Genetic Basis of Pigment Dispersion Syndrome and Pigmentary Glaucoma: An Update and Functional Insights
Abstract
1. Introduction
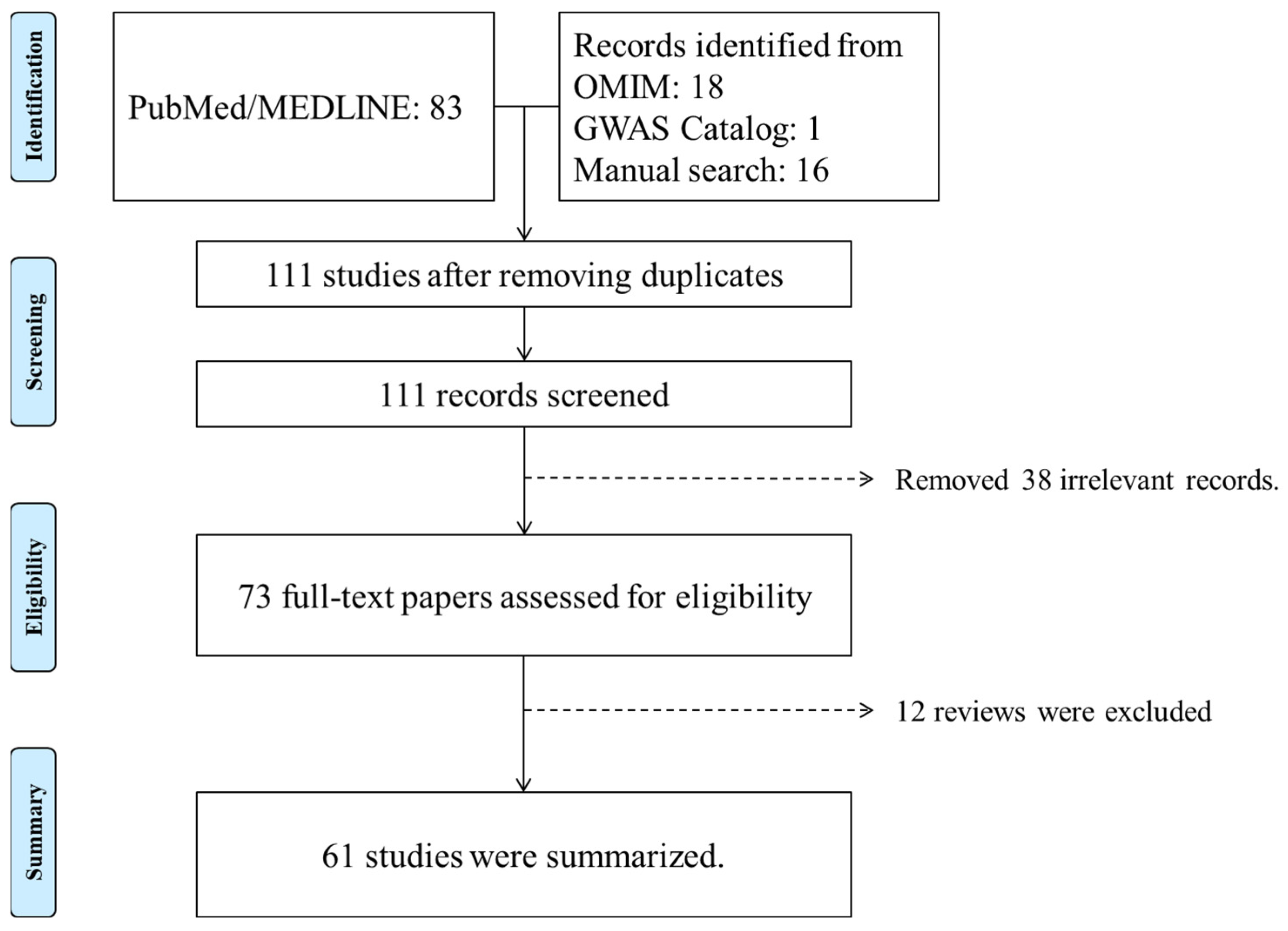
2. Identification of PDS/PG Genetic Studies
3. Genetic Factors Identified in PDS/PG
3.1. Genetic Loci Linked to PDS/PG
3.2. Candidate Gene Studies
3.3. Genome-Wide Association Study
3.4. Genetic Factors Identified by Exome Sequencing Studies
3.4.1. Identification of PMEL Variants in PDS/PG
3.4.2. Exome Sequencing-Based Survey of Relevant Genes in Large PDS Cohorts
3.4.3. CPAMD8 (C3 and PZP Like Alpha-2-Macroglobulin Domain Containing 8)
4. Shared Genetic Factors between PDS/PG and Other Conditions and Traits
5. Functional Insights into PDS/PG Pathogenesis Using Animal Models
5.1. Mouse Model
5.2. Canine Model
5.3. Zebrafish Model
6. Insights into Disease Pathogenic Pathways
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migliazzo, C.V.; Shaffer, R.N.; Nykin, R.; Magee, S. Long-term analysis of pigmentary dispersion syndrome and pigmentary glaucoma. Ophthalmology 1986, 93, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R.; Steinberger, D.; Liebmann, J.M. Prevalence of pigment dispersion syndrome in a population undergoing glaucoma screening. Am. J. Ophthalmol. 1993, 115, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.K.; Chaglasian, M.A.; Meetz, R.E. Clinical signs of the pigment dispersion syndrome in blacks. Optom. Vis. Sci. 1997, 74, 993–1006. [Google Scholar] [CrossRef]
- Qing, G.; Wang, N.; Tang, X.; Zhang, S.; Chen, H. Clinical characteristics of pigment dispersion syndrome in Chinese patients. Eye 2009, 23, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwase, A.; Araie, M.; Suzuki, Y.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The Tajimi Study report 2: Prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology 2005, 112, 1661–1669. [Google Scholar] [CrossRef]
- Becker, B.; Podos, S.M. Krukenberg’s spindles and primary open-angle glaucoma. Arch. Ophthalmol. 1966, 76, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Farrar, S.M.; Shields, M.B.; Miller, K.N.; Stoup, C.M. Risk factors for the development and severity of glaucoma in the pigment dispersion syndrome. Am. J. Ophthalmol. 1989, 108, 223–229. [Google Scholar] [CrossRef]
