HERVs: Expression Control Mechanisms and Interactions in Diseases and Human Immunodeficiency Virus Infection
Abstract
1. Introduction
2. ERV Expression Regulation Mechanisms
2.1. Methylation
2.1.1. DNA Methylation
2.1.2. Histone Methylation
2.2. Histone Acetylation
2.3. Cytosine Deamination
2.4. Chromatin Remodeling
3. HERVs in Inflammatory Diseases and Cancer
4. HERV Expression and HIV Infection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mager, D.L.; Stoye, J.P. Mammalian Endogenous Retroviruses. Microbiol. Spectr. 2015, 3, MDNA3-0009-2014. [Google Scholar] [CrossRef]
- Lee, Y.N.; Bieniasz, P.D. Reconstitution of an Infectious Human Endogenous Retrovirus. PLoS Pathog. 2007, 3, e10. [Google Scholar] [CrossRef]
- Marchi, E.; Kanapin, A.; Magiorkinis, G.; Belshaw, R. Unfixed Endogenous Retroviral Insertions in the Human Population. J. Virol. 2014, 88, 9529–9537. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Q.; Cong, Y.S. Human Endogenous Retroviruses in Development and Disease. Comput. Struct. Biotechnol. J. 2021, 19, 5978–5986. [Google Scholar] [CrossRef]
- Vargiu, L.; Rodriguez-Tomé, P.; Sperber, G.O.; Cadeddu, M.; Grandi, N.; Blikstad, V.; Tramontano, E.; Blomberg, J. Classification and Characterization of Human Endogenous Retroviruses; Mosaic Forms Are Common. Retrovirology 2016, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Benachenhou, F.; Blikstad, V.; Sperber, G.; Mayer, J. Classification and Nomenclature of Endogenous Retroviral Sequences (ERVs): Problems and Recommendations. Gene 2009, 448, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Fauquet, C.; Fargette, D. International Committee on Taxonomy of Viruses and the 3,142 Unassigned Species. Virol. J. 2005, 2, 64. [Google Scholar] [CrossRef]
- Seifarth, W.; Frank, O.; Zeilfelder, U.; Spiess, B.; Greenwood, A.D.; Hehlmann, R.; Leib-Mösch, C. Comprehensive Analysis of Human Endogenous Retrovirus Transcriptional Activity in Human Tissues with a Retrovirus-Specific Microarray. J. Virol. 2005, 79, 341–352. [Google Scholar] [CrossRef]
- Grandi, N.; Cadeddu, M.; Blomberg, J.; Tramontano, E. Contribution of Type W Human Endogenous Retroviruses to the Human Genome: Characterization of HERV-W Proviral Insertions and Processed Pseudogenes. Retrovirology 2016, 13, 67. [Google Scholar] [CrossRef]
- Göke, J.; Lu, X.; Chan, Y.-S.; Ng, H.-H.; Ly, L.-H.; Sachs, F.; Szczerbinska, I. Dynamic Transcription of Distinct Classes of Endogenous Retroviral Elements Marks Specific Populations of Early Human Embryonic Cells. Cell Stem Cell 2015, 16, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Sugimoto, R.; Nakaoka, H.; Yamada, S.; Kimura, T.; Hayano, T.; Inoue, I. Systematic Identification and Characterization of Regulatory Elements Derived from Human Endogenous Retroviruses. PLoS Genet. 2017, 13, e1006883. [Google Scholar] [CrossRef]
- Medstrand, P.; Landry, J.-R.; Mager, D.L. Long Terminal Repeats Are Used as Alternative Promoters for the Endothelin B Receptor and Apolipoprotein C-I Genes in Humans. J. Biol. Chem. 2001, 276, 1896–1903. [Google Scholar] [CrossRef]
- Jern, P.; Coffin, J.M. Effects of Retroviruses on Host Genome Function. Annu. Rev. Genet. 2008, 42, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Dolei, A. The Aliens inside Us: HERV-W Endogenous Retroviruses and Multiple Sclerosis. Mult. Scler. J. 2018, 24, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Dubowsky, M.; Theunissen, F.; Carr, J.M.; Rogers, M.-L. The Molecular Link Between TDP-43, Endogenous Retroviruses and Inflammatory Neurodegeneration in Amyotrophic Lateral Sclerosis: A Potential Target for Triumeq, an Antiretroviral Therapy. Mol. Neurobiol. 2023, 60, 6330–6345. [Google Scholar] [CrossRef] [PubMed]
