Selection and Verification of Standardized Reference Genes of Angelica dahurica under Various Abiotic Stresses by Real-Time Quantitative PCR
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
2.2. Total RNA Isolation and cDNA Synthesis
2.3. Selection of Candidate Reference Genes and Primer Design
2.4. RT-qPCR Conditions
2.5. Stability Evaluation of Candidate Reference Genes
2.6. Validation of Reference Gene Stability
3. Results
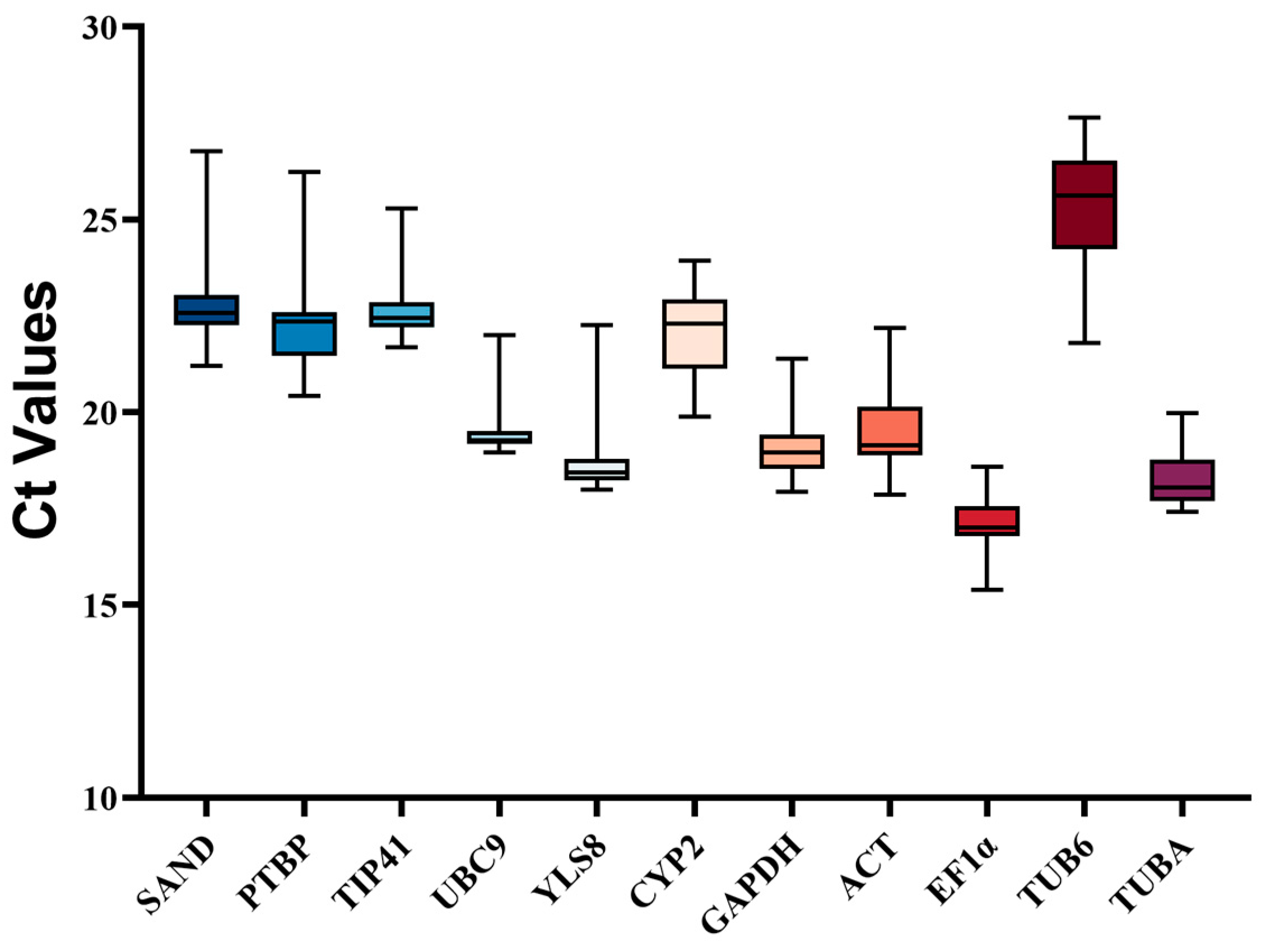
3.1. Selection of Candidate Reference Genes, Evaluation of Amplification Specificity, and PCR Efficiency
3.2. Expression Profile of Candidate Reference Genes
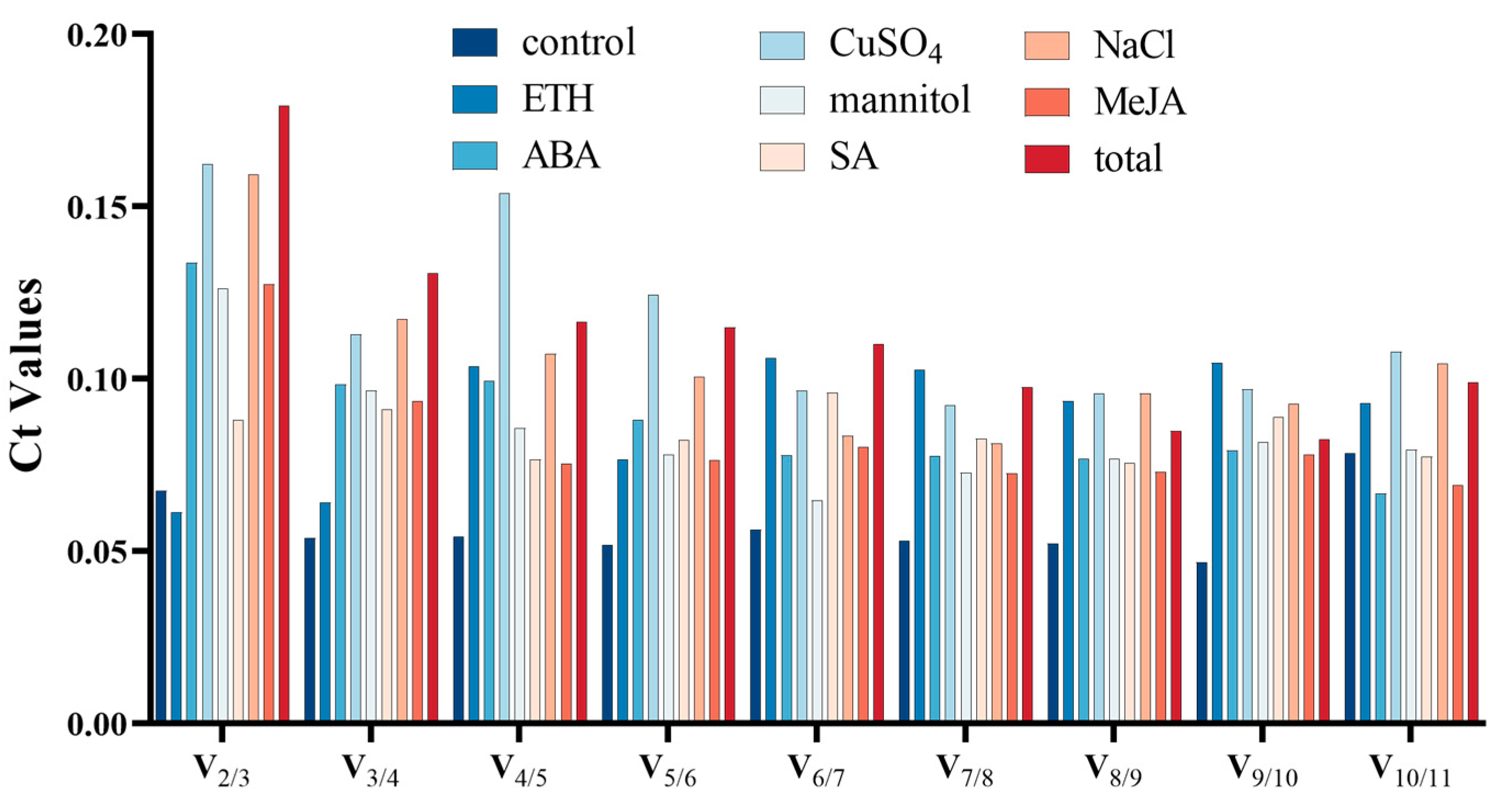
3.2.1. GeNorm Analysis
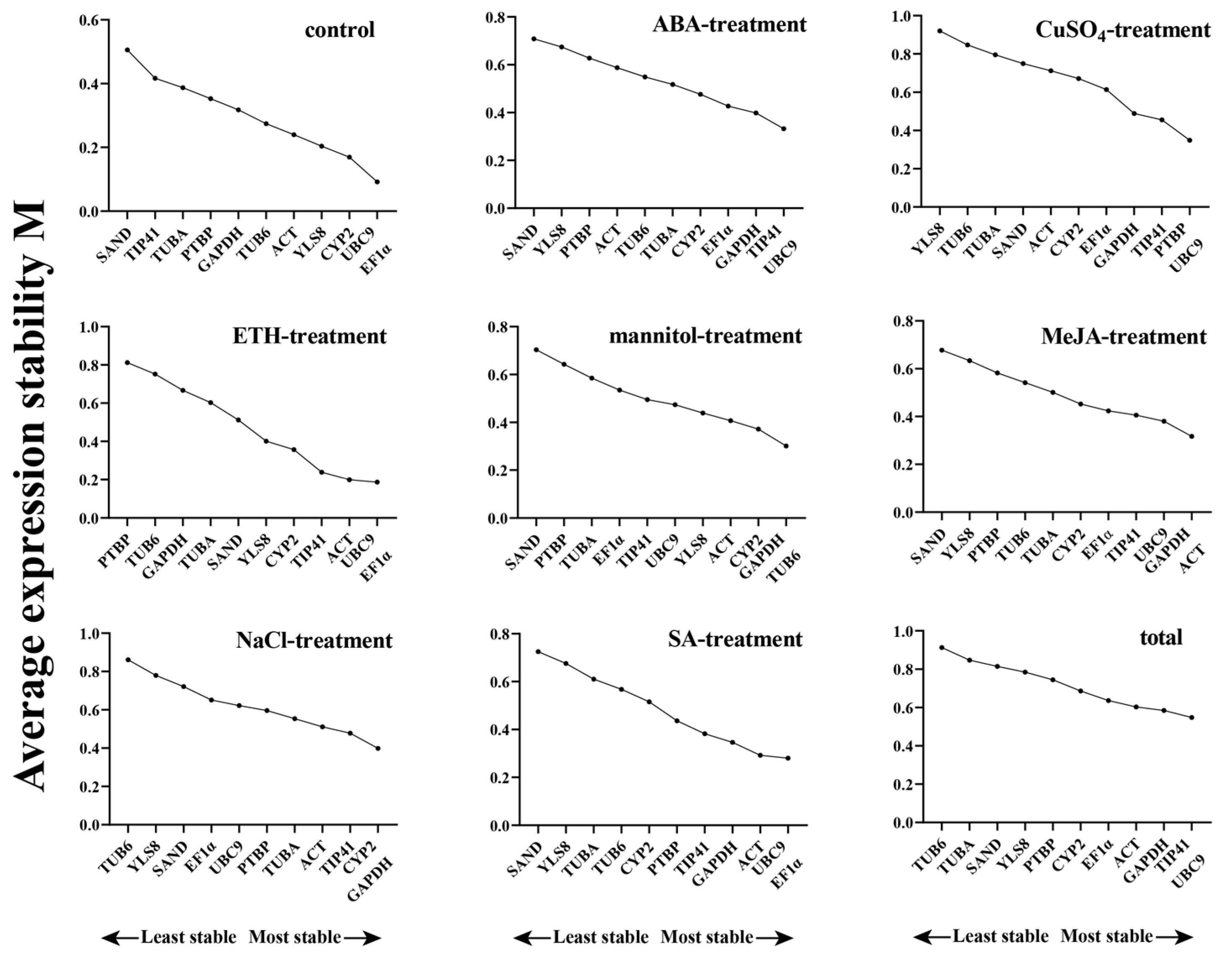
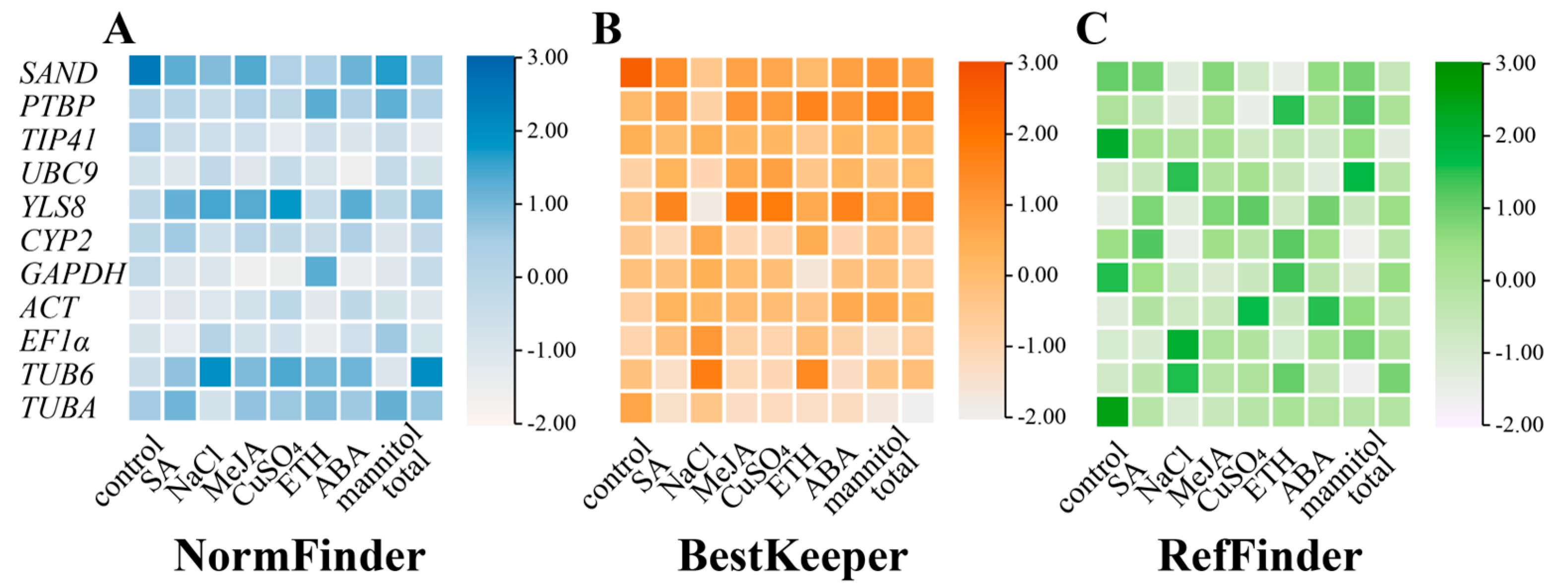
3.2.2. NormFinder Analysis
3.2.3. BestKeeper Analysis
3.2.4. RefFinder Analysis
3.2.5. Comprehensive Analysis
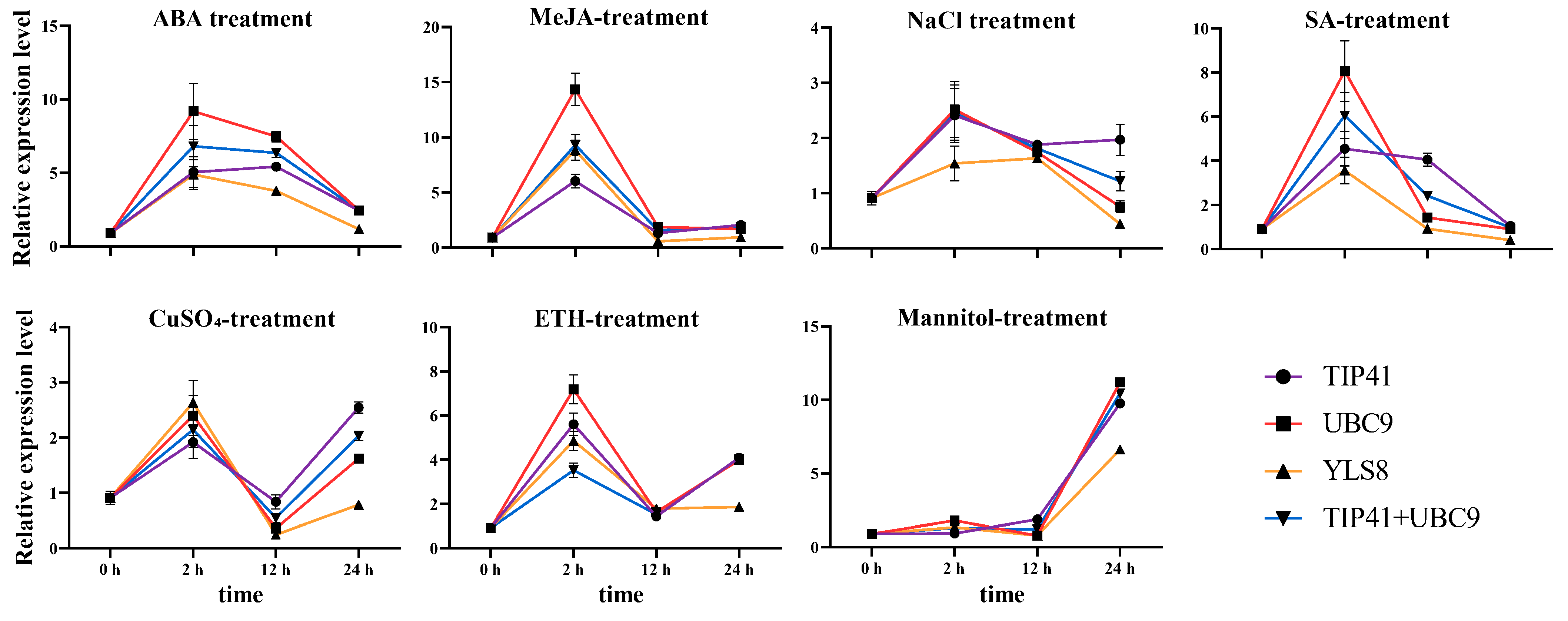
3.3. Reference Gene Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Feng, Y.-L.; Wang, M.; Wang, J.-J.; Liu, T.; Yu, J. The Angelica dahurica: A review of traditional uses, phytochemistry and pharmacology. Front. Pharmacol. 2022, 13, 896637. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Duan, Y.; Wang, Y.; Liu, A.; Chen, Y.; Guo, D.; Guo, W.; Li, S.; Su, Z.; Wu, Y.; et al. Phellopterin cream exerts an anti-inflammatory effect that facilitates diabetes-associated cutaneous wound healing via SIRT1. Phytomedicine 2022, 107, 154447. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.M.; Shen, J.Z.; Wang, Y.; Lu, A.X.; Ho, W.S. Anti-oxidant and anti-cancer activities of Angelica dahurica extract via induction of apoptosis in colon cancer cells. Phytomedicine 2016, 23, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, L. Coumarins from the roots of Angelica dahurica cause anti-allergic inflammation. Exp. Ther. Med. 2017, 14, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Nützmann, H.-W.; Huang, A.; Osbourn, A. Plant metabolic clusters—From genetics to genomics. New Phytol. 2016, 211, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, X.; Sun, M.; Cong, H.; Qiao, F. Evaluation of reference genes for normalizing RT-qPCR in leaves and suspension cells of Cephalotaxus hainanensis under various stimuli. Plant Methods 2019, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef]