- Tandon, A.; Zhang, Z.; Fingert, J.H.; Kwon, Y.H.; Wang, K.; Alward, W.L.M. The Heritability of Pigment Dispersion Syndrome and Pigmentary Glaucoma. Am. J. Ophthalmol. 2019, 202, 55–61. [Google Scholar] [CrossRef]
- Shen, L.; Melles, R.B.; Metlapally, R.; Barcellos, L.; Schaefer, C.; Risch, N.; Herrinton, L.J.; Wildsoet, C.; Jorgenson, E. The Association of Refractive Error with Glaucoma in a Multiethnic Population. Ophthalmology 2016, 123, 92–101. [Google Scholar] [CrossRef]
- Bovell, A.M.; Damji, K.F.; Dohadwala, A.A.; Hodge, W.G.; Allingham, R.R. Familial occurrence of pigment dispersion syndrome. Can. J. Ophthalmol. 2001, 36, 11–17. [Google Scholar] [CrossRef]
- Wiggs, J.L.; Del Bono, E.A.; Schuman, J.S.; Hutchinson, B.T.; Walton, D.S. Clinical features of five pedigrees genetically linked to the juvenile glaucoma locus on chromosome 1q21-q31. Ophthalmology 1995, 102, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Scheie, H.G.; Cameron, J.D. Pigment dispersion syndrome: A clinical study. Br. J. Ophthalmol. 1981, 65, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.U.; Richardson, T.M.; Grant, W.M. Pigmentary dispersion syndrome and pigmentary glaucoma. A prospective study of the natural history. Arch. Ophthalmol. 1986, 104, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Y.; Ten Hulzen, R.D.; Cameron, J.D.; Hodge, D.O.; Johnson, D.H. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am. J. Ophthalmol. 2003, 135, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Sakiyalak, D.; Krupin, T. Pigmentary glaucoma. J. Glaucoma 2001, 10, S30–S32. [Google Scholar] [CrossRef] [PubMed]
- Gramer, G.; Weber, B.H.; Gramer, E. Results of a patient-directed survey on frequency of family history of glaucoma in 2170 patients. Investig. Ophthalmol. Vis. Sci. 2014, 55, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Mandelkorn, R.M.; Hoffman, M.E.; Olander, K.W.; Zimmerman, T.; Harsha, D. Inheritance and the pigmentary dispersion syndrome. Ann. Ophthalmol. 1983, 15, 577–582. [Google Scholar] [CrossRef]
- Simcoe, M.J.; Weisschuh, N.; Wissinger, B.; Hysi, P.G.; Hammond, C.J. Genetic Heritability of Pigmentary Glaucoma and Associations With Other Eye Phenotypes. JAMA Ophthalmol. 2020, 138, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Simcoe, M.J.; Shah, A.; Fan, B.; Choquet, H.; Weisschuh, N.; Waseem, N.H.; Jiang, C.; Melles, R.B.; Ritch, R.; Mahroo, O.A.; et al. Genome-Wide Association Study Identifies Two Common Loci Associated with Pigment Dispersion Syndrome/Pigmentary Glaucoma and Implicates Myopia in its Development. Ophthalmology 2022, 129, 626–636. [Google Scholar] [CrossRef]