- Kitsou, K.; Lagiou, P.; Magiorkinis, G. Human Endogenous Retroviruses in Cancer: Oncogenesis Mechanisms and Clinical Implications. J. Med. Virol. 2023, 95, e28350. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Galindo, R.; González, M.; Almodovar-Camacho, S.; González-Ramírez, S.; Lorenzo, E.; Yamamura, Y. A New Real-Time-RT-PCR for Quantitation of Human Endogenous Retroviruses Type K (HERV-K) RNA Load in Plasma Samples: Increased HERV-K RNA Titers in HIV-1 Patients with HAART Non-Suppressive Regimens. J. Virol. Methods 2006, 136, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Yang, P.; Füchtbauer, A.C.; Füchtbauer, E.-M.; Silva, A.M.; Park, C.; Wu, W.; Nielsen, A.L.; Pedersen, F.S.; Macfarlan, T.S. The KRAB Zinc Finger Protein ZFP809 Is Required to Initiate Epigenetic Silencing of Endogenous Retroviruses. Genes Dev. 2015, 29, 538–554. [Google Scholar] [CrossRef]
- Feldman, N.; Gerson, A.; Fang, J.; Li, E.; Zhang, Y.; Shinkai, Y.; Cedar, H.; Bergman, Y. G9a-Mediated Irreversible Epigenetic Inactivation of Oct-3/4 during Early Embryogenesis. Nat. Cell Biol. 2006, 8, 188–194. [Google Scholar] [CrossRef]
- Khodosevich, K. Large-Scale Determination of the Methylation Status of Retrotransposons in Different Tissues Using a Methylation Tags Approach. Nucleic Acids Res. 2004, 32, 31e. [Google Scholar] [CrossRef]
- Ohtani, H.; Liu, M.; Zhou, W.; Liang, G.; Jones, P.A. Switching Roles for DNA and Histone Methylation Depend on Evolutionary Ages of Human Endogenous Retroviruses. Genome Res. 2018, 28, 1147–1157. [Google Scholar] [CrossRef]
- Geis, F.K.; Goff, S.P. Silencing and Transcriptional Regulation of Endogenous Retroviruses: An Overview. Viruses 2020, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Park, J.S.; Jeong, Y.S.; Jeon, K.; Kang, Y.-K. Dnmt1 Binds and Represses Genomic Retroelements via DNA Methylation in Mouse Early Embryos. Nucleic Acids Res. 2020, 48, 8431–8444. [Google Scholar] [CrossRef]
- Peng, H.; Begg, G.E.; Harper, S.L.; Friedman, J.R.; Speicher, D.W.; Rauscher, F.J. Biochemical Analysis of the Kruppel-Associated Box (KRAB) Transcriptional Repression Domain. J. Biol. Chem. 2000, 275, 18000–18010. [Google Scholar] [CrossRef] [PubMed]
- Schön, U.; Diem, O.; Leitner, L.; Gönzburg, W.H.; Mager, D.L.; Salmons, B.; Leib-Mösch, C. Human Endogenous Retroviral Long Terminal Repeat Sequences as Cell Type-Specific Promoters in Retroviral Vectors. J. Virol. 2009, 83, 12643–12650. [Google Scholar] [CrossRef]
- Ruda, V.M.; Akopov, S.B.; Trubetskoy, D.O.; Manuylov, N.L.; Vetchinova, A.S.; Zavalova, L.L.; Nikolaev, L.G.; Sverdlov, E.D. Tissue Specificity of Enhancer and Promoter Activities of a HERV-K(HML-2) LTR. Virus Res. 2004, 104, 11–16. [Google Scholar] [CrossRef]
- Turelli, P.; Castro-Diaz, N.; Marzetta, F.; Kapopoulou, A.; Raclot, C.; Duc, J.; Tieng, V.; Quenneville, S.; Trono, D. Interplay of TRIM28 and DNA Methylation in Controlling Human Endogenous Retroelements. Genome Res. 2014, 24, 1260–1270. [Google Scholar] [CrossRef]
- Wolf, D.; Goff, S.P. TRIM28 Mediates Primer Binding Site-Targeted Silencing of Murine Leukemia Virus in Embryonic Cells. Cell 2007, 131, 46–57. [Google Scholar] [CrossRef]
- Groner, A.C.; Meylan, S.; Ciuffi, A.; Zangger, N.; Ambrosini, G.; Dénervaud, N.; Bucher, P.; Trono, D. KRAB–Zinc Finger Proteins and KAP1 Can Mediate Long-Range Transcriptional Repression through Heterochromatin Spreading. PLoS Genet. 2010, 6, e1000869. [Google Scholar] [CrossRef]
- Yang, B.; Fang, L.; Gao, Q.; Xu, C.; Xu, J.; Chen, Z.-X.; Wang, Y.; Yang, P. Species-Specific KRAB-ZFPs Function as Repressors of Retroviruses by Targeting PBS Regions. Proc. Natl. Acad. Sci. USA 2022, 119, e2119415119. [Google Scholar] [CrossRef] [PubMed]