- Dheda, K.; Huggett, J.F.; Chang, J.S.; Kim, L.U.; Bustin, S.A.; Johnson, M.A.; Rook, G.A.W.; Zumla, A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal. Biochem. 2005, 344, 141–143. [Google Scholar] [CrossRef]
- Delporte, M.; Legrand, G.; Hilbert, J.-L.; Gagneul, D. Selection and validation of reference genes for quantitative real-time PCR analysis of gene expression in Cichorium intybus. Front. Plant Sci. 2015, 6, 651. [Google Scholar] [CrossRef]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-M.; Zhang, W.; Zhang, S.-B. Selection and validation of reference genes for quantitative real-time PCR analysis of development and tissue-dependent flower color formation in Cymbidium lowianum. Int. J. Mol. Sci. 2022, 23, 738. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, X.; Zhou, X.; Wu, Z.; Yuan, L.; Wang, Y.; Li, Y. Selection of reference genes for qRT-PCR analysis in medicinal plant glycyrrhiza under abiotic stresses and hormonal treatments. Plants 2020, 9, 1441. [Google Scholar] [CrossRef]
- Hou, S.; Zhao, T.; Yang, D.; Li, Q.; Liang, L.; Wang, G.; Ma, Q. Selection and validation of reference genes for quantitative RT-PCR analysis in Corylus heterophylla Fisch. × Corylus avellana L. Plants 2021, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Zou, X.; Carballar-Lejarazú, R.; Wu, L.; Sun, W.; Yuan, X.; Wu, S.; Li, P.; Ding, H.; Ni, L.; et al. Selection and evaluation of reference genes for qRT-PCR analysis in Euscaphis konishii Hayata based on transcriptome data. Plant Methods 2018, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhong, Y.; Bao, Z.; Wang, W.; Xu, X.; Gai, Y.; Wu, J. Evaluation of Angelica decursiva reference genes under various stimuli for RT-qPCR data normalization. Sci. Rep. 2021, 11, 18993. [Google Scholar] [CrossRef]
