Transcriptome Analysis of Key Genes Involved in the Initiation of Spermatogonial Stem Cell Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. SSC Self-Renewal and Differentiation Culture
2.2. Quantitative Real-Time PCR
2.3. RNA Sequencing and GO and KEGG Enrichment Analyses
2.4. Statistics
3. Results
3.1. Establishment of SSCs in In Vitro Differentiation Culture System
3.2. Analysis of Differentially Expressed Genes after Differentiation Culture
3.3. Gene Ontology (GO) Analysis of the Differentially Expressed Genes
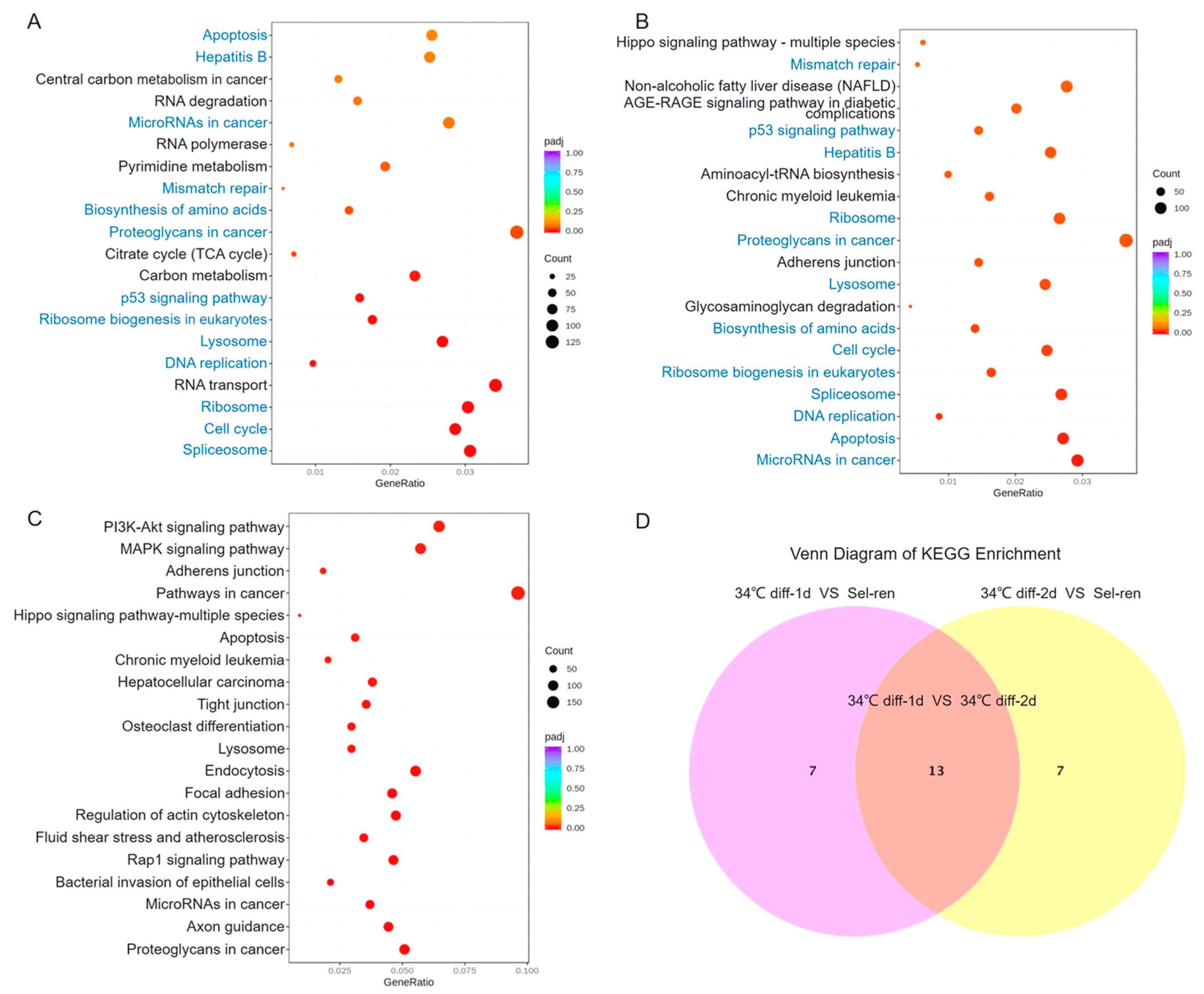
3.4. Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis of the Differentially Expressed Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanatsu, S.M.; Shinohara, T. Spermatogonial stem cell self-renewal and development. Annu. Rev. Cell Dev. Biol. 2013, 29, 163–187. [Google Scholar] [CrossRef]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 2015, 149, R159–R167. [Google Scholar] [CrossRef] [PubMed]
- Tagelenbosch, R.A.; de Rooij, D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. 1993, 290, 193–200. [Google Scholar] [CrossRef]
- Lord, T.; Oatley, J.M. A revised A(single) model to explain stem cell dynamics in the mouse male germline. Reproduction 2017, 154, R55–R64. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, J.; Toppari, J.; Rivero-Müller, A.; Ventelä, S. Reconstruction of mouse testicular cellular microenvironments in long-term seminiferous tubule culture. PLoS ONE 2014, 9, e90088. [Google Scholar] [CrossRef]
- Hara, K.; Nakagawa, T.; Enomoto, H.; Suzuki, M.; Yamamoto, M.; Simons, B.D.; Yoshida, S. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell 2014, 14, 658–672. [Google Scholar] [CrossRef]
- Carrieri, C.; Comazzetto, S.; Grover, A.; Morgan, M.; Buness, A.; Nerlov, C.; O’Carroll, D. A transit-amplifying population underpins the efficient regenerative capacity of the testis. J. Exp. Med. 2017, 214, 1631–1641. [Google Scholar] [CrossRef]
- Garbuzov, A.; Pech, M.F.; Hasegawa, K.; Sukhwani, M.; Zhang, R.J.; Orwig, K.E.; Artandi, S.E. Purification of GFRα1+ and GFRα1- Spermatogonial Stem Cells Reveals a Niche-Dependent Mechanism for Fate Determination. Stem Cell Rep. 2018, 10, 553–567. [Google Scholar] [CrossRef]
- Hermann, B.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.; Chen, I.; Gildersleeve, H.; Lehle, J.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e1658. [Google Scholar] [CrossRef]
- Mäkelä, J.; Hobbs, R. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction 2019, 158, R169–R187. [Google Scholar] [CrossRef]
- Ikami, K.; Tokue, M.; Sugimoto, R.; Noda, C.; Kobayashi, S.; Hara, K.; Yoshida, S. Hierarchical differentiation competence in response to retinoic acid ensures stem cell maintenance during mouse spermatogenesis. Development 2015, 142, 1582–1592. [Google Scholar] [CrossRef]