- Andersen, J.S.; Pralea, A.M.; DelBono, E.A.; Haines, J.L.; Gorin, M.B.; Schuman, J.S.; Mattox, C.G.; Wiggs, J.L. A gene responsible for the pigment dispersion syndrome maps to chromosome 7q35–q36. Arch. Ophthalmol. 1997, 115, 384–388. [Google Scholar] [CrossRef]
- Aragno, V.; Zeboulon, P.; Baudouin, C.; Labbé, A. A Severe Case of Pigmentary Glaucoma in a Child With a Family History of Pigment Dispersion Syndrome. J. Glaucoma 2016, 25, e745–e747. [Google Scholar] [CrossRef] [PubMed]
- Paglinauan, C.; Haines, J.L.; Del Bono, E.A.; Schuman, J.; Stawski, S.; Wiggs, J.L. Exclusion of chromosome 1q21-q31 from linkage to three pedigrees affected by the pigment-dispersion syndrome. Am. J. Hum. Genet. 1995, 56, 1240–1243. [Google Scholar] [PubMed]
- Johnson, A.T.; Drack, A.V.; Kwitek, A.E.; Cannon, R.L.; Stone, E.M.; Alward, W.L. Clinical features and linkage analysis of a family with autosomal dominant juvenile glaucoma. Ophthalmology 1993, 100, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zeng, L.; Wang, Y.; Liu, G.; Huang, L.; Chen, D.; Wang, X.; Fan, N.; He, Y.; Liu, X. Compound Heterozygous Variants of the CPAMD8 Gene Co-Segregating in Two Chinese Pedigrees with Pigment Dispersion Syndrome/Pigmentary Glaucoma. Front. Genet. 2022, 13, 845081. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, C.; Goar, W.; Meyer, K.J.; Alward, W.L.M.; Boese, E.A.; Sears, N.C.; Roos, B.R.; Kwon, Y.H.; DeLuca, A.P.; Siggs, O.M.; et al. Exome-based investigation of the genetic basis of human pigmentary glaucoma. BMC Genom. 2021, 22, 477. [Google Scholar] [CrossRef] [PubMed]
- Lahola-Chomiak, A.A.; Footz, T.; Nguyen-Phuoc, K.; Neil, G.J.; Fan, B.; Allen, K.F.; Greenfield, D.S.; Parrish, R.K.; Linkroum, K.; Pasquale, L.R.; et al. Non-Synonymous variants in premelanosome protein (PMEL) cause ocular pigment dispersion and pigmentary glaucoma. Hum. Mol. Genet. 2019, 28, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Bocchini, C.A.; Scott, A.F.; Hamosh, A. Online Mendelian Inheritance in Man (OMIM). Available online: https://www.omim.org/ (accessed on 28 November 2023).