- Song, H.; Mao, W.; Duan, Z.; Que, Q.; Zhou, W.; Chen, X.; Li, P. Selection and validation of reference genes for measuring gene expression in Toona ciliata under different experimental conditions by quantitative real-time PCR analysis. BMC Plant Biol. 2020, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, R.; Zhou, Z. Identification and validation of reference genes for gene expression analysis in Schima superba. Genes 2021, 12, 732. [Google Scholar] [CrossRef]
- Yi, S.; Lin, Q.; Zhang, X.; Wang, J.; Miao, Y.; Tan, N. Selection and validation of appropriate reference genes for quantitative RT-PCR analysis in Rubia yunnanensis Diels based on transcriptome data. Biomed Res. Int. 2020, 2020, 5824841. [Google Scholar] [CrossRef]
- Zhong, Y.; Gai, Y.; Gao, J.; Nie, W.; Bao, Z.; Wang, W.; Xu, X.; Wu, J.; He, Y. Selection and validation of reference genes for quantitative real-time PCR normalization in Psoralea corylifolia (Babchi) under various abiotic stress. J. Plant Physiol. 2022, 274, 153722. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Liu, Y.; Chen, J.; Lin, H.; Huang, Y.; Bian, X.; Zhao, Y. Selection and validation of appropriate reference genes for real-time quantitative PCR snalysis in Momordica charantia. Phytochemistry 2019, 164, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mu, D.; Wang, L.; Wang, X.; Wilson, I.W.; Chen, W.; Wang, J.; Liu, Z.; Qiu, D.; Tang, Q. Reference genes screening and fene expression patterns analysis involved in gelsenicine biosynthesis under different hormone treatments in Gelsemium elegans. Int. J. Mol. Sci. 2023, 24, 15973. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Ma, P.; Ding, K.; Liu, S.; Jia, Y.; Ru, M.; Dong, J.; Liang, Z. SmJAZ8 acts as a core repressor regulating JA-induced biosynthesis of salvianolic acids and tanshinones in Salvia miltiorrhiza Hairy roots. J. Exp. Bot. 2018, 69, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Postharvest UV-C irradiation increased the flavonoids and anthocyanins accumulation, phenylpropanoid pathway gene expression, and antioxidant activity in sweet sherries (Prunus avium L.). Postharvest Biol. Technol. 2021, 175, 111490. [Google Scholar] [CrossRef]