- Tie, C.H.; Fernandes, L.; Conde, L.; Robbez-Masson, L.; Sumner, R.P.; Peacock, T.; Rodriguez-Plata, M.T.; Mickute, G.; Gifford, R.; Towers, G.J.; et al. KAP1 Regulates Endogenous Retroviruses in Adult Human Cells and Contributes to Innate Immune Control. EMBO Rep. 2018, 19, e45000. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Kimura, I.; Soper, A.; Coudray, A.; Koyanagi, Y.; Nakaoka, H.; Inoue, I.; Turelli, P.; Trono, D.; Sato, K. Endogenous Retroviruses Drive KRAB Zinc-Finger Protein Family Expression for Tumor Suppression. Sci. Adv. 2020, 6, eabc3020. [Google Scholar] [CrossRef]
- Seczynska, M.; Bloor, S.; Cuesta, S.M.; Lehner, P.J. Genome Surveillance by HUSH-Mediated Silencing of Intronless Mobile Elements. Nature 2022, 601, 440–445. [Google Scholar] [CrossRef]
- Tchasovnikarova, I.A.; Timms, R.T.; Matheson, N.J.; Wals, K.; Antrobus, R.; Göttgens, B.; Dougan, G.; Dawson, M.A.; Lehner, P.J. Epigenetic Silencing by the HUSH Complex Mediates Position-Effect Variegation in Human Cells. Science 2015, 348, 1481–1485. [Google Scholar] [CrossRef]
- Chougui, G.; Munir-Matloob, S.; Matkovic, R.; Martin, M.M.; Morel, M.; Lahouassa, H.; Leduc, M.; Ramirez, B.C.; Etienne, L.; Margottin-Goguet, F. HIV-2/SIV Viral Protein X Counteracts HUSH Repressor Complex. Nat. Microbiol. 2018, 3, 891–897. [Google Scholar] [CrossRef]
- Seczynska, M.; Lehner, P.J. The Sound of Silence: Mechanisms and Implications of HUSH Complex Function. Trends Genet. 2023, 39, 251–267. [Google Scholar] [CrossRef]
- Robbez-Masson, L.; Tie, C.H.C.; Conde, L.; Tunbak, H.; Husovsky, C.; Tchasovnikarova, I.A.; Timms, R.T.; Herrero, J.; Lehner, P.J.; Rowe, H.M. The HUSH Complex Cooperates with TRIM28 to Repress Young Retrotransposons and New Genes. Genome Res. 2018, 28, 836–845. [Google Scholar] [CrossRef]
- Rowe, H.M.; Friedli, M.; Offner, S.; Verp, S.; Mesnard, D.; Marquis, J.; Aktas, T.; Trono, D. De Novo DNA Methylation of Endogenous Retroviruses Is Shaped by KRAB-ZFPs/KAP1 and ESET. Development 2013, 140, 519–529. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Shvedunova, M.; Akhtar, A. Modulation of Cellular Processes by Histone and Non-Histone Protein Acetylation. Nat. Rev. Mol. Cell Biol. 2022, 23, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Hurst, T.; Pace, M.; Katzourakis, A.; Phillips, R.; Klenerman, P.; Frater, J.; Magiorkinis, G. Human Endogenous Retrovirus (HERV) Expression Is Not Induced by Treatment with the Histone Deacetylase (HDAC) Inhibitors in Cellular Models of HIV-1 Latency. Retrovirology 2016, 13, 10. [Google Scholar] [CrossRef]
- White, C.H.; Beliakova-Bethell, N.; Lada, S.M.; Breen, M.S.; Hurst, T.P.; Spina, C.A.; Richman, D.D.; Frater, J.; Magiorkinis, G.; Woelk, C.H. Transcriptional Modulation of Human Endogenous Retroviruses in Primary CD4+ T Cells Following Vorinostat Treatment. Front. Immunol. 2018, 9, 603. [Google Scholar] [CrossRef]
- Esnault, C.; Priet, S.; Ribet, D.; Heidmann, O.; Heidmann, T. Restriction by APOBEC3 Proteins of Endogenous Retroviruses with an Extracellular Life Cycle: Ex Vivo Effects and in Vivo “Traces” on the Murine IAPE and Human HERV-K Elements. Retrovirology 2008, 5, 75. [Google Scholar] [CrossRef]
- Delviks-Frankenberry, K.A.; Desimmie, B.A.; Pathak, V.K. Structural Insights into APOBEC3-Mediated Lentiviral Restriction. Viruses 2020, 12, 587. [Google Scholar] [CrossRef]
- Chiu, Y.-L.; Greene, W.C. The APOBEC3 Cytidine Deaminases: An Innate Defensive Network Opposing Exogenous Retroviruses and Endogenous Retroelements. Annu. Rev. Immunol. 2008, 26, 317–353. [Google Scholar] [CrossRef]
- Ito, J.; Gifford, R.J.; Sato, K. Retroviruses Drive the Rapid Evolution of Mammalian APOBEC3 Genes. Proc. Natl. Acad. Sci. USA 2020, 117, 610–618. [Google Scholar] [CrossRef]
- Wilson, B.G.; Roberts, C.W.M. SWI/SNF Nucleosome Remodellers and Cancer. Nat. Rev. Cancer 2011, 11, 481–492. [Google Scholar] [CrossRef]