- Sollis, E.; Mosaku, A.; Abid, A.; Buniello, A.; Cerezo, M.; Gil, L.; Groza, T.; Gunes, O.; Hall, P.; Hayhurst, J.; et al. The NHGRI-EBI GWAS Catalog: Knowledgebase and deposition resource. Nucleic Acids Res. 2023, 51, D977–D985. [Google Scholar] [CrossRef]
- Wagner, S.H.; DelBono, E.; Greenfield, D.S.; Parrish, R.K.; Haines, J.L.; Wiggs, J.L. A Second Locus for Pigment Dispersion Syndrome Maps to Chromosome 18q21. Investig. Ophthalmol. Vis. Sci. 2005, 46, 29. [Google Scholar]
- Mikelsaar, R.; Molder, H.; Bartsch, O.; Punab, M. Two novel deletions (array CGH findings) in pigment dispersion syndrome. Ophthalmic Genet. 2007, 28, 216–219. [Google Scholar] [CrossRef]
- Lynch, S.; Yanagi, G.; DelBono, E.; Wiggs, J.L. DNA sequence variants in the tyrosinase-related protein 1 (TYRP1) gene are not associated with human pigmentary glaucoma. Mol. Vis. 2002, 8, 127–129. [Google Scholar]
- Jaksic, V.; Markovic, V.; Milenkovic, S.; Stefanovic, I.; Jakovic, N.; Knezevic, M. MTHFR C677T homozygous mutation in a patient with pigmentary glaucoma and central retinal vein occlusion. Ophthalmic Res. 2010, 43, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Fingert, J.H.; Robin, A.L.; Scheetz, T.E.; Kwon, Y.H.; Liebmann, J.M.; Ritch, R.; Alward, W.L. Tank-Binding Kinase 1 (TBK1) Gene and Open-Angle Glaucomas (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2016, 114, T6. [Google Scholar] [PubMed]
- Giardina, E.; Oddone, F.; Lepre, T.; Centofanti, M.; Peconi, C.; Tanga, L.; Quaranta, L.; Frezzotti, P.; Novelli, G.; Manni, G. Common sequence variants in the LOXL1 gene in pigment dispersion syndrome and pigmentary glaucoma. BMC Ophthalmol. 2014, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Gramer, E.; Müller-Myhsok, B.; Pasutto, F.; Gramer, G.; Wissinger, B.; Weisschuh, N. Lysyl oxidase-like 1 gene polymorphisms in German patients with normal tension glaucoma, pigmentary glaucoma and exfoliation glaucoma. J. Glaucoma 2010, 19, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Ritch, R.; Dorairaj, S.K.; Kaur, I.; Liebmann, J.M.; Thomas, R.; Chakrabarti, S. Exfoliation syndrome and exfoliation glaucoma-associated LOXL1 variations are not involved in pigment dispersion syndrome and pigmentary glaucoma. Mol. Vis. 2008, 14, 1254–1262. [Google Scholar]
- Hodges, E.D.; Chrystal, P.W.; Footz, T.; Doucette, L.P.; Noel, N.C.L.; Li, Z.; Walter, M.A.; Allison, W.T. Disrupting the Repeat Domain of Premelanosome Protein (PMEL) Produces Dysamyloidosis and Dystrophic Ocular Pigment Reflective of Pigmentary Glaucoma. Int. J. Mol. Sci. 2023, 24, 14423. [Google Scholar] [CrossRef]
- Ma, A.; Yousoof, S.; Grigg, J.R.; Flaherty, M.; Minoche, A.E.; Cowley, M.J.; Nash, B.M.; Ho, G.; Gayagay, T.; Lai, T.; et al. Revealing hidden genetic diagnoses in the ocular anterior segment disorders. Genet. Med. 2020, 22, 1623–1632. [Google Scholar] [CrossRef]
- Bonet-Fernandez, J.M.; Aroca-Aguilar, J.D.; Corton, M.; Ramirez, A.I.; Alexandre-Moreno, S.; Garcia-Anton, M.T.; Salazar, J.J.; Ferre-Fernandez, J.J.; Atienzar-Aroca, R.; Villaverde, C.; et al. CPAMD8 loss-of-function underlies non-dominant congenital glaucoma with variable anterior segment dysgenesis and abnormal extracellular matrix. Hum. Genet. 2020, 139, 1209–1231. [Google Scholar] [CrossRef]
- Cheong, S.S.; Hentschel, L.; Davidson, A.E.; Gerrelli, D.; Davie, R.; Rizzo, R.; Pontikos, N.; Plagnol, V.; Moore, A.T.; Sowden, J.C.; et al. Mutations in CPAMD8 Cause a Unique Form of Autosomal-Recessive Anterior Segment Dysgenesis. Am. J. Hum. Genet. 2016, 99, 1338–1352. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Xiao, X.; Fang, L.; Li, S.; Liu, X.; Zhang, Q. Biallelic variants in CPAMD8 are associated with primary open-angle glaucoma and primary angle-closure glaucoma. Br. J. Ophthalmol. 2022, 106, 1710–1715. [Google Scholar] [CrossRef]