- He, Y.; Zhong, X.; Jiang, X.; Cong, H.; Sun, H.; Qiao, F. Characterisation, expression and functional analysis of PAL gene family in Cephalotaxus hainanensis. Plant Physiol. Biochem. PPB 2020, 156, 461–470. [Google Scholar] [CrossRef]
- Sui, Z.; Luo, J.; Yao, R.; Huang, C.; Zhao, Y.; Kong, L. Functional characterization and correlation analysis of phenylalanine ammonia-lyase (PAL) in coumarin biosynthesis from Peucedanum praeruptorum Dunn. Phytochemistry 2019, 158, 35–45. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; He, Y.; Liu, H.; Wang, C.; Guan, G.; Zhao, Y.; Tian, Y.; Zhong, X.; Lu, X. Two O-methyltransferases are responsible for multiple O-methylation steps in the biosynthesis of furanocoumarins from Angelica decursiva. Plant Physiol. Biochem. 2023, 204, 108142. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Mu, D.; Lu, Y.; Chen, W.; Zhang, Y.; Zhang, R.; Qin, Y.; Yuan, J.; Pan, L.; et al. Selection of reference genes in Evodia rutaecarpa Var. officinalis and expression patterns of genes Iinvolved in its limonin biosynthesis. Plants 2023, 12, 3197. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and aample integrity: BestKeeper--excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-J.; Tian, C.; Jiang, Q.; Li, X.-H.; Zhuang, J. Selection of suitable reference genes for qRT-PCR normalization during leaf development and hormonal stimuli in tea plant (Camellia sinensis). Sci. Rep. 2016, 6, 19748. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A Web-Based Tool for Comprehensively Analyzing and Identifying Reference Genes. Funct. Integr. Genomics 2023, 23, 125. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. MiRDeepFinder: A MiRNA Analysis Tool for Deep Sequencing of Plant Small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, X.; Jia, B.; Liu, L.; Wei, P.; Manzoor, M.A.; Wang, F.; Li, B.Y.; Wang, G.; Chen, C.; et al. Comparative transcriptomics unveil the crucial genes involved in coumarin biosynthesis in Peucedanum praeruptorum Dunn. Front. Plant Sci. 2022, 13, 899819. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Olofsson, L.; Engström, A.; Lundgren, A.; Brodelius, P.E. Relative expression of genes of terpene metabolism in different tissues of Artemisia annua L. BMC Plant Biol. 2011, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002, 29, 23–39. [Google Scholar] [CrossRef]
- Xiao, Z.; Sun, X.; Liu, X.; Li, C.; He, L.; Chen, S.; Su, J. Selection of reliable reference genes for gene expression studies on Rhododendron molle G. Don. Front. Plant Sci. 2016, 7, 1547. [Google Scholar] [CrossRef]
- Hu, A.; Yang, X.; Zhu, J.; Wang, X.; Liu, J.; Wang, J.; Wu, H.; Zhang, H.; Zhang, H. Selection and validation of appropriate reference genes for RT-qPCR analysis of Nitraria sibirica under various abiotic stresses. BMC Plant Biol. 2022, 22, 592. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Shi, Y.; Ma, H. Selection of candidate reference genes for gene expression analysis in Kentucky bluegrass (Poa pratensis L.) under abiotic stress. Front. Plant Sci. 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, L.; Duan, H.; Chong, P.; Li, Y. Selection and validation of reference genes for quantitative real-time PCR analysis of Nitraria tangutorum. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Yi, S.; Lu, H.; Tian, C.; Xu, T.; Song, C.; Wang, W.; Wei, P.; Gu, F.; Liu, D.; Cai, Y.; et al. Selection of suitable reference genes for gene expression normalization studies in Dendrobium huoshanense. Genes 2022, 13, 1486. [Google Scholar] [CrossRef] [PubMed]
- Payyavula, R.S.; Shakya, R.; Sengoda, V.G.; Munyaneza, J.E.; Swamy, P.; Navarre, D.A. Synthesis and regulation of chlorogenic acid in potato: Rerouting phenylpropanoid flux in HQT-silenced lines. Plant Biotechnol. J. 2015, 13, 551–564. [Google Scholar] [CrossRef]
| Gene Symbol | Gene Name | Forward/Reverse Primer (5′-3′) | Tm (°C) | Size (bp) | E (%) | R2 |
|---|---|---|---|---|---|---|
| SAND | SAND family protein | GCTTGCCTTGTTGGATAA | 59.4 | 101 | 100.40 | 0.9964 |
| GATGCTGTGTTCTCTTCTC | 59.4 | |||||