- Zhou, M.; Leung, J.Y.; Gessner, K.H.; Hepperla, A.J.; Simon, J.M.; Davis, I.J.; Kim, W.Y. PBRM1 Inactivation Promotes Upregulation of Human Endogenous Retroviruses in a HIF-Dependent Manner. Cancer Immunol. Res. 2022, 10, 285–290. [Google Scholar] [CrossRef]
- Groh, S.; Milton, A.V.; Marinelli, L.K.; Sickinger, C.V.; Russo, A.; Bollig, H.; de Almeida, G.P.; Schmidt, A.; Forné, I.; Imhof, A.; et al. Morc3 Silences Endogenous Retroviruses by Enabling Daxx-Mediated Histone H3.3 Incorporation. Nat. Commun. 2021, 12, 5996. [Google Scholar] [CrossRef]
- Garland, W.; Müller, I.; Wu, M.; Schmid, M.; Imamura, K.; Rib, L.; Sandelin, A.; Helin, K.; Jensen, T.H. Chromatin Modifier HUSH Co-Operates with RNA Decay Factor NEXT to Restrict Transposable Element Expression. Mol. Cell 2022, 82, 1691–1707.e8. [Google Scholar] [CrossRef]
- Tovo, P.-A.; Marozio, L.; Abbona, G.; Calvi, C.; Frezet, F.; Gambarino, S.; Dini, M.; Benedetto, C.; Galliano, I.; Bergallo, M. Pregnancy Is Associated with Impaired Transcription of Human Endogenous Retroviruses and of TRIM28 and SETDB1, Particularly in Mothers Affected by Multiple Sclerosis. Viruses 2023, 15, 710. [Google Scholar] [CrossRef]
- Brudek, T.; Christensen, T.; Aagaard, L.; Petersen, T.; Hansen, H.J.; Møller-Larsen, A. B Cells and Monocytes from Patients with Active Multiple Sclerosis Exhibit Increased Surface Expression of Both HERV-H Env and HERV-W Env, Accompanied by Increased Seroreactivity. Retrovirology 2009, 6, 104. [Google Scholar] [CrossRef]
- Tegla, C.A.; Azimzadeh, P.; Andrian-Albescu, M.; Martin, A.; Cudrici, C.D.; Trippe, R.; Sugarman, A.; Chen, H.; Boodhoo, D.; Vlaicu, S.I.; et al. SIRT1 Is Decreased during Relapses in Patients with Multiple Sclerosis. Exp. Mol. Pathol. 2014, 96, 139–148. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Zhang, N.; Fan, D. Human Endogenous Retrovirus K (HERV-K) Env in Neuronal Extracellular Vesicles: A New Biomarker of Motor Neuron Disease. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 100–107. [Google Scholar] [CrossRef]
- Li, W.; Lee, M.-H.; Henderson, L.; Tyagi, R.; Bachani, M.; Steiner, J.; Campanac, E.; Hoffman, D.A.; von Geldern, G.; Johnson, K.; et al. Human Endogenous Retrovirus-K Contributes to Motor Neuron Disease. Sci. Transl. Med. 2015, 7, 307ra153. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, M.E.; Faulkner, G.J. Endogenous Retroviruses Can Propagate TDP-43 Proteinopathy. Trends Neurosci. 2023, 46, 413–414. [Google Scholar] [CrossRef] [PubMed]
- HERVÉ, C.A.; LUGLI, E.B.; BRAND, A.; GRIFFITHS, D.J.; VENABLES, P.J.W. Autoantibodies to Human Endogenous Retrovirus-K Are Frequently Detected in Health and Disease and React with Multiple Epitopes. Clin. Exp. Immunol. 2002, 128, 75–82. [Google Scholar] [CrossRef]
- Nelson, P.N.; Roden, D.; Nevill, A.; Freimanis, G.L.; Trela, M.; Ejtehadi, H.D.; Bowman, S.; Axford, J.; Veitch, A.M.; Tugnet, N.; et al. Rheumatoid Arthritis Is Associated with IgG Antibodies to Human Endogenous Retrovirus Gag Matrix: A Potential Pathogenic Mechanism of Disease? J. Rheumatol. 2014, 41, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.; Wang, X.; Ni, K.; Smith, S.E.B.; Najjar, R.; Whitmore, L.S.; Yacoub, M.; Bays, A.; Gale, M.; Mustelin, T. Expression of Envelope Protein Encoded by Endogenous Retrovirus K102 in Rheumatoid Arthritis Neutrophils. Microorganisms 2023, 11, 1310. [Google Scholar] [CrossRef]
- Wang, X.; Hefton, A.; Ni, K.; Ukadike, K.C.; Bowen, M.A.; Eckert, M.; Stevens, A.; Lood, C.; Mustelin, T. Autoantibodies Against Unmodified and Citrullinated Human Endogenous Retrovirus K Envelope Protein in Patients With Rheumatoid Arthritis. J. Rheumatol. 2022, 49, 26–35. [Google Scholar] [CrossRef]