- Wiggs, J.L. CPAMD8, a New Gene for Anterior Segment Dysgenesis and Childhood Glaucoma. Ophthalmology 2020, 127, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Siggs, O.M.; Souzeau, E.; Taranath, D.A.; Dubowsky, A.; Chappell, A.; Zhou, T.; Javadiyan, S.; Nicholl, J.; Kearns, L.S.; Staffieri, S.E.; et al. Biallelic CPAMD8 Variants Are a Frequent Cause of Childhood and Juvenile Open-Angle Glaucoma. Ophthalmology 2020, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.M.; Fingert, J.H.; Alward, W.L.; Nguyen, T.D.; Polansky, J.R.; Sunden, S.L.; Nishimura, D.; Clark, A.F.; Nystuen, A.; Nichols, B.E.; et al. Identification of a gene that causes primary open angle glaucoma. Science 1997, 275, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, V.C.; Stone, E.M.; Alward, W.L.; Drack, A.V.; Johnson, A.T.; Streb, L.M.; Nichols, B.E. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat. Genet. 1993, 4, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.L.; Billingsley, G.; Buys, Y.; Levin, A.V.; Priston, M.; Trope, G.; Williams-Lyn, D.; Héon, E. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am. J. Hum. Genet. 2002, 70, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Faucher, M.; Anctil, J.L.; Rodrigue, M.A.; Duchesne, A.; Bergeron, D.; Blondeau, P.; Cote, G.; Dubois, S.; Bergeron, J.; Arseneault, R.; et al. Founder TIGR/myocilin mutations for glaucoma in the Quebec population. Hum. Mol. Genet. 2002, 11, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Alward, W.L.; Kwon, Y.H.; Khanna, C.L.; Johnson, A.T.; Hayreh, S.S.; Zimmerman, M.B.; Narkiewicz, J.; Andorf, J.L.; Moore, P.A.; Fingert, J.H.; et al. Variations in the myocilin gene in patients with open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 1189–1197. [Google Scholar] [CrossRef]
- Bruttini, M.; Longo, I.; Frezzotti, P.; Ciappetta, R.; Randazzo, A.; Orzalesi, N.; Fumagalli, E.; Caporossi, A.; Frezzotti, R.; Renieri, A. Mutations in the myocilin gene in families with primary open-angle glaucoma and juvenile open-angle glaucoma. Arch. Ophthalmol. 2003, 121, 1034–1038. [Google Scholar] [CrossRef]
- Pokrovskaya, O.; O’Brien, C. What’s in a Gene? Pseudoexfoliation Syndrome and Pigment Dispersion Syndrome in the Same Patient. Case Rep. Ophthalmol. 2016, 7, 54–60. [Google Scholar] [CrossRef]
- Sertie, A.L.; Sossi, V.; Camargo, A.A.; Zatz, M.; Brahe, C.; Passos-Bueno, M.R. Collagen XVIII, containing an endogenous inhibitor of angiogenesis and tumor growth, plays a critical role in the maintenance of retinal structure and in neural tube closure (Knobloch syndrome). Hum. Mol. Genet. 2000, 9, 2051–2058. [Google Scholar] [CrossRef]
- Joyce, S.; Tee, L.; Abid, A.; Khaliq, S.; Mehdi, S.Q.; Maher, E.R. Locus heterogeneity and Knobloch syndrome. Am. J. Med. Genet. Part A 2010, 152A, 2880–2881. [Google Scholar] [CrossRef]
- Kuchtey, J.; Chang, T.C.; Panagis, L.; Kuchtey, R.W. Marfan syndrome caused by a novel FBN1 mutation with associated pigmentary glaucoma. Am. J. Med. Genet. Part A 2013, 161A, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, T.; Spaeth, G. An Overlap Syndrome of Pigment Dispersion and Pigmentary Glaucoma accompanied by Marfan Syndrome: Case Report with Literature Review. J. Curr. Glaucoma Pract. 2013, 7, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, N.J.; Traboulsi, E.I.; Enger, C.; Maumenee, I.H. Glaucoma in the Marfan syndrome. Trans. Am. Ophthalmol. Soc. 1992, 90, 111–117; discussion 118–122. [Google Scholar] [PubMed]