| PTBP | Polypyrimidine tract-binding protein | GTGTGATTGGAACTTGATG | 58.1 | 149 | 94.53 | 0.9886 |
| TTACTGATGCTGAGACTG | 57.4 | |||||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | GTATCCGTTGTTGACCTT | 58.4 | 120 | 100.12 | 0.9961 |
| ATCACAGACCTCCAGAAT | 58.6 | |||||
| ACT | Actin | TTCACCATTCCAGTTCCA | 59.9 | 147 | 107.35 | 0.9902 |
| TATCCGCTTCATCGTTACA | 60.1 | |||||
| TIP41 | TIP41-like protein | GCAAGTGAGAGGTCTGAA | 60.4 | 184 | 106.82 | 0.9857 |
| ACAAGTCGGAATATGGAAGT | 60.5 | |||||
| CYP2 | Cyclophilin 2 | AACAGCGACATAATCAAGC | 60.1 | 125 | 105.68 | 0.9901 |
| CTCTCCAGCACCAAGTAA | 60.0 | |||||
| EF1α | Elongation factor 1 α | GATTGGAGGTATTGGAACT | 58.0 | 158 | 107.59 | 0.9973 |
| CATTGTCACCAGGAAGAG | 58.8 | |||||
| UBC9 | Ubiquitin-protein ligase 9 | CCTCCAGATAGTCCTTATTC | 57.9 | 165 | 109.07 | 0.9973 |
| GCTCCATTGTTCTTTCAG | 57.4 | |||||
| TUB6 | Tubulin β-6 | TGAGATAAGGAAGAGTAGTG | 57.1 | 92 | 102.06 | 0.9911 |
| ACATAGGCATAAAGGGATAC | 58.2 | |||||
| YLS8 | Thioredoxin-like protein YLS8 | CTGTTGCGGAGACGATAA | 60.9 | 191 | 100.67 | 0.9953 |
| CTGCTTGTCCTTCATTGC | 60.5 | |||||
| TUBA | (Tubulin-α) | AAGGTATGGACGAGATGG | 59.6 | 181 | 101.41 | 0.9974 |
| TCAGCAGTTCACACAGTA | 59.6 | |||||
| PAL | Phenylalanine ammonia lyase | GGTGATGGAGAGTATGAA | 56.7 | 170 | 105.61 | 0.9989 |
| GTGGTAATGTGTTGGAAG | 57.0 |
| Rank | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | |||||||||||
| geNorm | EF1α/UBC9 | / | ACT | GAPDH | TIP41 | PTBP | CYP2 | TUB6 | TUBA | YLS8 | SAND |
| NormFinder | EF1α | UBC9 | ACT | GAPDH | TIP41 | PTBP | CYP2 | TUB6 | TUBA | YLS8 | SAND |
| BestKeeper | TUBA | TUB6 | CYP2 | GAPDH | EF1α | TIP41 | ACT | UBC9 | PTBP | SAND | YLS8 |
| Comprehensive Ranking | EF1α | UBC9 | ACT | GAPDH | TUBA | TIP41 | TUB6 | CYP2 | PTBP | YLS8 | SAND |
| NaCl | |||||||||||
| geNorm | GAPDH/CYP2 | / | TIP41 | ACT | TUBA | PTBP | UBC9 | EF1α | SAND | YLS8 | TUB6 |
| NormFinder | ACT | GADPH | TUBA | TIP41 | CYP2 | PTBP | UBC9 | EF1α | SAND | YLS8 | YUB6 |
| BestKeeper | YLS8 | UBC9 | PTBP | SAND | TUBA | ACT | GAPDH | TIP41 | CYP2 | EF1α | TUB6 |
