Optimized Pepper Target SNP-Seq Applied in Population Structure and Genetic Diversity Analysis of 496 Pepper (Capsicum spp.) Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and DNA Isolation
2.2. Resequencing and Genome-Wide SNP Discovery
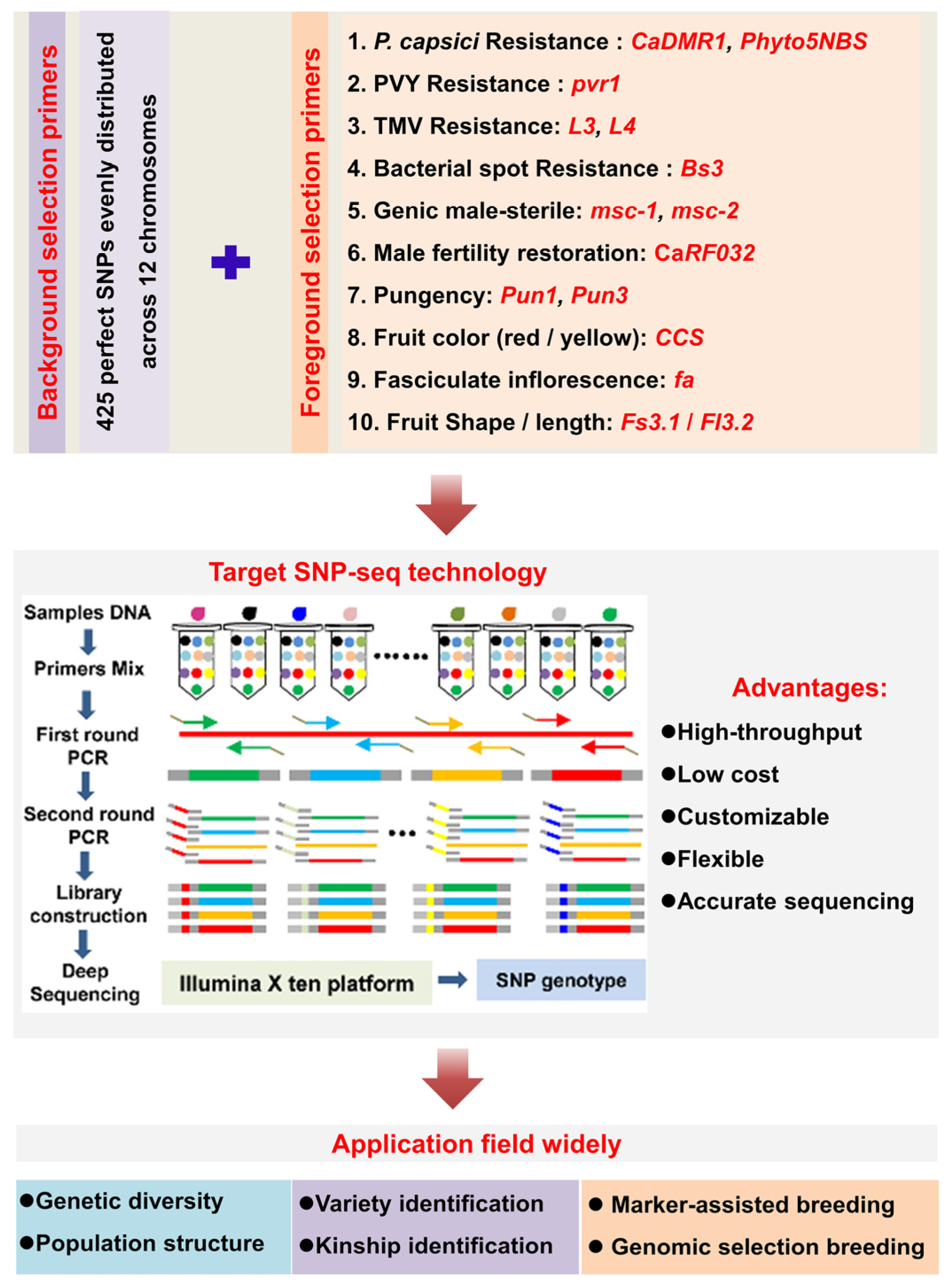
2.3. SNP Genotype Analysis by Target SNP-Seq