- Nair, K.S.; Cosma, M.; Raghupathy, N.; Sellarole, M.A.; Tolman, N.G.; de Vries, W.; Smith, R.S.; John, S.W. YBR/EiJ mice: A new model of glaucoma caused by genes on chromosomes 4 and 17. Dis. Models Mech. 2016, 9, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.S.; Barbay, J.; Smith, R.S.; Masli, S.; John, S.W. Determining immune components necessary for progression of pigment dispersing disease to glaucoma in DBA/2J mice. BMC Genet. 2014, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Trantow, C.M.; Cuffy, T.L.; Fingert, J.H.; Kuehn, M.H.; Anderson, M.G. Microarray analysis of iris gene expression in mice with mutations influencing pigmentation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 237–248. [Google Scholar] [CrossRef][Green Version]
- Lu, H.; Wang, X.; Pullen, M.; Guan, H.; Chen, H.; Sahu, S.; Zhang, B.; Chen, H.; Williams, R.W.; Geisert, E.E.; et al. Genetic dissection of the Gpnmb network in the eye. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4132–4142. [Google Scholar] [CrossRef][Green Version]
- Raymond, I.D.; Pool, A.L.; Vila, A.; Brecha, N.C. A Thy1-CFP DBA/2J mouse line with cyan fluorescent protein expression in retinal ganglion cells. Vis. Neurosci. 2009, 26, 453–465. [Google Scholar] [CrossRef]
- Calkins, D.J.; Horner, P.J.; Roberts, R.; Gradianu, M.; Berkowitz, B.A. Manganese-enhanced MRI of the DBA/2J mouse model of hereditary glaucoma. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5083–5088. [Google Scholar] [CrossRef]
- Zhou, X.; Li, F.; Ge, J.; Sarkisian, S.R., Jr.; Tomita, H.; Zaharia, A.; Chodosh, J.; Cao, W. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev. Neurobiol. 2007, 67, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.A.; Brown, R.E. Age-related changes in visual acuity, learning and memory in C57BL/6J and DBA/2J mice. Neurobiol. Aging 2007, 28, 1577–1593. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.G.; Libby, R.T.; Mao, M.; Cosma, I.M.; Wilson, L.A.; Smith, R.S.; John, S.W. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, F.; Kong, L.; Tomita, H.; Li, C.; Cao, W. Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J. Biol. Chem. 2005, 280, 31240–31248. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Smith, R.S.; Hawes, N.L.; Anderson, M.G.; Zabaleta, A.; Savinova, O.; Roderick, T.H.; Heckenlively, J.R.; Davisson, M.T.; John, S.W. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat. Genet. 1999, 21, 405–409. [Google Scholar] [CrossRef]
- John, S.W.; Smith, R.S.; Savinova, O.V.; Hawes, N.L.; Chang, B.; Turnbull, D.; Davisson, M.; Roderick, T.H.; Heckenlively, J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 1998, 39, 951–962. [Google Scholar]
- Anderson, M.G.; Smith, R.S.; Hawes, N.L.; Zabaleta, A.; Chang, B.; Wiggs, J.L.; John, S.W. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat. Genet. 2002, 30, 81–85. [Google Scholar] [CrossRef]
- Williams, P.A.; Howell, G.R.; Barbay, J.M.; Braine, C.E.; Sousa, G.L.; John, S.W.; Morgan, J.E. Retinal ganglion cell dendritic atrophy in DBA/2J glaucoma. PLoS ONE 2013, 8, e72282. [Google Scholar] [CrossRef]
- Howell, G.R.; Libby, R.T.; Jakobs, T.C.; Smith, R.S.; Phalan, F.C.; Barter, J.W.; Barbay, J.M.; Marchant, J.K.; Mahesh, N.; Porciatti, V.; et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007, 179, 1523–1537. [Google Scholar] [CrossRef]
- Libby, R.T.; Anderson, M.G.; Pang, I.H.; Robinson, Z.H.; Savinova, O.V.; Cosma, I.M.; Snow, A.; Wilson, L.A.; Smith, R.S.; Clark, A.F.; et al. Inherited glaucoma in DBA/2J mice: Pertinent disease features for studying the neurodegeneration. Vis. Neurosci. 2005, 22, 637–648. [Google Scholar] [CrossRef]
- Anderson, M.G.; Hawes, N.L.; Trantow, C.M.; Chang, B.; John, S.W. Iris phenotypes and pigment dispersion caused by genes influencing pigmentation. Pigment. Cell Melanoma Res. 2008, 21, 565–578. [Google Scholar] [CrossRef]
