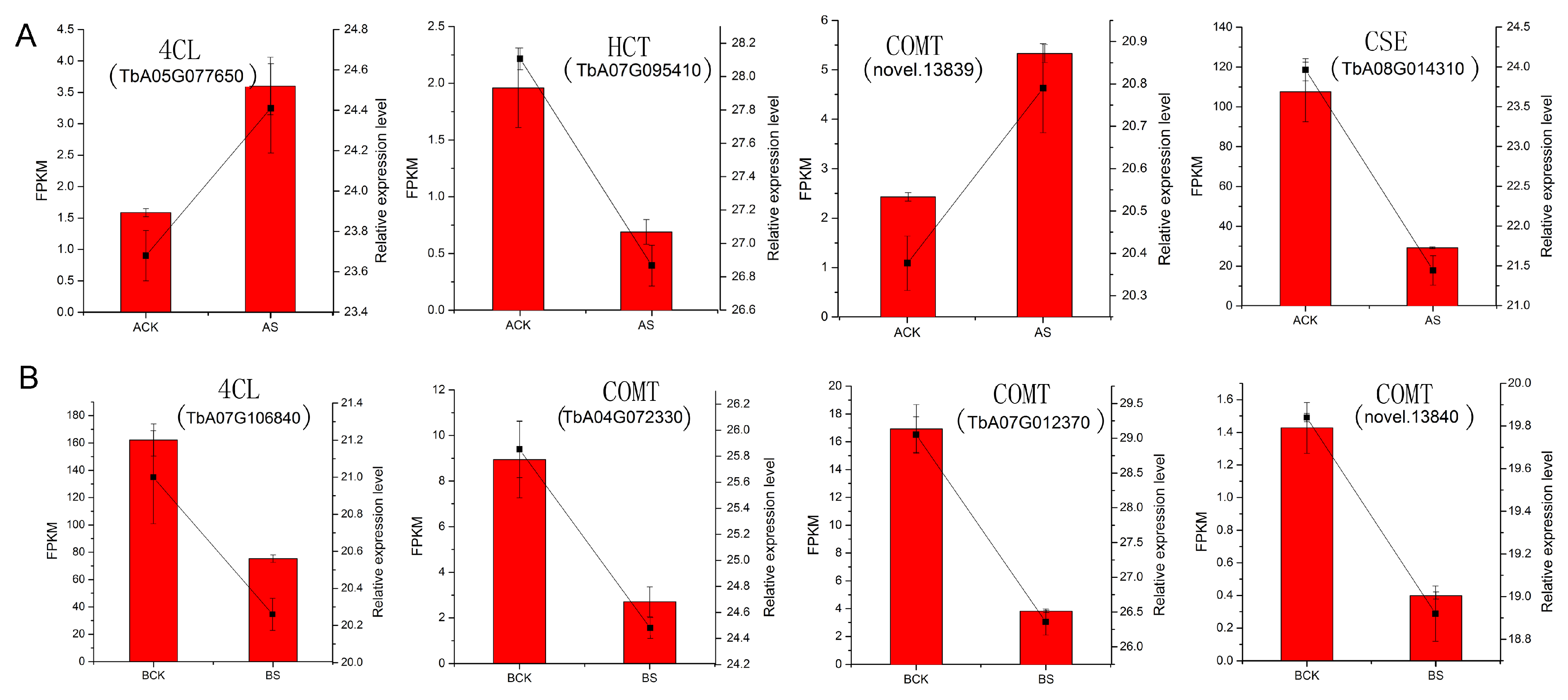
3.9. Verification of RNA-seq Data
4. Discussion
4.1. Morphological and Physiological Adaptation to Saline Stress
4.2. DEGs in Dandelion under Saline Stress
4.3. Specific Expression of TFs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Xue, Z.; Li, H.; Zhang, X.; Wang, X.; Lu, X. Cultivation of dandelion (Taraxacum erythropodium) on coastal saline land based on the control of salinity and fertilizer. Folia Hortic. 2019, 31, 277–284. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, Z.; Lu, X.; Jia, Y.; Wang, X.; Zhang, X. Salt-tolerance identification and quality evaluation of Abelmoschus manihot (L.) Medik. Can. J. Plant Sci. 2020, 100, 568–574. [Google Scholar] [CrossRef]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant Salt Stress: Adaptive Responses, Tolerance Mechanism and Bioengineering for Salt Tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Wang, W. Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Sci. Plant Nutr. 2009, 55, 698–704. [Google Scholar] [CrossRef]
- Şirin, S.; Aslım, B. Determination of antioxidant capacity, phenolic acid composition and antiproliferative effect associated with phenylalanine ammonia lyase (PAL) activity in some plants naturally growing under salt stress. Med. Chem. Res. 2019, 28, 229–238. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, W.; Tian, R.; Li, C.; Ji, Y.; Li, T.; Wei, C.; Chen, Z. Morphological, physiological, and secondary metabolic responses of Taraxacum officinale to salt stress. Plant Physiol. Biochem. 2022, 189, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Z.; Feng, W.; Meng, R.; Wang, X.; Wu, C. The breeding of high-quality dandelions by NaCl induced callus variation combined with a Drosophila tumor cell migration test. Horticulturae 2022, 8, 1167. [Google Scholar] [CrossRef]
- Bao, Y.-F.; Shen, X.-C.; Wang, F. The research progress and development prospects of biosynthesis, structure activity relationship and biological activity of caffeic acid and its main types of derivatives. Nat. Prod. Res. Dev. 2018, 30, 1825–1833. [Google Scholar]
- Amirbakhtiar, N.; Ismaili, A.; Ghaffari, M.R.; Nazarian Firouzabadi, F.; Shobbar, Z.-S. Transcriptome response of roots to salt stress in a salinity-tolerant bread wheat cultivar. PLoS ONE 2019, 14, e0213305. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Xu, Y.; Yao, L.; Liu, Z.; Cheng, H.; Ma, M.; Wu, J.; Wang, W.; Ning, W. Overexpression of and RNA interference with hydroxycinnamoyl-CoA quinate hydroxycinnamoyl transferase affect the chlorogenic acid metabolic pathway and enhance salt tolerance in Taraxacum antungense Kitag. Phytochem. Lett. 2018, 28, 116–123. [Google Scholar] [CrossRef]