| Comprehensive Ranking | GAPDH | ACT | CYP2 | TUBA | TIP41 | PTBP | UBC9 | YLS8 | SAND | EF1α | TUB6 |
| MeJA | |||||||||||
| geNorm | ACT/GAPDH | / | UBC9 | TIP41 | EF1α | CYP2 | TUBA | TUB6 | PTBP | YLS8 | SAND |
| NormFinder | GAPDH | UBC9 | ACT | EF1α | TIP41 | CYP2 | PTBP | TUBA | TUB6 | YLS8 | SAND |
| BestKeeper | TUBA | TUB6 | CYP2 | EF1α | GAPDH | ACT | TIP41 | UBC9 | SAND | PTBP | YLS8 |
| Comprehensive Ranking | GAPDH | ACT | UBC9 | EF1α | TUBA | CYP2 | TIP41 | TUB6 | PTBP | YLS8 | SAND |
| CuSO4 | |||||||||||
| geNorm | UBC9/PTBP | / | TIP41 | GAPDH | EF1α | CYP2 | ACT | SAND | TUBA | TUB6 | YLS8 |
| NormFinder | GAPDH | TIP41 | EF1α | UBC9 | CYP2 | ACT | PTBP | SAND | TUBA | TUB6 | YLS8 |
| BestKeeper | TUBA | TUB6 | CYP2 | EF1α | GAPDH | ACT | TIP41 | SAND | UBC9 | PTBP | YLS8 |
| Comprehensive Ranking | GAPDH | TIP41 | UBC9 | EF1α | PTBP | CYP2 | TUBA | ACT | TUB6 | SAND | YLS8 |
| ETH | |||||||||||
| geNorm | EF1α/UBC9 | / | ACT | TIP41 | CYP2 | YLS8 | SAND | TUBA | GAPDH | TUB6 | PTBP |
| NormFinder | EF1α | ACT | UBC9 | TIP41 | CYP2 | YLS8 | SAND | TUBA | TUB6 | PTBP | GAPDH |
| BestKeeper | GAPDH | TUBA | TIP41 | UBC9 | ACT | EF1α | SAND | CYP2 | YLS8 | TUB6 | PTBP |
| Comprehensive Ranking | EF1α | UBC9 | ACT | TIP41 | GAPDH | CYP2 | TUBA | YLS8 | SAND | TUB6 | PTBP |
| ABA | |||||||||||
| geNorm | UBC9/TIP41 | / | GAPDH | EF1α | CYP2 | TUBA | TUB6 | ACT | PTBP | YLS8 | SAND |
| NormFinder | UBC9 | GAPDH | TIP41 | EF1α | ACT | PTBP | CYP2 | TUBA | TUB6 | SAND | YLS8 |
| BestKeeper | TUB6 | TUBA | CYP2 | EF1α | GAPDH | UBC9 | TIP41 | ACT | SAND | PTBP | YLS8 |
| Comprehensive Ranking | UBC9 | GAPDH | TIP41 | EF1α | TUB6 | CYP2 | TUBA | ACT | PTBP | SAND | YLS8 |
| mannitol | |||||||||||
| geNorm | TUB6/GAPDH | / | CYP2 | ACT | YLS8 | UBC9 | TIP41 | EF1α | TUBA | PTBP | SAND |
| NormFinder | GAPDH | TUB6 | CYP2 | ACT | TIP41 | UBC9 | YLS8 | EF1α | TUBA | PTBP | SAND |
| BestKeeper | TUBA | EF1α | TUB6 | UBC9 | GAPDH | CYP2 | TIP41 | ACT | YLS8 | SAND | PTBP |
| Comprehensive Ranking | GAPDH | TUB6 | CYP2 | ACT | TUBA | UBC9 | EF1α | TIP41 | YLS8 | PTBP | SAND |