2.4. Genetic Structure Analysis of Pepper Lines
2.5. Population Diversity Analysis of Pepper Lines
2.6. Selection of the Core-SNPs Set for Inbred Line Discrimination
2.7. Selection of Core Pepper Lines
3. Results
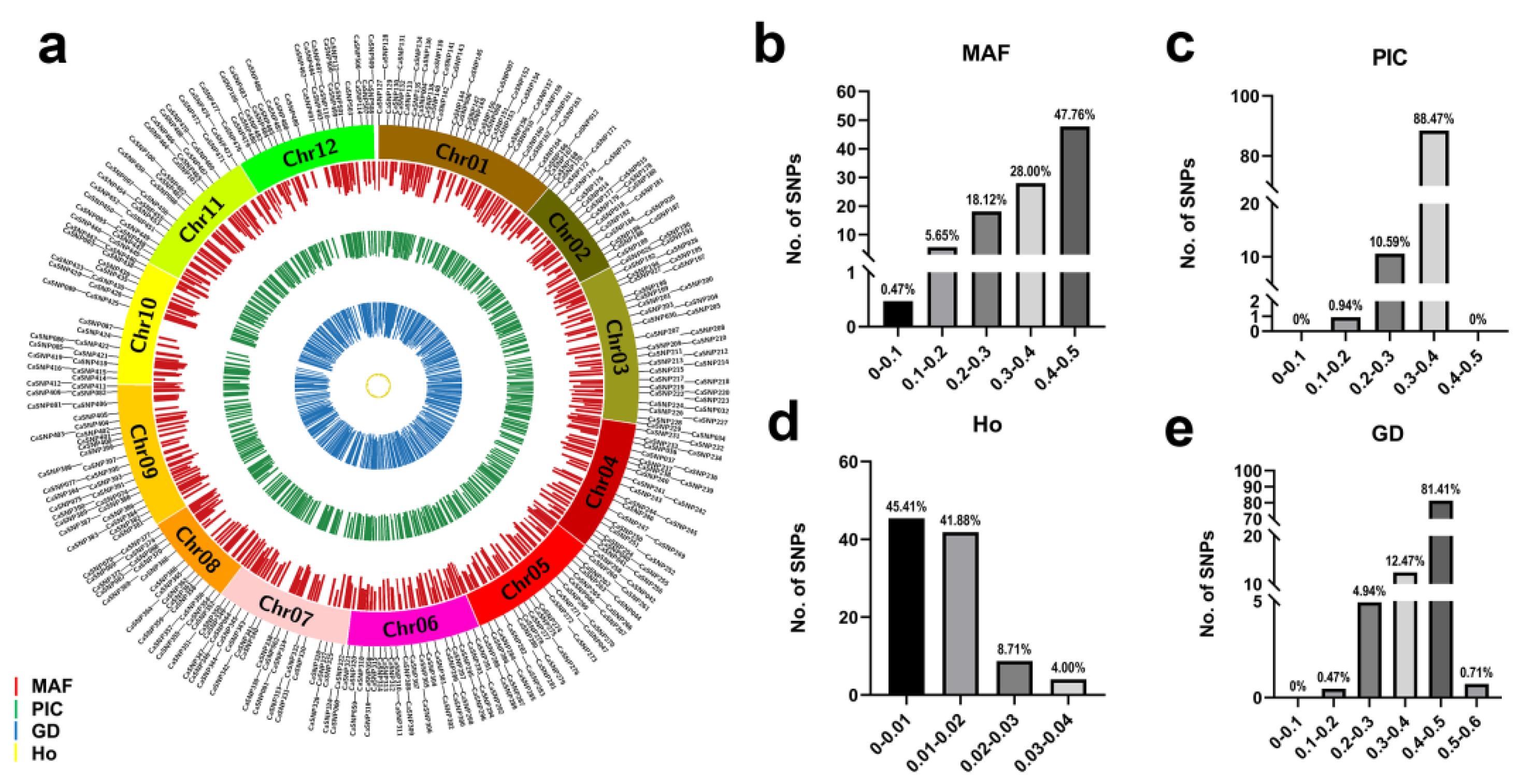
3.1. Genome-Wide Perfect SNPs Identified