- Tokuyama, M.; Gunn, B.M.; Venkataraman, A.; Kong, Y.; Kang, I.; Rakib, T.; Townsend, M.J.; Costenbader, K.H.; Alter, G.; Iwasaki, A. Antibodies against Human Endogenous Retrovirus K102 Envelope Activate Neutrophils in Systemic Lupus Erythematosus. J. Exp. Med. 2021, 218, e20191766. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, M.; Kong, Y.; Song, E.; Jayewickreme, T.; Kang, I.; Iwasaki, A. ERVmap Analysis Reveals Genome-Wide Transcription of Human Endogenous Retroviruses. Proc. Natl. Acad. Sci. USA 2018, 115, 12565–12572. [Google Scholar] [CrossRef]
- Liu, X.; Ding, Y.; Zheng, X.; Huang, H.; Shi, L.; Yang, X.; Wei, J.; Li, Y.; Kao, W.; Zhang, F.; et al. Small RNAs Encoded by Human Endogenous Retrovirus K Overexpressed in PBMCs May Contribute to the Diagnosis and Evaluation of Systemic Lupus Erythematosus as Novel Biomarkers. Hum. Mol. Genet. 2022, 31, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Liu, Y.; Zhou, P.; Yan, Q.; Yu, H.; Chen, X.; Zhu, F. Implication of Human Endogenous Retrovirus W Family Envelope in Hepatocellular Carcinoma Promotes MEK/ERK-Mediated Metastatic Invasiveness and Doxorubicin Resistance. Cell Death Discov. 2021, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Salavatiha, Z.; Soleimani-Jelodar, R.; Jalilvand, S. The Role of Endogenous retroviruses-K in Human Cancer. Rev. Med. Virol. 2020, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. HERV Envelope Proteins: Physiological Role and Pathogenic Potential in Cancer and Autoimmunity. Front. Microbiol. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Shah, N.M.; Du, A.Y.; Dailey, Z.Z.; Pehrsson, E.C.; Godoy, P.M.; Zhang, D.; Li, D.; Xing, X.; Kim, S.; et al. Transposable Elements Drive Widespread Expression of Oncogenes in Human Cancers. Nat. Genet. 2019, 51, 611–617. [Google Scholar] [CrossRef]
- Camargo-Forero, N.; Orozco-Arias, S.; Perez Agudelo, J.M.; Guyot, R. HERV-K (HML-2) Insertion Polymorphisms in the 8q24.13 Region and Their Potential Etiological Associations with Acute Myeloid Leukemia. Arch. Virol. 2023, 168, 125. [Google Scholar] [CrossRef]
- La Ferlita, A.; Nigita, G.; Tsyba, L.; Palamarchuk, A.; Alaimo, S.; Pulvirenti, A.; Balatti, V.; Rassenti, L.; Tsichlis, P.N.; Kipps, T.; et al. Expression Signature of Human Endogenous Retroviruses in Chronic Lymphocytic Leukemia. Proc. Natl. Acad. Sci. USA 2023, 120, e2307593120. [Google Scholar] [CrossRef]
- Masuda, Y.; Ishihara, R.; Murakami, Y.; Watanabe, S.; Asao, Y.; Gotoh, N.; Kasamatsu, T.; Takei, H.; Kobayashi, N.; Saitoh, T.; et al. Clinical Significance of Human Endogenous Retrovirus K (HERV-K) in Multiple Myeloma Progression. Int. J. Hematol. 2023, 117, 563–577. [Google Scholar] [CrossRef]
- Shah, A.H.; Rivas, S.R.; Doucet-O’Hare, T.T.; Govindarajan, V.; DeMarino, C.; Wang, T.; Ampie, L.; Zhang, Y.; Banasavadi-Siddegowda, Y.K.; Walbridge, S.; et al. Human Endogenous Retrovirus K Contributes to a Stem Cell Niche in Glioblastoma. J. Clin. Investig. 2023, 133, e167929. [Google Scholar] [CrossRef]
- Wen, X.; Shen, J.; De Miglio, M.R.; Zeng, D.; Sechi, L.A. Endogenous Retrovirus Group FRD Member 1 Is a Potential Biomarker for Prognosis and Immunotherapy for Kidney Renal Clear Cell Carcinoma. Front. Cell Infect. Microbiol. 2023, 13, 1252905. [Google Scholar] [CrossRef]
- Lemaître, C.; Tsang, J.; Bireau, C.; Heidmann, T.; Dewannieux, M. A Human Endogenous Retrovirus-Derived Gene That Can Contribute to Oncogenesis by Activating the ERK Pathway and Inducing Migration and Invasion. PLoS Pathog. 2017, 13, e1006451. [Google Scholar] [CrossRef]