| control | |||||||||||
| geNorm | EF1α/UBC9 | / | CYP2 | YLS8 | ACT | TUB6 | GAPDH | PTBP | TUBA | TIP41 | SAND |
| NormFinder | ACT | EF1α | UBC9 | TUB6 | GAPDH | YLS8 | CYYP2 | PTBP | TUBA | TIP41 | SAND |
| BestKeeper | EF1α | UBC9 | ACT | CYP2 | YLS8 | TUB6 | GAPDH | PTBP | TIP41 | TUBA | SAND |
| Comprehensive Ranking | EF1α | ACT | UBC9 | TUB6 | CYP2 | YLS8 | GAPDH | PTBP | TUBA | TIP41 | SAND |
| Total | |||||||||||
| geNorm | UBC9/TIP41 | / | GAPDH | ACT | EF1α | CYP2 | PTBP | YLS8 | SAND | TUBA | TUB6 |
| NormFinder | TIP41 | ACT | EF1α | UBC9 | GAPDH | CYP2 | PTBP | SAND | TUBA | YLS8 | TUB6 |
| BestKeeper | TUBA | CYP2 | EF1α | GAPDH | TUB6 | UBC9 | TIP41 | ACT | SAND | YLS8 | PTBP |
| Comprehensive Ranking | TIP41 | UBC9 | ACT | EF1α | GAPDH | CYP2 | TUBA | PTBP | SAND | TUB6 | YLS8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; He, X.; Zhou, J.; Dong, Z.; Yu, H.; Tang, Q.; Yuan, L.; Peng, S.; Zhong, X.; He, Y. Selection and Verification of Standardized Reference Genes of Angelica dahurica under Various Abiotic Stresses by Real-Time Quantitative PCR. Genes 2024, 15, 79. https://doi.org/10.3390/genes15010079
Zhang J, He X, Zhou J, Dong Z, Yu H, Tang Q, Yuan L, Peng S, Zhong X, He Y. Selection and Verification of Standardized Reference Genes of Angelica dahurica under Various Abiotic Stresses by Real-Time Quantitative PCR. Genes. 2024; 15(1):79. https://doi.org/10.3390/genes15010079
Chicago/Turabian StyleZhang, Jing, Xinyi He, Jun Zhou, Zhuang Dong, Han Yu, Qi Tang, Lei Yuan, Siqing Peng, Xiaohong Zhong, and Yuedong He. 2024. "Selection and Verification of Standardized Reference Genes of Angelica dahurica under Various Abiotic Stresses by Real-Time Quantitative PCR" Genes 15, no. 1: 79. https://doi.org/10.3390/genes15010079
APA StyleZhang, J., He, X., Zhou, J., Dong, Z., Yu, H., Tang, Q., Yuan, L., Peng, S., Zhong, X., & He, Y. (2024). Selection and Verification of Standardized Reference Genes of Angelica dahurica under Various Abiotic Stresses by Real-Time Quantitative PCR. Genes, 15(1), 79. https://doi.org/10.3390/genes15010079