3.2. Genotyping of Pepper Lines Using Target SNP-seq
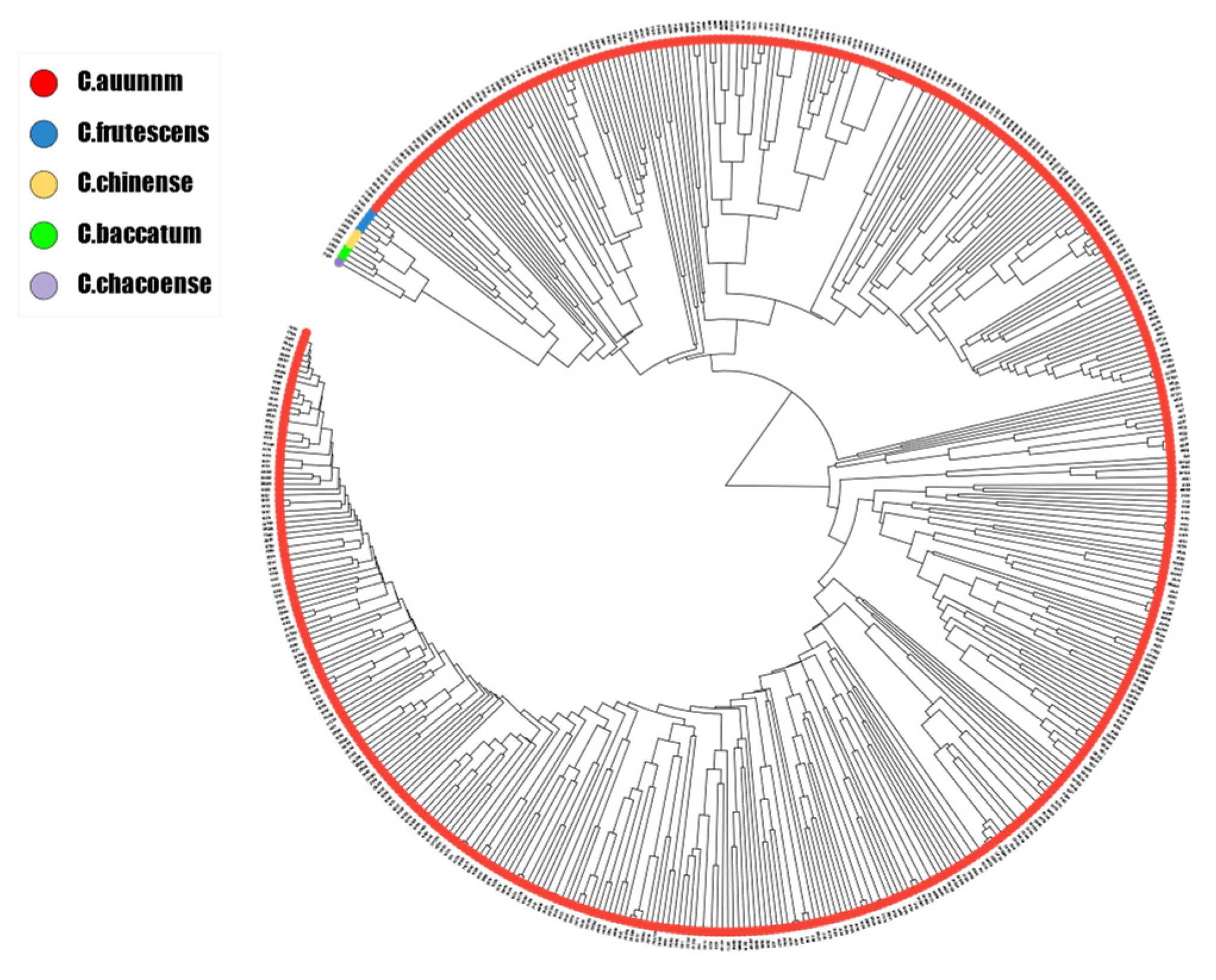
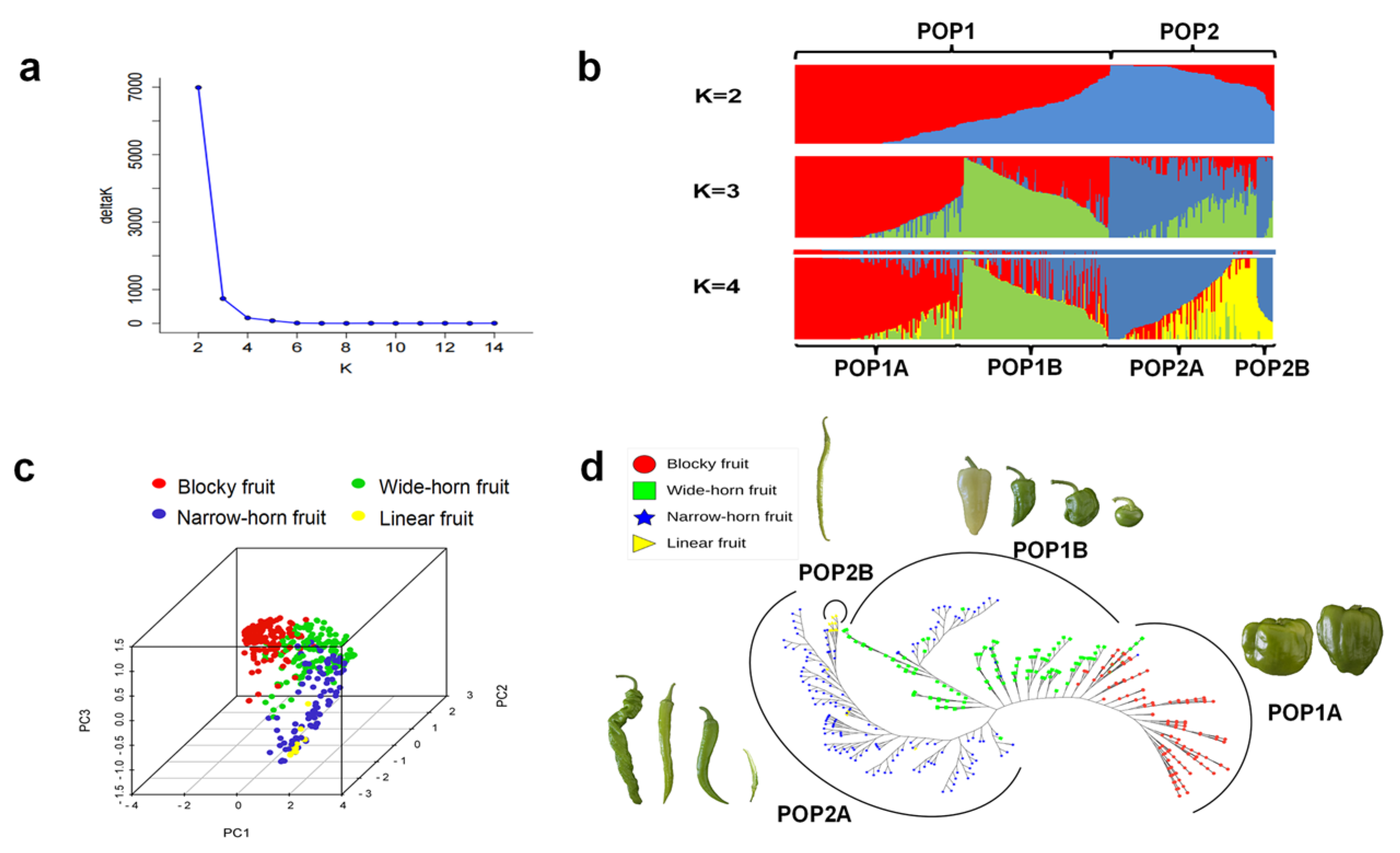
3.3. Genetic Structure of Pepper Lines
3.4. Identification of a Core-SNP Set
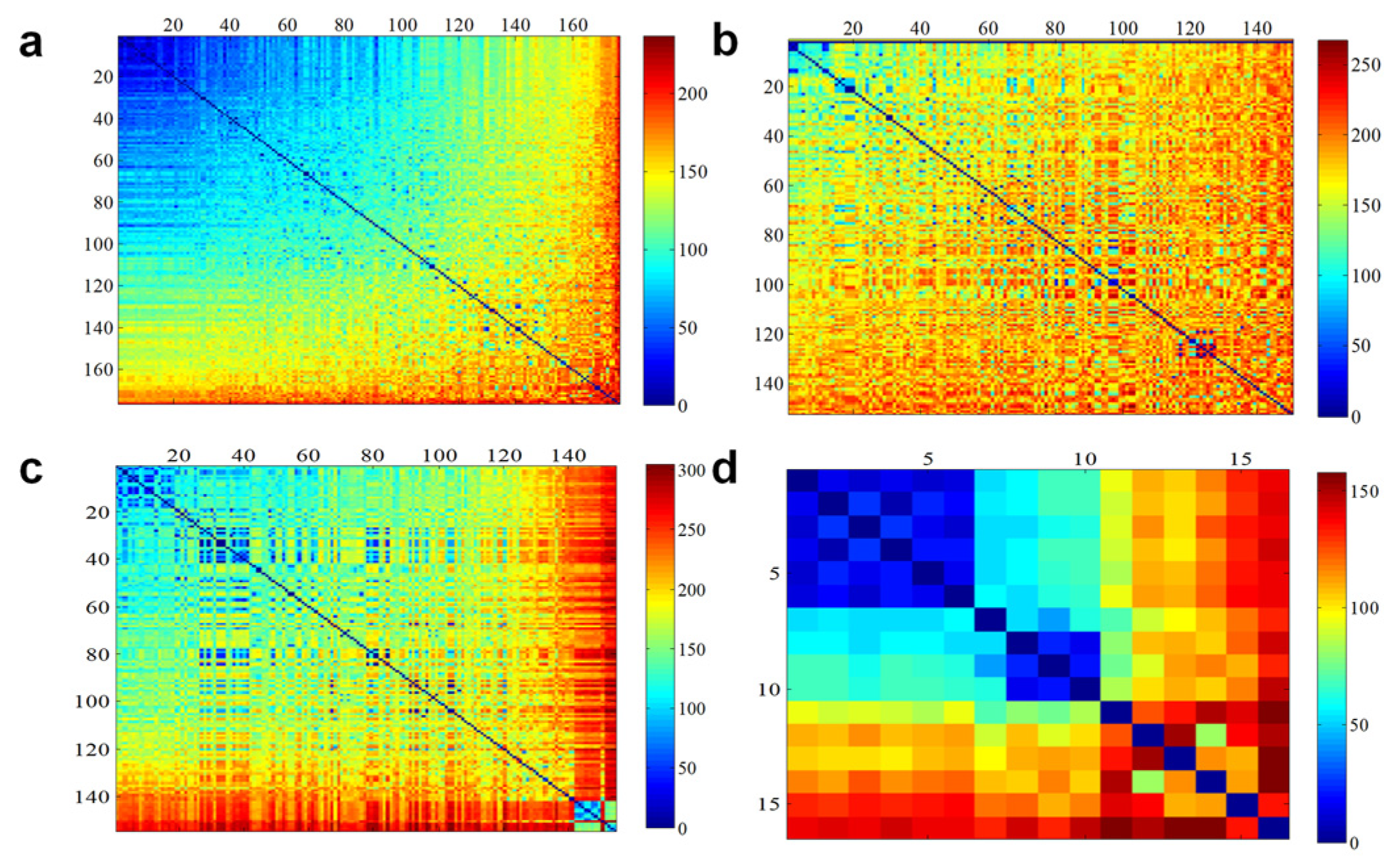
3.5. Genetic Variation in Pepper Populations
3.6. Genetic Diversity and Core Pepper Lines of Subpopulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, C.; Yu, C.S.; Shen, Y.O.; Fang, X.D.; Chen, L.; Min, J.M.; Cheng, J.W.; Zhao, S.C.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations, FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 8 August 2023).