- Johnson, R.; Jackson, I.J. Light is a dominant mouse mutation resulting in premature cell death. Nat. Genet. 1992, 1, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Zdarsky, E.; Favor, J.; Jackson, I.J. The molecular basis of brown, an old mouse mutation, and of an induced revertant to wild type. Genetics 1990, 126, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Lu, H.; Williams, R.W.; Lu, L.; Jablonski, M.M. Genetic modulation of the iris transillumination defect: A systems genetics analysis using the expanded family of BXD glaucoma strains. Pigment. Cell Melanoma Res. 2013, 26, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, M.; Colman, M.A.; Stevens, G.; Zwane, E.; Kromberg, J.; Farrall, M.; Jenkins, T. The tyrosinase-positive oculocutaneous albinism locus maps to chromosome 15q11.2-q12. Am. J. Hum. Genet. 1992, 51, 879–884. [Google Scholar] [PubMed]
- Sulem, P.; Gudbjartsson, D.F.; Stacey, S.N.; Helgason, A.; Rafnar, T.; Magnusson, K.P.; Manolescu, A.; Karason, A.; Palsson, A.; Thorleifsson, G.; et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat. Genet. 2007, 39, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Watt, B.; Tenza, D.; Lemmon, M.A.; Kerje, S.; Raposo, G.; Andersson, L.; Marks, M.S. Mutations in or near the transmembrane domain alter PMEL amyloid formation from functional to pathogenic. PLoS Genet. 2011, 7, e1002286. [Google Scholar] [CrossRef]
- Theos, A.C.; Berson, J.F.; Theos, S.C.; Herman, K.E.; Harper, D.C.; Tenza, D.; Sviderskaya, E.V.; Lamoreux, M.L.; Bennett, D.C.; Raposo, G.; et al. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell 2006, 17, 3598–3612. [Google Scholar] [CrossRef]
- Kwon, B.S.; Halaban, R.; Ponnazhagan, S.; Kim, K.; Chintamaneni, C.; Bennett, D.; Pickard, R.T. Mouse silver mutation is caused by a single base insertion in the putative cytoplasmic domain of Pmel 17. Nucleic Acids Res. 1995, 23, 154–158. [Google Scholar] [CrossRef]
- Mo, J.S.; Anderson, M.G.; Gregory, M.; Smith, R.S.; Savinova, O.V.; Serreze, D.V.; Ksander, B.R.; Streilein, J.W.; John, S.W. By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. J. Exp. Med. 2003, 197, 1335–1344. [Google Scholar] [CrossRef]
- Shiflett, S.L.; Kaplan, J.; Ward, D.M. Chediak-Higashi Syndrome: A rare disorder of lysosomes and lysosome related organelles. Pigment. Cell Res. 2002, 15, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Trantow, C.M.; Mao, M.; Petersen, G.E.; Alward, E.M.; Alward, W.L.; Fingert, J.H.; Anderson, M.G. Lyst mutation in mice recapitulates iris defects of human exfoliation syndrome. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.N.; Wu, X.S.; Rachel, R.A.; Huang, J.D.; Swing, D.A.; Matesic, L.E.; Hammer, J.A., 3rd; Copeland, N.G.; Jenkins, N.A. dsu functions in a MYO5A-independent pathway to suppress the coat color of dilute mice. Proc. Natl. Acad. Sci. USA 2004, 101, 16831–16836. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Bowers, B.; Rao, K.; Wei, Q.; Hammer, J.A., 3rd. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. J. Cell Biol. 1998, 143, 1899–1918. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Bowers, B.; Wei, Q.; Kocher, B.; Hammer, J.A., 3rd. Myosin V associates with melanosomes in mouse melanocytes: Evidence that myosin V is an organelle motor. J. Cell Sci. 1997, 110 Pt 7, 847–859. [Google Scholar] [CrossRef]
- Scott, G.A.; Arioka, M.; Jacobs, S.E. Lysophosphatidylcholine mediates melanocyte dendricity through PKCzeta activation. J. Investig. Dermatol. 2007, 127, 668–675. [Google Scholar] [CrossRef]
- Turque, N.; Denhez, F.; Martin, P.; Planque, N.; Bailly, M.; Begue, A.; Stehelin, D.; Saule, S. Characterization of a new melanocyte-specific gene (QNR-71) expressed in v-myc-transformed quail neuroretina. EMBO J. 1996, 15, 3338–3350. [Google Scholar] [CrossRef]
- Esson, D.; Armour, M.; Mundy, P.; Schobert, C.S.; Dubielzig, R.R. The histopathological and immunohistochemical characteristics of pigmentary and cystic glaucoma in the Golden Retriever. Vet. Ophthalmol. 2009, 12, 361–368. [Google Scholar] [CrossRef]