- Tokue, M.; Ikami, K.; Mizuno, S.; Takagi, C.; Miyagi, A.; Takada, R. SHISA6 Confers Resistance to Differentiation-Promoting Wnt/β-Catenin Signaling in Mouse Spermatogenic Stem Cells. Stem Cell Rep. 2017, 8, 561–575. [Google Scholar] [CrossRef]
- Zhou, Z.; Kawabe, H.; Suzuki, A.; Shinmyozu, K.; Saga, Y. NEDD4 controls spermatogonial stem cell homeostasis and stress response by regulating messenger ribonucleoprotein complexes. Nat. Commun. 2017, 8, 15662. [Google Scholar] [CrossRef]
- Hobbs, R.; La, H.; Mäkelä, J.; Kobayashi, T.; Noda, T.; Pandolfi, P. Distinct germline progenitor subsets defined through Tsc2-mTORC1 signaling. EMBO Rep. 2015, 16, 467–480. [Google Scholar] [CrossRef]
- Hobbs, R.; Seandel, M.; Falciatori, I.; Rafii, S.; Pandolfi, P. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell 2010, 142, 468–479. [Google Scholar] [CrossRef]
- La, H.; Chan, A.; Legrand, J.; Rossello, F.; Gangemi, C.; Papa, A.; Cheng, Q.; Morand, E.; Hobbs, R. GILZ-dependent modulation of mTORC1 regulates spermatogonial maintenance. Development 2018, 145, 165324. [Google Scholar] [CrossRef]
- Yeh, J.; Zhang, X.; Nagano, M. Indirect effects of Wnt3a/β-catenin signalling support mouse spermatogonial stem cells in vitro. PLoS ONE 2012, 7, e40002. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Nusse, R. Paracrine Wnt/β-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc. Natl. Acad. Sci. USA 2016, 113, E1489–E1497. [Google Scholar] [CrossRef] [PubMed]
- Chassot, A.; Le Rolle, M.; Jourden, M.; Taketo, M.; Ghyselinck, N.; Chaboissier, M. Constitutive WNT/CTNNB1 activation triggers spermatogonial stem cell proliferation and germ cell depletion. Dev. Biol. 2017, 426, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Gely-Pernot, A.; Raverdeau, M.; Célébi, C.; Dennefeld, C.; Feret, B.; Klopfenstein, M.; Yoshida, S.; Ghyselinck, N.; Mark, M. Spermatogonia differentiation requires retinoic acid receptor γ. Endocrinology 2012, 153, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Lord, T.; Oatley, M.; Oatley, J. Testicular Architecture Is Critical for Mediation of Retinoic Acid Responsiveness by Undifferentiated Spermatogonial Subtypes in the Mouse. Stem Cell Rep. 2018, 10, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Vernet, N.; Dennefeld, C.; Rochette-Egly, C.; Oulad-Abdelghani, M.; Chambon, P.; Ghyselinck, N.; Mark, M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 2006, 147, 96–110. [Google Scholar] [CrossRef]
- MacLean, G.; Li, H.; Metzger, D.; Chambon, P.; Petkovich, M. Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 2007, 148, 4560–4567. [Google Scholar] [CrossRef]