- Barboza, G.; García, C.; Scaldaferro, M.; Bohs, L. Capsicum An amazing new (Solanaceae) species from the Andean-Amazonian Piedmont. PhytoKeys 2020, 167, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Taitano, N.E.K. Exploring Genetic Diversity Underlying Fruit Diversity in Tomato and Pepper. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2020. [Google Scholar]
- Tommasini, L.; Batley, J.; Arnold, G.; Cooke, R.; Donini, P.; Lee, D.; Law, J.; Lowe, C.; Moule, C.; Trick, M.; et al. The development of multiplex simple sequence repeat (SSR) markers to complement distinctness, uniformity and stability testing of rape (Brassica napus L.) varieties. Theor. Appl. Genet. 2003, 106, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Han, R.; Zhang, F.; Mao, A.; Luo, J.; Dong, B.; Liu, H.; Tang, H.; Zhang, J.; et al. Target SSR-Seq: A Novel SSR Genotyping Technology Associate with Perfect SSRs in Genetic Analysis of Cucumber Varieties. Front. Plant Sci. 2019, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.; Yeom, S.; Kim, Y.; Lee, J.; Lee, H.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, J.; Zhu, Z.; Liu, Y.; Chen, J.; Zhou, Y.; Liu, F.; Lei, J.; Gaut, B.; Cao, B.; et al. The 3D architecture of the pepper genome and its relationship to function and evolution. Nat. Commun. 2022, 13, 3479. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, Z.H.; Gu, X.Z.; Mao, S.L.; Li, X.X.; Chadoeuf, J.; Palloix, A.; Wang, L.H.; Zhang, B.X. Genetic diversity of pepper (Capsicum spp.) germplasm resources in China reflects selection for cultivar types and spatial distribution. J. Integr. Agric. 2016, 15, 1991–2001. [Google Scholar] [CrossRef]
- Wu, B.D.; Fan, R.; Hu, L.S.; Wu, H.S.; Hao, C.Y. Genetic diversity in the germplasm of black pepper determined by EST-SSR markers. Genet. Mol. Res. 2016, 15, 9. [Google Scholar] [CrossRef]
- Gu, X.Z.; Cao, Y.C.; Zhang, Z.H.; Zhang, B.X.; Zhao, H.; Zhang, X.M.; Wang, H.P.; Li, X.X.; Wang, L.H. Genetic diversity and population structure analysis of Capsicum germplasm accessions. J. Integr. Agric. 2019, 18, 1312–1320. [Google Scholar] [CrossRef]
- Jo, Y.D.; Park, J.; Kim, J.; Song, W.; Hur, C.G.; Lee, Y.H.; Kang, B.C. Complete sequencing and comparative analyses of the pepper (Capsicum annuum L.) plastome revealed high frequency of tandem repeats and large insertion/deletions on pepper plastome. Plant Cell Rep. 2011, 30, 217–229. [Google Scholar] [CrossRef]
- Esposito, S.; Cigliano, R.A.; Cardi, T.; Tripodi, P. Whole-genome resequencing reveals genomic footprints of Italian sweet and hot pepper heirlooms giving insight into genes underlying key agronomic and qualitative traits. BMC Genom. Data 2022, 23, 16. [Google Scholar] [CrossRef]
- Yasuda, K.; Yamaguchi, H. Genetic diversity of vegetable water pepper (Persicaria hydropiper (L.) Spach) as revealed by RAPD markers. Breed. Sci. 2005, 55, 7–14. [Google Scholar] [CrossRef]
- Lefebvre, V.; Palloix, A.; Rives, M. Nuclear RFLP between pepper cultivars (Capsicum annuum L.). Euphytica 1993, 71, 189–199. [Google Scholar] [CrossRef]
- Lee, H.; Ro, N.; Jeong, H.; Kwon, J.; Jo, J.; Ha, Y.; Jung, A.; Han, J.; Venkatesh, J.; Kang, B. Genetic diversity and population structure analysis to construct a core collection from a large Capsicum germplasm. BMC Genet. 2016, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Lozada, D.N.; Bhatta, M.; Coon, D.; Bosland, P.W. Single nucleotide polymorphisms reveal genetic diversity in New Mexican chile peppers (Capsicum spp.). BMC Genom. 2021, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Bjornstad, A.; Ndjiondjop, M.N. An overview of molecular marker methods for plants. AFR. J. Biotechnol. 2006, 5, 2540–2568. [Google Scholar]
- Yanagisawa, T.; Kiribuchi-Otobe, C.; Hirano, H.; Suzuki, Y.; Fujita, M. Detection of single nucleotide polymorphism (SNP) controlling the waxy character in wheat by using a derived cleaved amplified polymorphic sequence (dCAPS) marker. Theor. Appl. Genet. 2003, 107, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2014, 33, 1–14. [Google Scholar] [CrossRef]