- Lin, T.; Xu, X.; Du, H.; Fan, X.; Chen, Q.; Hai, C.; Zhou, Z.; Su, X.; Kou, L.; Gao, Q.; et al. Extensive sequence divergence between the reference genomes of Taraxacum kok-saghyz and Taraxacum mongolicum. Sci. China Life Sci. 2022, 65, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Xu, X.; Ruan, J.; Liu, S.; Wu, S.; Shao, X.; Wang, X.; Gan, L.; Qin, B.; Yang, Y.; et al. Genome analysis of Taraxacum kok-saghyz Rodin provides new insights into rubber biosynthesis. Nat. Sci. Rev. 2017, 5, 78–87. [Google Scholar] [CrossRef]
- Yazıcı, S.Ö. Optimization of all extraction process for phenolic compounds with maximum antioxidant activity from extract of Taraxacum assemanii by statistical strategies. J. Food Meas. Charact. 2021, 15, 4388–4402. [Google Scholar] [CrossRef]
- Li, C.; Tian, Y.; Zhao, C.; Li, S.; Wang, T.; Qiao, B.; Fu, Y. Application of fingerprint combined with quantitative analysis and multivariate chemometric methods in quality evaluation of dandelion (Taraxacum mongolicum). R. Soc. Open Sci. 2021, 8, 210614. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lv, Q.; Liu, A.; Wang, J.; Sun, X.; Deng, J.; Chen, Q.; Wu, Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, Z.; Zhang, R.; Liu, P.; Liu, M.; Liu, Z.; Zhao, Z.; Wang, L.; Chen, X. The regulation of cell wall lignification and lignin biosynthesis during pigmentation of winter jujube. Hortic. Res. 2021, 8, 238. [Google Scholar] [CrossRef]
- Wu, Z.; Feng, W.; Lu, X.; Wang, X. Changes of Phenolic Acids Related Genes in Taraxacum mongolicum Hand.-Mazz. under Saline Stress. Open Access Libr. J. 2023, 10, 1–14. [Google Scholar] [CrossRef]
- Kesawat, M.S.; Satheesh, N.; Kherawat, B.S.; Kumar, A.; Kim, H.-U.; Chung, S.-M.; Kumar, M. Regulation of Reactive Oxygen Species during Salt Stress in Plants and Their Crosstalk with Other Signaling Molecules-Current Perspectives and Future Directions. Plants 2023, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.O.; Gomez-Perez, D.; Probst, N.; Dietz, K.-J. Combinatorial signal integration by APETALA2/Ethylene Response Factor (ERF)-transcription factors and the involvement of AP2-2 in starvation response. Int. J. Mol. Sci. 2012, 13, 5933–5951. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, R.; Alemzadeh, A.; Razi, H. Microarray analysis of transcriptional responses to salt and drought stress in Arabidopsis thaliana. Heliyon 2019, 5, e02614. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Aghaei, K.; Komatsu, S. Crop and medicinal plants proteomics in response to salt stress. Front. Plant Sci. 2013, 4, 8. [Google Scholar] [CrossRef]
- Nazari, M.; Ghasemi-Soloklui, A.A.; Kordrostami, M.; Abdel Latef, A.A.H. Deciphering the response of medicinal plants to abiotic stressors: A focus on drought and salinity. Plant Stress 2023, 10, 100255. [Google Scholar] [CrossRef]