- DeFalco, T.; Potter, S.; Williams, A.; Waller, B.; Kan, M.; Capel, B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015, 12, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, N.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, 167502. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef]
- Schrans-Stassen, B.; van de Kant, H.; de Rooij, D.; van Pelt, A. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 1999, 140, 5894–5900. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Nie, R.; Friel, P.; Mitchell, D.; Evanoff, R.; Pouchnik, D.; Banasik, B.; McCarrey, J.; Small, C.; et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol. Reprod. 2008, 78, 537–545. [Google Scholar] [CrossRef]
- Pellegrini, M.; Filipponi, D.; Gori, M.; Barrios, F.; Lolicato, F.; Grimaldi, P.; Rossi, P.; Jannini, E.; Geremia, R.; Dolci, S. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle 2008, 7, 3878–3888. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, Y.; Feng, X.; Liao, S.; Wang, X.; Gan, H.; Wang, L.; Lin, X.; Han, C. BMP4 Cooperates with Retinoic Acid to Induce the Expression of Differentiation Markers in Cultured Mouse Spermatogonia. Stem Cells Int. 2016, 2016, 9536192. [Google Scholar] [CrossRef] [PubMed]
- Nasimi, M.; Jorsaraei, S.; Fattahi, E.; Tabari, M.; Neyshaburi, E. SCF Improves In Vitro Differentiation of SSCs Through Transcriptionally Up-regulating PRTM1, STRA8, c-KIT, PIWIL2, and OCT4 Genes. Reprod. Sci. 2021, 28, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Wijayarathna, R.; de Kretser, D. Activins in reproductive biology and beyond. Hum. Reprod. Update 2016, 22, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Nagano, M.; Ryu, B.; Brinster, C.; Avarbock, M.; Brinster, R. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Whiley, P.; Nathaniel, B.; Stanton, P.; Hobbs, R.; Loveland, K. Spermatogonial fate in mice with increased activin A bioactivity and testicular somatic cell tumours. Front. Cell Dev. Biol. 2023, 11, 1237273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhang, X.; Sun, J.; Hao, J. BMP4/Smad signaling pathway induces the differentiation of mouse spermato-gonial stem cells via upregulation of Sohlh2. Anat. Rec. 2014, 297, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chen, W.; Wu, S.; Wan, C.; He, Z. In Vitro generation of male germ cells from the stem cells. Asian J. Androl. 2023, 25, 13–20. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, L.; Cheng, Q.; Diao, F.; Zeng, Q.; Yang, X.; Wu, Y.; Zhang, H.; Huang, M.; Chen, J.; et al. In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Res. 2020, 30, 244–255. [Google Scholar] [CrossRef]