- Meaburn, E.; Butcher, L.; Schalkwyk, L.; Plomin, R. Genotyping pooled DNA using 100K SNP microarrays: A step towards genomewide association scans. Nucleic Acids Res. 2006, 34, e27. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.; Spacek, D.; Snyder, M. High-throughput sequencing technologies. Mol. Cell 2015, 58, 586–597. [Google Scholar] [CrossRef]
- Ma, J.; Cao, Y.Y.; Wang, Y.Z.; Ding, Y. Development of the maize 5.5K loci panel for genomic prediction through genotyping by target sequencing. Front. Plant Sci. 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, J.A.; Shi, X.L.; Chen, L.; Qin, J.; Zhang, M.C.; Yang, C.Y.; Song, Q.J.; Yan, L. Development of SNP marker panels for genotyping by target sequencing (GBTS) and its application in soybean. Mol. Breed. 2023, 43, 11. [Google Scholar] [CrossRef]
- Guo, Z.F.; Wang, H.W.; Tao, J.J.; Ren, Y.H.; Xu, C.; Wu, K.S.; Zou, C.; Zhang, J.N.; Xu, Y.B. Development of multiple SNP marker panels affordable to breeders through genotyping by target sequencing (GBTS) in maize. Mol. Breed. 2019, 39, 12. [Google Scholar] [CrossRef]
- Shen, Y.S.; Wang, J.S.; Shaw, R.K.; Yu, H.F.; Sheng, X.G.; Zhao, Z.Q.; Li, S.J.; Gu, H.H. Development of GBTS and KASP Panels for Genetic Diversity, Population Structure, and Fingerprinting of a Large Collection of Broccoli (Brassica oleracea L. var. italica) in China. Front. Plant Sci. 2021, 12, 17. [Google Scholar] [CrossRef]
- Guo, Z.F.; Yang, Q.; Huang, F.F.; Zheng, H.J.; Sang, Z.Q.; Xu, Y.F.; Zhang, C.; Wu, K.S.; Tao, J.J.; Prasanna, B.M.; et al. Development of high-resolution multiple-SNP arrays for genetic analyses and molecular breeding through genotyping by target sequencing and liquid chip. Plant Commun. 2021, 2, 15. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.J.; Zhang, L.K.; Luo, J.; Zhao, H.; Zhang, J.A.; Wen, C.L. A new SNP genotyping technology Target SNP-seq and its application in genetic analysis of cucumber varieties. Sci. Rep. 2020, 10, 11. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Lv, Y.; Zhang, X.; Xia, C.; Zhao, H.; Wen, C. Genetic diversity analysis and variety identification using SSR and SNP markers in melon. BMC Plant Biol. 2023, 23, 39. [Google Scholar] [CrossRef]
- Liu, W.L.; Qian, Z.W.; Zhang, J.; Yang, J.J.; Wu, M.S.; Barchi, L.; Zhao, H.Y.; Sun, H.H.; Cui, Y.L.; Wen, C.L. Impact of fruit shape selection on genetic structure and diversity uncovered from genome-wide perfect SNPs genotyping in eggplant. Mol. Breed. 2019, 39, 13. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhang, J.; Du, H.S.; Zhao, H.; Mao, A.J.; Zhang, X.F.; Jiang, L.; Zhang, H.Y.; Wen, C.L.; Xu, Y. Genetic relationship and pedigree of Chinese watermelon varieties based on diversity of perfect SNPs. Hortic. Plant J. 2022, 8, 489–498. [Google Scholar] [CrossRef]
- Du, H.; Yang, J.; Chen, B.; Zhang, X.; Zhang, J.; Yang, K.; Geng, S.; Wen, C. Target sequencing reveals genetic diversity, population structure, core-SNP markers, and fruit shape-associated loci in pepper varieties. BMC Plant Biol. 2019, 19, 578. [Google Scholar] [CrossRef]
- Fulton, T.M.; Tanksley, C.S.D. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol. Biol. Rep. 1995, 13, 207–209. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Husson, F.; Josse, J.; Pagès, J. Principal component methods—Hierarchical clustering—Partitional clustering: Why would we need to choose for visualizing data? Unpublished work. 2010. [Google Scholar]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Smouse, P.; Quattro, J. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- de Meeûs, T.; Goudet, J. A step-by-step tutorial to use HierFstat to analyse populations hierarchically structured at multiple levels. Infect. Genet. Evol. 2007, 7, 731–735. [Google Scholar] [CrossRef]
- Jones, H.; Norris, C.; Smith, D.; Cockram, J.; Lee, D.; O’Sullivan, D.M.; Mackay, I. Evaluation of the use of high-density SNP genotyping to implement UPOV Model 2 for DUS testing in barley. Theor. Appl. Genet. 2013, 126, 901–911. [Google Scholar] [CrossRef]