- Kitsou, K.; Iliopoulou, M.; Spoulou, V.; Lagiou, P.; Magiorkinis, G. Viral Causality of Human Cancer and Potential Roles of Human Endogenous Retroviruses in the Multi-Omics Era: An Evolutionary Epidemiology Review. Front. Oncol. 2021, 11, 687631. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Padmanabhan Nair, V.; Bauer, T.; Sprinzl, M.F.; Protzer, U.; Vincendeau, M. Increased HERV-K(HML-2) Transcript Levels Correlate with Clinical Parameters of Liver Damage in Hepatitis C Patients. Cells 2021, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Kitsou, K.; Kotanidou, A.; Paraskevis, D.; Karamitros, T.; Katzourakis, A.; Tedder, R.; Hurst, T.; Sapounas, S.; Kotsinas, A.; Gorgoulis, V.; et al. Upregulation of Human Endogenous Retroviruses in Bronchoalveolar Lavage Fluid of COVID-19 Patients. Microbiol. Spectr. 2021, 9, e01260-21. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Erbì, M.C.; Scognamiglio, S.; Tramontano, E. Human Endogenous Retrovirus (HERV) Transcriptome Is Dynamically Modulated during SARS-CoV-2 Infection and Allows Discrimination of COVID-19 Clinical Stages. Microbiol. Spectr. 2023, 11, e02516-22. [Google Scholar] [CrossRef] [PubMed]
- Petrone, V.; Fanelli, M.; Giudice, M.; Toschi, N.; Conti, A.; Maracchioni, C.; Iannetta, M.; Resta, C.; Cipriani, C.; Miele, M.T.; et al. Expression Profile of HERVs and Inflammatory Mediators Detected in Nasal Mucosa as a Predictive Biomarker of COVID-19 Severity. Front. Microbiol. 2023, 14, 1155624. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.R.W.; Shinn, P.; Chen, H.; Berry, C.; Ecker, J.R.; Bushman, F. HIV-1 Integration in the Human Genome Favors Active Genes and Local Hotspots. Cell 2002, 110, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Srinivasachar Badarinarayan, S.; Shcherbakova, I.; Langer, S.; Koepke, L.; Preising, A.; Hotter, D.; Kirchhoff, F.; Sparrer, K.M.J.; Schotta, G.; Sauter, D. HIV-1 Infection Activates Endogenous Retroviral Promoters Regulating Antiviral Gene Expression. Nucleic Acids Res. 2020, 48, 10890–10908. [Google Scholar] [CrossRef] [PubMed]
- Vincendeau, M.; Göttesdorfer, I.; Schreml, J.M.H.; Wetie, A.N.G.; Mayer, J.; Greenwood, A.D.; Helfer, M.; Kramer, S.; Seifarth, W.; Hadian, K.; et al. Modulation of Human Endogenous Retrovirus (HERV) Transcription during Persistent and de Novo HIV-1 Infection. Retrovirology 2015, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Esqueda, D.; Xu, F.; Moore, Y.; Yang, Z.; Huang, G.; Lennon, P.A.; Hu, P.C.; Dong, J. Lack of Correlation between HERV-K Expression and HIV-1 Viral Load in Plasma Specimens. Ann. Clin. Lab. Sci. 2013, 43, 122–125. [Google Scholar]
- Mantovani, F.; Kitsou, K.; Paraskevis, D.; Lagiou, P.; Magiorkinis, G. The Interaction of Human Immunodeficiency Virus-1 and Human Endogenous Retroviruses in Patients (Primary Cell Cultures) and Cell Line Models. Microbiol. Spectr. 2023, 11, e01379-23. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Galindo, R.; Kaplan, M.H.; Contreras-Galindo, A.C.; Gonzalez-Hernandez, M.J.; Ferlenghi, I.; Giusti, F.; Lorenzo, E.; Gitlin, S.D.; Dosik, M.H.; Yamamura, Y.; et al. Characterization of Human Endogenous Retroviral Elements in the Blood of HIV-1-Infected Individuals. J. Virol. 2012, 86, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Young, G.R.; Terry, S.N.; Manganaro, L.; Cuesta-Dominguez, A.; Deikus, G.; Bernal-Rubio, D.; Campisi, L.; Fernandez-Sesma, A.; Sebra, R.; Simon, V.; et al. HIV-1 Infection of Primary CD4+ T Cells Regulates the Expression of Specific Human Endogenous Retrovirus HERV-K (HML-2) Elements. J. Virol. 2018, 92, e01507-17. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, E.; Magiorkinis, G. Interplay between Endogenous and Exogenous Human Retroviruses. Trends Microbiol. 2023, 31, 933–946. [Google Scholar] [CrossRef]
- Li, L.; Dahiya, S.; Kortagere, S.; Aiamkitsumrit, B.; Cunningham, D.; Pirrone, V.; Nonnemacher, M.R.; Wigdahl, B. Impact of Tat Genetic Variation on HIV-1 Disease. Adv. Virol. 2012, 2012, 123605. [Google Scholar] [CrossRef]