- Murakami, T.; Saitoh, I.; Inada, E.; Kurosawa, M.; Iwase, Y.; Noguchi, H.; Terao, Y.; Yamasaki, Y.; Hayasaki, H.; Sato, M. STO Feeder Cells Are Useful for Propagation of Primarily Cultured Human Deciduous Dental Pulp Cells by Eliminating Contaminating Bacteria and Promoting Cellular Outgrowth. Cell Med. 2013, 6, 75–81. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, Q.; Niu, M.; Wang, H.; Wen, L.; Yao, C.; Hou, J.; Chen, Z.; Fu, H.; Zhou, F.; et al. Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death Differ. 2018, 25, 749–766. [Google Scholar] [CrossRef]
- Wang, P.; Suo, L.; Shang, H.; Li, Y.; Li, G.; Li, Q.; Hu, J. Differentiation of spermatogonial stem cell-like cells from murine testicular tissue into haploid male germ cells in vitro. Cytotechnology 2014, 66, 365–372. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Hu, X.; Tian, G.; Ma, W.; Pei, X.; Wang, Y.; Wu, J. MicroRNA-10b regulates the renewal of spermatogonial stem cells through Kruppel-like factor 4. Cell Biochem. Funct. 2017, 35, 184–191. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, M.; Yuan, Y.; Wang, X.; Fu, R.; Wan, H. Complete Meiosis from Embryonic Stem Cell-Derived Germ Cells In Vitro. Cell Stem Cell 2016, 18, 330–340. [Google Scholar] [CrossRef]
- Gao, W.; Li, H.; Feng, J.; Lu, X.; Yin, P.; Jia, H.; Ma, W. Transcriptome Analysis in High Temperature Inhibiting Spermatogonial Stem Cell Differentiation In Vitro. Reprod. Sci. 2023, 30, 1938–1951. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.; Liu, T.; Feng, X.; Yang, N.; Zhou, H. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, G.; Han, L.; Liu, L.; Ren, F.; Li, L.; Ma, M.; Shan, B. P-Hydroxycinnamaldehyde Induces B16-F1 Melanoma Cell Differentiation via the RhoA-MAPK Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 38, 2247–2260. [Google Scholar] [CrossRef]
- Phillips, B.; Gassei, K.; Orwig, K. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef]
- Yeh, J.; Zhang, X.; Nagano, M. Establishment of a short-term in vitro assay for mouse spermatogonial stem cells. Biol. Reprod. 2007, 77, 897–904. [Google Scholar] [CrossRef][Green Version]
- Zhang, P.; Wu, Y.; Dai, Q.; Fang, B.; Jiang, L. p38-MAPK signaling pathway is not involved in osteogenic differentiation during early response of mesenchymal stem cells to continuous mechanical strain. Mol. Cell. Biochem. 2013, 378, 19–28. [Google Scholar] [CrossRef]
- Jang, Y.; Koo, H.; Sohn, E.; Kang, S.; Rhee, D.; Pyo, S. Theobromine inhibits differentiation of 3T3-L1 cells during the early stage of adipogenesis via AMPK and MAPK signaling pathways. Food Funct. 2015, 6, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Kunath, T.; Saba-El-Leil, M.; Almousailleakh, M.; Wray, J.; Meloche, S.; Smith, A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 2007, 134, 2895–2902. [Google Scholar] [CrossRef]