- Gamage Kaushalya Madhavi, B.; Mun Choi, G.; Entaz Bahar, M.; Eun Moon, B.; Eun Kim, N.; Lee, H.-W.; Tae Kim, H. Assessment of different salt concentrations on the growth and phytochemical change of the ice plants. J. King Saud Univ. Sci. 2022, 34, 102168. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Dhifi, W.; Limam, F.; Marzouk, B. Effect of salinity on plant growth and biological activities of Carthamus tinctorius L. extracts at two flowering stages. Acta Physiol. Plant. 2014, 36, 433–445. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2023, 51, 16–34. [Google Scholar] [CrossRef]
- Gengmao, Z.; Yu, H.; Xing, S.; Shihui, L.; Quanmei, S.; Changhai, W. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind. Crops Prod. 2015, 64, 175–181. [Google Scholar] [CrossRef]
- Iaffaldano, B.J.; Zhang, Y.; Cardina, J.; Cornish, K. Genome size variation among common dandelion accessions informs their mode of reproduction and suggests the absence of sexual diploids in North America. Plant Syst. Evol. 2017, 303, 719–725. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Y.; Wu, Z.; Qian, J.; Han, B.; Wang, G.; Gao, Y.; Liang, C.; Kai, G. Understanding the biosynthesis and regulatory mechanisms of bioactive compounds in Taraxacum species (dandelions), a model system for natural rubber, food, and medicinal plant biology. Crit. Rev. Plant Sci. 2022, 41, 406–425. [Google Scholar] [CrossRef]
- Mrázová, A.; Belay, S.A.; Eliášová, A.; Perez-Delgado, C.; Kaducová, M.; Betti, M.; Vega, J.M.; Paľove-Balang, P. Expression, activity of phenylalanine-ammonia-lyase and accumulation of phenolic compounds in Lotus japonicus under salt stress. Biologia 2017, 72, 36–42. [Google Scholar] [CrossRef]
- Liu, T.; Yao, R.; Zhao, Y.; Xu, S.; Huang, C.; Luo, J.; Kong, L. Cloning, functional characterization and site-directed mutagenesis of 4-coumarate: Coenzyme A ligase (4CL) involved in coumarin biosynthesis in Peucedanum praeruptorum Dunn. Front. Plant Sci. 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.M.; Choi, B.S.; Park, S.U.; Lim, H.-S.; Bae, H. Transcriptional analysis of hydroxycinnamoyl transferase (HCT) in various tissues of Hibiscus cannabinus in response to abiotic stress conditions. Plant Omics 2012, 5, 305. [Google Scholar]
- Neves, G.; Marchiosi, R.; Ferrarese, M.; Siqueira-Soares, R.; Ferrarese-Filho, O. Root growth inhibition and lignification induced by salt stress in soybean. J. Agron. Crop Sci. 2010, 196, 467–473. [Google Scholar] [CrossRef]
- Fernandez-Garcia, N.; Lopez-Perez, L.; Hernandez, M.; Olmos, E. Role of phi cells and the endodermis under salt stress in Brassica oleracea. New Phytol. 2009, 181, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.M.; Escamilla-Trevino, L.; Yarce, J.C.S.; Kim, H.; Ralph, J.; Chen, F.; Dixon, R.A. An essential role of caffeoyl shikimate esterase in monolignol biosynthesis in Medicago truncatula. Plant J. 2016, 86, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wen, D.; Yang, W.; Meng, Q.; Shi, Q.; Gong, B. Overexpression of caffeic acid O-methyltransferase 1 (COMT1) increases melatonin level and salt stress tolerance in tomato plant. J. Plant Growth Regul. 2020, 39, 1221–1235. [Google Scholar] [CrossRef]