- Curty, G.; Iniguez, L.P.; Soares, M.A.; Nixon, D.F.; de Mulder Rougvie, M. Off-Target Effect of Activation of NF-ΚB by HIV Latency Reversal Agents on Transposable Elements Expression. Viruses 2022, 14, 1571. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, M.J.; Swanson, M.D.; Contreras-Galindo, R.; Cookinham, S.; King, S.R.; Noel, R.J.; Kaplan, M.H.; Markovitz, D.M. Expression of Human Endogenous Retrovirus Type K (HML-2) Is Activated by the Tat Protein of HIV-1. J. Virol. 2012, 86, 7790–7805. [Google Scholar] [CrossRef]
- O’Carroll, I.P.; Fan, L.; Kroupa, T.; McShane, E.K.; Theodore, C.; Yates, E.A.; Kondrup, B.; Ding, J.; Martin, T.S.; Rein, A.; et al. Structural Mimicry Drives HIV-1 Rev-Mediated HERV-K Expression. J. Mol. Biol. 2020, 432, 166711. [Google Scholar] [CrossRef]
- Hohn, O.; Hanke, K.; Bannert, N. HERV-K(HML-2), the Best Preserved Family of HERVs: Endogenization, Expression, and Implications in Health and Disease. Front. Oncol. 2013, 3, 246. [Google Scholar] [CrossRef]
- Yang, J.; Bogerd, H.P.; Peng, S.; Wiegand, H.; Truant, R.; Cullen, B.R. An Ancient Family of Human Endogenous Retroviruses Encodes a Functional Homolog of the HIV-1 Rev Protein. Proc. Natl. Acad. Sci. USA 1999, 96, 13404–13408. [Google Scholar] [CrossRef]
- Monde, K.; Terasawa, H.; Nakano, Y.; Soheilian, F.; Nagashima, K.; Maeda, Y.; Ono, A. Molecular Mechanisms by Which HERV-K Gag Interferes with HIV-1 Gag Assembly and Particle Infectivity. Retrovirology 2017, 14, 27. [Google Scholar] [CrossRef]
- Hurst, T.; Magiorkinis, G. Epigenetic Control of Human Endogenous Retrovirus Expression: Focus on Regulation of Long-Terminal Repeats (LTRs). Viruses 2017, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Michaud, H.A.; de Mulder, M.; SenGupta, D.; Deeks, S.G.; Martin, J.N.; Pilcher, C.D.; Hecht, F.M.; Sacha, J.B.; Nixon, D.F. Trans-Activation, Post-Transcriptional Maturation, and Induction of Antibodies to HERV-K (HML-2) Envelope Transmembrane Protein in HIV-1 Infection. Retrovirology 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Salamango, D.J.; Harris, R.S. Dual Functionality of HIV-1 Vif in APOBEC3 Counteraction and Cell Cycle Arrest. Front. Microbiol. 2021, 11, 622012. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.E.; Katzourakis, A.; de Oliveira, T.; Welch, J.J.; Belshaw, R.; Bishop, K.N.; Kramer, B.; McMichael, A.J.; Rambaut, A.; Iversen, A.K.N. Conserved Footprints of APOBEC3G on Hypermutated Human Immunodeficiency Virus Type 1 and Human Endogenous Retrovirus HERV-K(HML2) Sequences. J. Virol. 2008, 82, 8743–8761. [Google Scholar] [CrossRef]
- Jones, R.B.; Garrison, K.E.; Mujib, S.; Mihajlovic, V.; Aidarus, N.; Hunter, D.V.; Martin, E.; John, V.M.; Zhan, W.; Faruk, N.F.; et al. HERV-K-Specific T Cells Eliminate Diverse HIV-1/2 and SIV Primary Isolates. J. Clin. Investig. 2012, 122, 4473–4489. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, L.; Guney, M.H.; Kim, K.; Goh, S.L.; McCauley, S.; Dauphin, A.; Diehl, W.E.; Luban, J. Primate Immunodeficiency Virus Proteins Vpx and Vpr Counteract Transcriptional Repression of Proviruses by the HUSH Complex. Nat. Microbiol. 2018, 3, 1354–1361. [Google Scholar] [CrossRef]
- Goyal, A.; Bauer, J.; Hey, J.; Papageorgiou, D.N.; Stepanova, E.; Daskalakis, M.; Scheid, J.; Dubbelaar, M.; Klimovich, B.; Schwarz, D.; et al. DNMT and HDAC Inhibition Induces Immunogenic Neoantigens from Human Endogenous Retroviral Element-Derived Transcripts. Nat. Commun. 2023, 14, 6731. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, G.; Curtin, F. Temelimab, an IgG4 Anti-Human Endogenous Retrovirus Monoclonal Antibody: An Early Development Safety Review. Drug Saf. 2020, 43, 1287–1296. [Google Scholar] [CrossRef]