- Moreira, A.F.P.; Ruas, P.M.; Ruas, C.d.F.; Baba, V.Y.; Giordani, W.; Arruda, I.M.; Rodrigues, R.; Gonalves, L.S.A. Genetic diversity, population structure and genetic parameters of fruit traits in Capsicum chinense. Sci. Hortic. 2018, 236, 1–9. [Google Scholar] [CrossRef]
- Colonna, V.; D’Agostino, N.; Garrison, E.; Albrechtsen, A.; Tripodi, P. Genomic diversity and novel genome-wide association with fruit morphology in Capsicum, from 746k polymorphic sites. Sci. Rep. 2019, 9, 10067. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, S.; Garcés-Claver, A.; Mallor, C.; Sáenz de Miera, L.; Fayos, O.; Pomar, F.; Merino, F.; Silvar, C. New insights into Capsicum spp. relatedness and the diversification process of Capsicum annuum in Spain. PLoS ONE 2014, 9, e116276. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, A.; Eguiarte, L.; Mercer, K.; Martínez-Ainsworth, N.; McHale, L.; van der Knaap, E.; Jardón-Barbolla, L. Genetic diversity, gene flow, and differentiation among wild, semiwild, and landrace chile pepper (Capsicum annuum) populations in Oaxaca, Mexico. Am. J. Bot. 2022, 109, 1157–1176. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Liu, Z.; Hu, Y.; Tian, J.; Yang, T.; Wei, A. Genomic analysis reveals the genetic diversity, population structure, evolutionary history and relationships of Chinese pepper. Hortic. Res. 2020, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.; Cantet, M.; Lefebvre, V.; Sage-Palloix, A.M.; Palloix, A. Genotyping a large collection of pepper (Capsicum spp.) with SSR loci brings new evidence for the wild origin of cultivated C. annuum and the structuring of genetic diversity by human selection of cultivar types. Genet. Resour. Crop Evol. 2013, 60, 2375–2390. [Google Scholar] [CrossRef]
- Pereira-Dias, L.; Vilanova, S.; Fita, A.; Prohens, J.; Rodríguez-Burruezo, A. Genetic diversity, population structure, and relationships in a collection of pepper (Capsicum spp.) landraces from the Spanish centre of diversity revealed by genotyping-by-sequencing (GBS). Hortic. Res. 2019, 6, 54. [Google Scholar] [CrossRef]
- Ibiza, V.P.; Blanca, J.; Cañizares, J.; Nuez, F. Taxonomy and genetic diversity of domesticated Capsicum species in the Andean region. Genet. Resour. Crop Evol. 2012, 59, 1077–1088. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, K.; Yu, H.; Chen, S.; Xu, D.; Zhao, H.; Zhang, Z.; Yang, Y.; Gu, X.; Liu, X.; et al. Pepper variome reveals the history and key loci associated with fruit domestication and diversification. Mol. Plant 2022, 15, 1744–1758. [Google Scholar] [CrossRef]
- Costa, M.; do Rêgo, M.; da Silva, A.; do Rêgo, E.; Barroso, P. Characterization and genetic diversity of pepper (Capsicum spp.) parents and interspecific hybrids. Genet. Mol. Res. GMR 2016, 15, gmr.15027652. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.; Tikunov, Y.; de Vos, R.; Pelgrom, K.; Maharijaya, A.; Sudarmonowati, E.; Bino, R.; Bovy, A. Metabolomics and molecular marker analysis to explore pepper (Capsicum spp.) biodiversity. Metabolomics 2013, 9, 130–144. [Google Scholar] [CrossRef]
- Mcleod, M.J.; Guttman, S.I.; Eshbaugh, W.H.; Rayle, R.E. An Electrophoretic Study of Evolution in Capsicum (Solanaceae). Evolution 1983, 37, 562–574. [Google Scholar] [CrossRef]
- Moscone, E.A.; Scaldaferro, M.A.; Grabiele, M.; Cecchini, N.M.; Sánchez García, Y.; Jarret, R.; Daviña, J.R.; Ducasse, D.A.; Barboza, G.E.; Ehrendorfer, F. The evolution of chili peppers (Capsicum-Solanaceae): A cytogenetic perspective. Acta Hortic. 2007, 745, 137–170. [Google Scholar] [CrossRef]
- Xu, W.; Huang, H.; Li, X.; Yang, M.; Chi, S.; Pan, Y.; Li, N.; Paterson, A.; Chai, Y.; Lu, K. CaHMA1 promotes Cd accumulation in pepper fruit. J. Hazard. Mater. 2023, 460, 132480. [Google Scholar] [CrossRef]
- Tam, S.; Lefebvre, V.; Palloix, A.; Sage-Palloix, A.; Mhiri, C.; Grandbastien, M. LTR-retrotransposons Tnt1 and T135 markers reveal genetic diversity and evolutionary relationships of domesticated peppers. Theor. Appl. Genet. 2009, 119, 973–989. [Google Scholar] [CrossRef][Green Version]