- Yang, R.; Liu, J.; Lin, Z.; Sun, W.; Wu, Z.; Hu, H.; Zhang, Y. ERF transcription factors involved in salt response in tomato. Plant Growth Regul. 2018, 84, 573–582. [Google Scholar] [CrossRef]
- Zhang, Q.; Dai, X.; Wang, H.; Wang, F.; Tang, D.; Jiang, C.; Zhang, X.; Guo, W.; Lei, Y.; Ma, C. Transcriptomic Profiling Provides Molecular Insights into Hydrogen Peroxide-Enhanced Arabidopsis Growth and Its Salt Tolerance. Front. Plant Sci. 2022, 13, 866063. [Google Scholar] [CrossRef]
- Rong, W.; Qi, L.; Wang, A.; Ye, X.; Du, L.; Liang, H.; Xin, Z.; Zhang, Z. The ERF transcription factor Ta ERF 3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol. J. 2014, 12, 468–479. [Google Scholar] [CrossRef]



| Raw Reads (Mb) | Clean Reads (Mb) | Clean Base (G) | Q20 (%) | GC Content (%) | Mapped Reads | Mapped Ratio | |
|---|---|---|---|---|---|---|---|
| ACK 1 | 47.35 ± 1.21 | 46.27 ± 1.02 | 6.94 ± 0.15 | 97.41 ± 0.08 | 43.69 ± 0.15 | 35.95 ± 0.73 | 0.78 ± 0.003 |
| AS 1 | 47.39 ± 0.48 | 46.29 ± 0.3 | 6.94 ± 0.05 | 97.33 ± 0.12 | 43.49 ± 0.16 | 36.27 ± 0.18 | 0.78 ± 0.001 |
| BCK 1 | 50.39 ± 1.5 | 48.86 ± 1.83 | 7.33 ± 0.27 | 97.39 ± 0.04 | 43.81 ± 0.54 | 42.13 ± 1.73 | 0.86 ± 0.003 |
| BS 1 | 48.65 ± 0.83 | 47.35 ± 0.83 | 7.10 ± 0.12 | 97.34 ± 0.15 | 44.52 ± 0.16 | 40.60 ± 0.7 | 0.86 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Meng, R.; Feng, W.; Wongsnansilp, T.; Li, Z.; Lu, X.; Wang, X. Study of Dandelion (Taraxacum mongolicum Hand.-Mazz.) Salt Response and Caffeic Acid Metabolism under Saline Stress by Transcriptome Analysis. Genes 2024, 15, 220. https://doi.org/10.3390/genes15020220
Wu Z, Meng R, Feng W, Wongsnansilp T, Li Z, Lu X, Wang X. Study of Dandelion (Taraxacum mongolicum Hand.-Mazz.) Salt Response and Caffeic Acid Metabolism under Saline Stress by Transcriptome Analysis. Genes. 2024; 15(2):220. https://doi.org/10.3390/genes15020220
Chicago/Turabian StyleWu, Zhe, Ran Meng, Wei Feng, Tassnapa Wongsnansilp, Zhaojia Li, Xuelin Lu, and Xiuping Wang. 2024. "Study of Dandelion (Taraxacum mongolicum Hand.-Mazz.) Salt Response and Caffeic Acid Metabolism under Saline Stress by Transcriptome Analysis" Genes 15, no. 2: 220. https://doi.org/10.3390/genes15020220
APA StyleWu, Z., Meng, R., Feng, W., Wongsnansilp, T., Li, Z., Lu, X., & Wang, X. (2024). Study of Dandelion (Taraxacum mongolicum Hand.-Mazz.) Salt Response and Caffeic Acid Metabolism under Saline Stress by Transcriptome Analysis. Genes, 15(2), 220. https://doi.org/10.3390/genes15020220