- Krishnamurthy, J.; Rabinovich, B.A.; Mi, T.; Switzer, K.C.; Olivares, S.; Maiti, S.N.; Plummer, J.B.; Singh, H.; Kumaresan, P.R.; Huls, H.M.; et al. Genetic Engineering of T Cells to Target HERV-K, an Ancient Retrovirus on Melanoma. Clin. Cancer Res. 2015, 21, 3241–3251. [Google Scholar] [CrossRef]
- Zhou, F.; Krishnamurthy, J.; Wei, Y.; Li, M.; Hunt, K.; Johanning, G.L.; Cooper, L.J.; Wang-Johanning, F. Chimeric Antigen Receptor T Cells Targeting HERV-K Inhibit Breast Cancer and Its Metastasis through Downregulation of Ras. Oncoimmunology 2015, 4, e1047582. [Google Scholar] [CrossRef]
- Ng, K.W.; Boumelha, J.; Enfield, K.S.S.; Almagro, J.; Cha, H.; Pich, O.; Karasaki, T.; Moore, D.A.; Salgado, R.; Sivakumar, M.; et al. Antibodies against Endogenous Retroviruses Promote Lung Cancer Immunotherapy. Nature 2023, 616, 563–573. [Google Scholar] [CrossRef]
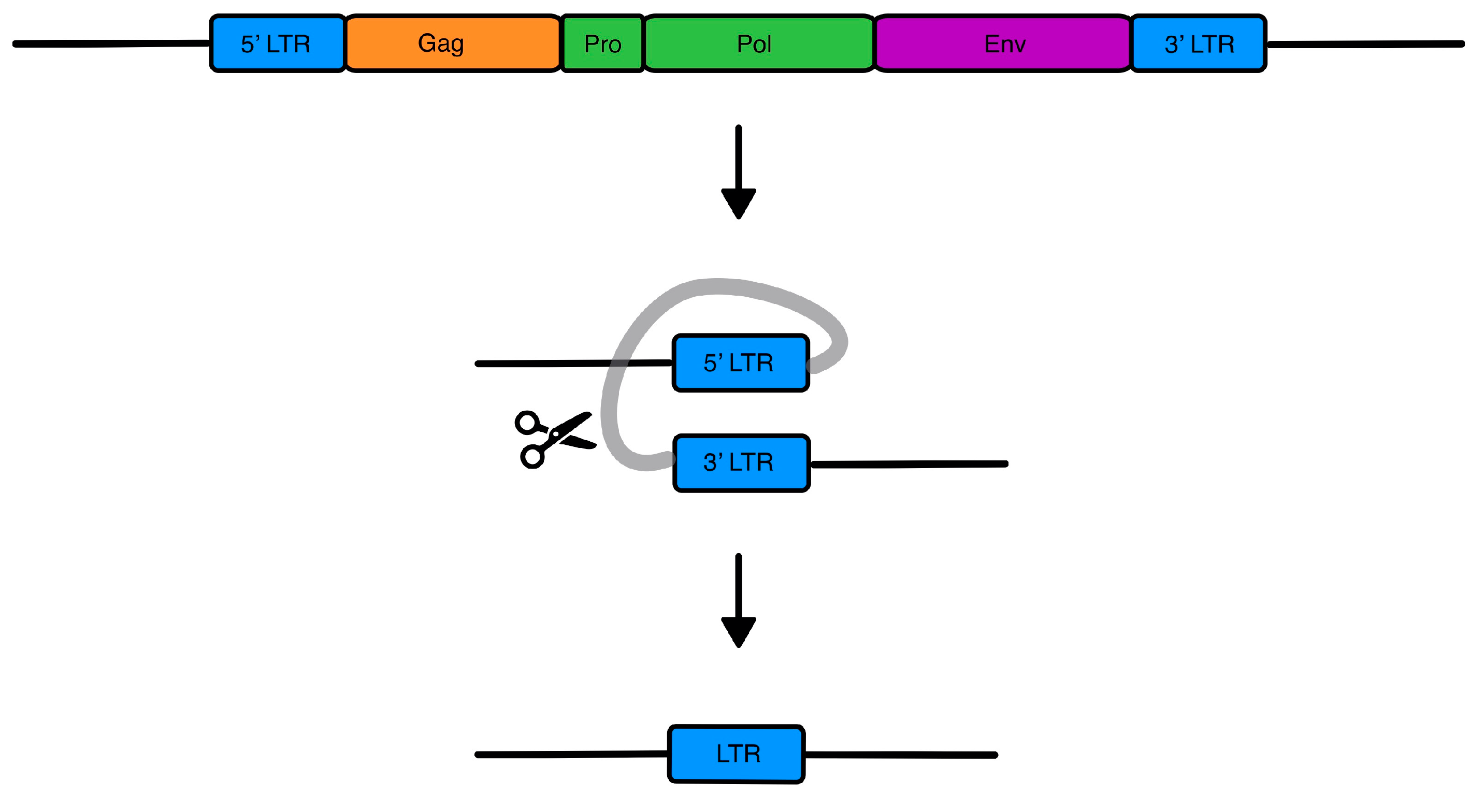

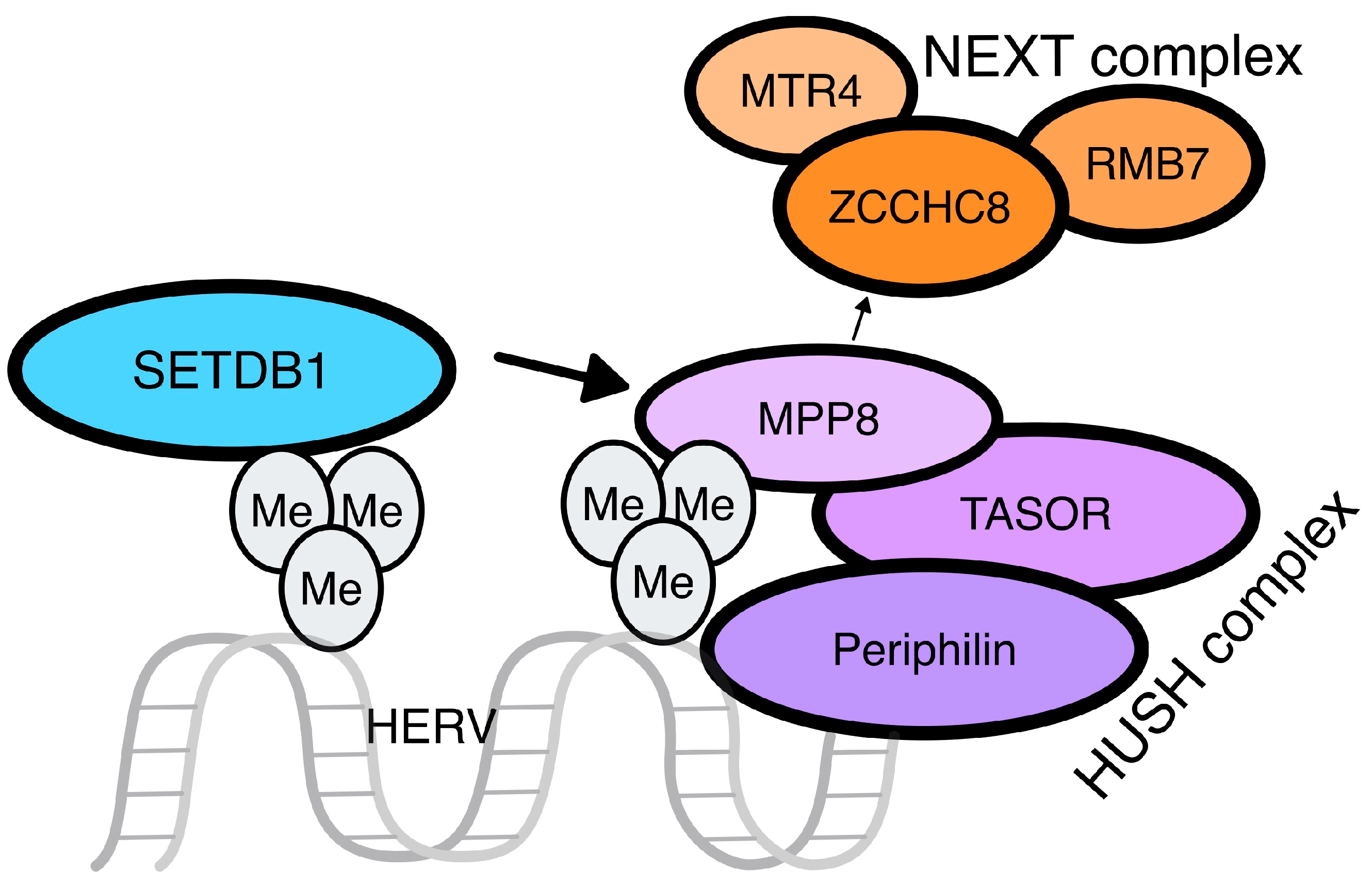
| Disease | HERV |
|---|---|
| Multiple Sclerosis | Increased HERV-W and HERV-H during disease relapses, early detection of HERV-W in cerebrospinal fluid during early stages linked to worse prognosis [51,52] |
| Amyotrophic Lateral Sclerosis | HERV-K Env expression correlation to disease stage, severity, phenotype, and its link to neuroinflammation pathogenesis [54,55] |
| Rheumatoid Arthritis | Increased levels of antibodies against HERV-K10 Gag; HERV-K102 increased expression on neutrophils and production of autoantibodies against this Env epitope [59] |
| Systemic Lupus Erythematosus | Anti-HERV-K102 IgG linked to innate immunity activation and interferon responses [58] |
| Juvenile Idiopathic Arthritis | HERV-K antigenicity [60] |
| Acute Myeloid Leukemia | 70 HERV-K (HML-2) polymorphisms in 8q24.13 to 8q24.21 linked to disease development [68] |
| Chronic Lymphocytic Leukemia | Locus-specific HERV expression profiles correlating each form of the disease (indolent and aggressive); these differentially expressed HERV loci are associated with distinct patterns of signaling pathway modifications [69] |
| Multiple Myeloma | HERV-K env and LTR increased expression as a distinctive characteristic between multiple myeloma and monoclonal gammopathy of undetermined significance or controls; carcinogenic effects [70] |
| Glioblastoma | HERV-K HML-2 expression linked to pluripotency and stemness [71] |
| Kidney Renal Clear Cell Carcinoma | HERV-FRD-1 expression linked to better prognosis [72] |
| HCV-induced Liver Cirrhosis | HERV-K (HML-2) transcription linked to resistance to HCV clearance after antiviral therapy [75] |
| SARS-CoV-2 | HERV-K and HERV-W as discriminative characteristics among infection stages; HERV upregulation linked to systemic inflammation [77,78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantovani, F.; Kitsou, K.; Magiorkinis, G. HERVs: Expression Control Mechanisms and Interactions in Diseases and Human Immunodeficiency Virus Infection. Genes 2024, 15, 192. https://doi.org/10.3390/genes15020192
Mantovani F, Kitsou K, Magiorkinis G. HERVs: Expression Control Mechanisms and Interactions in Diseases and Human Immunodeficiency Virus Infection. Genes. 2024; 15(2):192. https://doi.org/10.3390/genes15020192
Chicago/Turabian StyleMantovani, Federica, Konstantina Kitsou, and Gkikas Magiorkinis. 2024. "HERVs: Expression Control Mechanisms and Interactions in Diseases and Human Immunodeficiency Virus Infection" Genes 15, no. 2: 192. https://doi.org/10.3390/genes15020192
APA StyleMantovani, F., Kitsou, K., & Magiorkinis, G. (2024). HERVs: Expression Control Mechanisms and Interactions in Diseases and Human Immunodeficiency Virus Infection. Genes, 15(2), 192. https://doi.org/10.3390/genes15020192