- Solomon, A.; Han, K.; Lee, J.; Lee, H.; Jang, S.; Kang, B. Genetic diversity and population structure of Ethiopian Capsicum germplasms. PLoS ONE 2019, 14, e0216886. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wang, P.; Liu, J.; Wu, L.; Zhang, Z.; Li, T.; Gao, W.; Yang, W.; Sun, L.; Shen, H. Identification of candidate genes underlying genic male-sterile msc-1 locus via genome resequencing in Capsicum annuum L. Theor. Appl. Genet. 2018, 131, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Li, T.; Ai, Y.; Lu, Q.; Wang, Y.; Wu, L.; Liu, J.; Sun, L.; Shen, H. Phenotypic, genetic, and molecular function of msc-2, a genic male sterile mutant in pepper (Capsicum annuum L.). Theor. Appl. Genet. 2020, 133, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Kang, B.; Liu, K.; Mazourek, M.; Moore, S.; Yoo, E.; Kim, B.; Paran, I.; Jahn, M. The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J. 2005, 42, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jang, S.; Lee, J.; Lee, D.; Kwon, J.; Kang, B. A MYB transcription factor is a candidate to control pungency in Capsicum annuum. Theor. Appl. Genet. 2019, 132, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Y.; Cao, Y.; Yu, H.; Bai, R.; Zhao, H.; Zhang, B.; Wang, L. Fine mapping of the male fertility restoration gene CaRf032 in Capsicum annuum L. Theor. Appl. Genet. 2020, 133, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, Y.; Liu, Z.; Wang, J.; Zou, X. Mapping and identifying candidate genes involved in the novel fasciculate inflorescence in pepper (Capsicum annuum L.). Mol. Breed. 2019, 39, 148. [Google Scholar] [CrossRef]
- Popovsky, S.; Paran, I. Molecular genetics of the y locus in pepper: Its relation to capsanthin-capsorubin synthase and to fruit color. Theor. Appl. Genet. 2000, 101, 86–89. [Google Scholar] [CrossRef]

| Source of Variation | Degrees of Freedom | Sum of Squares | Mean Square | Variation Component | Percentage of Variation |
|---|---|---|---|---|---|
| Between populations | 1 | 3612.23 | 3612.23 | 1.52 | 1.53 |
| Between subpopulations within populations | 2 | 3886.47 | 1943.24 | 9.26 | 9.32 |
| Between samples within subpopulations | 480 | 85,948.64 | 174.69 | 86.08 | 86.6 |
| Within samples | 484 | 1257.78 | 2.54 | 2.54 | 2.55 |
| Total | 967 | 94,705.12 | 95.57 | 99.4 | 100 |
| (Sub) Populations | POP1 | POP1B | POP2A | POP2B |
|---|---|---|---|---|
| POP2 | 0.3027 | |||
| POP1A | 0.1988 | 0.4363 | 0.6229 | |
| POP1B | 0.2215 | 0.3982 | ||
| POP2A | 0.1672 |
| Subpopulation | Number of Pepper Lines | Number of Polymorphic Markers | PIC | MAF | Ho | GD | Inbreeding Coefficient |
|---|---|---|---|---|---|---|---|
| POP1A | 183 | 420 | 0.223 | 0.189 | 0.006 | 0.27 | 0.972 |
| POP1B | 170 | 424 | 0.312 | 0.319 | 0.007 | 0.4 | 0.981 |
| POP2A | 120 | 425 | 0.301 | 0.285 | 0.021 | 0.379 | 0.936 |
| POP2B | 11 | 291 | 0.23 | 0.197 | 0.01 | 0.279 | 0.956 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, X.; Yang, J.; Chen, B.; Zhang, J.; Li, W.; Du, H.; Geng, S. Optimized Pepper Target SNP-Seq Applied in Population Structure and Genetic Diversity Analysis of 496 Pepper (Capsicum spp.) Lines. Genes 2024, 15, 214. https://doi.org/10.3390/genes15020214
Wang Y, Zhang X, Yang J, Chen B, Zhang J, Li W, Du H, Geng S. Optimized Pepper Target SNP-Seq Applied in Population Structure and Genetic Diversity Analysis of 496 Pepper (Capsicum spp.) Lines. Genes. 2024; 15(2):214. https://doi.org/10.3390/genes15020214
Chicago/Turabian StyleWang, Yihao, Xiaofen Zhang, Jingjing Yang, Bin Chen, Jian Zhang, Wenyue Li, Heshan Du, and Sansheng Geng. 2024. "Optimized Pepper Target SNP-Seq Applied in Population Structure and Genetic Diversity Analysis of 496 Pepper (Capsicum spp.) Lines" Genes 15, no. 2: 214. https://doi.org/10.3390/genes15020214
APA StyleWang, Y., Zhang, X., Yang, J., Chen, B., Zhang, J., Li, W., Du, H., & Geng, S. (2024). Optimized Pepper Target SNP-Seq Applied in Population Structure and Genetic Diversity Analysis of 496 Pepper (Capsicum spp.) Lines. Genes, 15(2), 214. https://doi.org/10.3390/genes15020214