- van de Sandt, R.R.; Boeve, M.H.; Stades, F.C.; Kik, M.J. Abnormal ocular pigment deposition and glaucoma in the dog. Vet. Ophthalmol. 2003, 6, 273–278. [Google Scholar] [CrossRef]
- Petersen-Jones, S.M.; Forcier, J.; Mentzer, A.L. Ocular melanosis in the Cairn Terrier: Clinical description and investigation of mode of inheritance. Vet. Ophthalmol. 2007, 10 (Suppl. S1), 63–69. [Google Scholar] [CrossRef]
- Winkler, P.A.; Bartoe, J.T.; Quinones, C.R.; Venta, P.J.; Petersen-Jones, S.M. Exclusion of eleven candidate genes for ocular melanosis in Cairn terriers. J. Negat. Results Biomed. 2013, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Vander Wall, R.; Gupta, V.; Klistorner, A.; Graham, S.L. DBA/2J mouse model for experimental glaucoma: Pitfalls and problems. Clin. Exp. Ophthalmol. 2017, 45, 911–922. [Google Scholar] [CrossRef] [PubMed]

| First Author | Year | Phenotype | Number of Families | Method of Genetic Testing | Genetic Loci Identified | Gene Identified | Ancestry of Study Population | Ref. |
|---|---|---|---|---|---|---|---|---|
| Wagner, S. H. | 2005 | PG | 4 | Microsatellite repeat markers | 18q21 | Not specified | Caucasian | [29] |
| Andersen, J. S. | 1997 | PDS | 4 | Genome screen using microsatellite repeat markers | 7q35-q36 | Not specified | White (Irish and mixed western European descent) | [20] |
| First Author | Year | Phenotype | Study Population | Sample Size | Method of Genetic Testing | Study Population | Gene | Ref. |
|---|---|---|---|---|---|---|---|---|
| Tan, J. | 2022 | PDS/PG | Family & unrelated samples | 2 pedigrees; 38 sporadic patients | WES | Chinese | CPAMD8 | [24] |
| van der Heide, C. | 2021 | PG | Unrelated samples | 415 cases; 1645 controls | WES | Caucasian | MRAP | [25] |
| Lahola-Chomiak, A. A. | 2019 | PG | Family | 2 pedigrees; 394 in cohorts | WES; Targeted screening | Caucasian | PMEL | [26] |
| Candidate Genes | Potential Pathogenesis Pathways | Evidence Level (Y/N) | |
|---|---|---|---|
| Human Genetics | Animal Models | ||
| PMEL | Melanosome function, melanin synthesis, storage | Yes | Yes (Zebrafish, Mouse) |
| GSAP | Pigmentation processes | Yes (GWAS) | No |
| GRM5/TYR | Ocular pigmentation, retinal detachment | Yes (GWAS) | No |
| CPAMD8 | Ocular phenotypes, anterior segment dysgenesis | Yes | No |
| TYRP1 | Iris pigment dispersion, melanin synthesis | No | Yes (Mouse) |
| GPNMB | Melanosome structural integrity, pigmentation | No | Yes (Mouse) |
| LYST | Melanosome function, melanin synthesis intermediates | No | Yes (Mouse) |
| MITF | Regulation of melanocyte identity and function | No | Yes (Mouse) |
| DCT | Plays a critical role in melanin synthesis | No | Yes (Mouse) |
| TBK1 | Distinct genetic basis from other glaucoma types | No | No |
| LOXL1 | Influence on expression and risk of PDS/PG | No | No |
| MRAP | Possible link to PDS, role in iris and ocular tissues | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, S.; Yu, X.; Wiggs, J.L. Genetic Basis of Pigment Dispersion Syndrome and Pigmentary Glaucoma: An Update and Functional Insights. Genes 2024, 15, 142. https://doi.org/10.3390/genes15020142
Rong S, Yu X, Wiggs JL. Genetic Basis of Pigment Dispersion Syndrome and Pigmentary Glaucoma: An Update and Functional Insights. Genes. 2024; 15(2):142. https://doi.org/10.3390/genes15020142
Chicago/Turabian StyleRong, Shisong, Xinting Yu, and Janey L. Wiggs. 2024. "Genetic Basis of Pigment Dispersion Syndrome and Pigmentary Glaucoma: An Update and Functional Insights" Genes 15, no. 2: 142. https://doi.org/10.3390/genes15020142
APA StyleRong, S., Yu, X., & Wiggs, J. L. (2024). Genetic Basis of Pigment Dispersion Syndrome and Pigmentary Glaucoma: An Update and Functional Insights. Genes, 15(2), 142. https://doi.org/10.3390/genes15020142