- Bissonauth, V.; Roy, S.; Gravel, M.; Guillemette, S.; Charron, J. Requirement for Map2k1 (Mek1) in extra-embryonic ectoderm during placentogenesis. Development 2006, 133, 3429–3440. [Google Scholar] [CrossRef]
- Crews, C.; Alessandrini, A.; Erikson, R. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 1992, 258, 478–480. [Google Scholar] [CrossRef]
- Chu, Y.; Corey, D. RNA sequencing: Platform selection, experimental design, and data interpretation. Nucleic Acid Ther. 2012, 22, 271–274. [Google Scholar] [CrossRef]
- Ali, N.; Zirak, B.; Rodriguez, R.; Pauli, M.; Truong, H.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.; Scharschmidt, T.; et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169, 1119–1129.e1111. [Google Scholar] [CrossRef]
- Luo, S.; Shi, Q.; Li, W.; Wu, W.; Zha, Z. ITGB1 promotes the chondrogenic differentiation of human adipose-derived mesenchymal stem cells by activating the ERK signaling. J. Mol. Histol. 2020, 51, 729–739. [Google Scholar] [CrossRef]
- Park, G.; Kim, H.; Park, H.; Seo, Y.; Kim, J.; Shin, S.; Kwon, H.; Sung, E.; Lee, J.; Lee, B. Tensin-3 Regulates Integrin-Mediated Proliferation and Differentiation of Tonsil-Derived Mesenchymal Stem Cells. Cells 2019, 9, 89. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Xue, P.; Ma, X.; Li, J.; Zhang, J. FN1 promotes chondrocyte differentiation and collagen production via TGF-β/PI3K/Akt pathway in mice with femoral fracture. Gene 2021, 769, 145253. [Google Scholar] [CrossRef]
- Jiang, X.; Teng, M.; Ji, R.; Zhang, D.; Zhang, Z.; Lv, Y.; Zhang, Q.; Zhang, J.; Huang, Y. CD9 regulates keratinocyte differentiation and motility by recruiting E-cadherin to the plasma membrane and activating the PI3K/Akt pathway. Biochim. Et Biophys. Acta. Mol. Cell Res. 2020, 1867, 118574. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, H.; Hu, Y.; Yao, R.; Liu, P.; Yang, Y.; Li, S. RNA binding motif protein 3 (RBM3) promotes protein kinase B (AKT) activation to enhance glucose metabolism and reduce apoptosis in skeletal muscle of mice under acute cold exposure. Cell Stress Chaperones 2022, 27, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, K.; Kim, E.; Jang, W. Hypothermia-induced RNA-binding motif protein 3 (RBM3) stimulates osteoblast differentiation via the ERK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 498, 459–465. [Google Scholar] [CrossRef]
- Wellmann, S.; Truss, M.; Bruder, E.; Tornillo, L.; Zelmer, A.; Seeger, K.; Bührer, C. The RNA-binding protein RBM3 is required for cell proliferation and protects against serum deprivation-induced cell death. Pediatr. Res. 2010, 67, 35–41. [Google Scholar] [CrossRef]
- Yang, H.; Ju, F.; Guo, X.; Ma, S.; Wang, L.; Cheng, B.; Zhuang, R.; Zhang, B.; Shi, X.; Feng, Z.; et al. RNA-binding protein RBM3 prevents NO-induced apoptosis in human neuroblastoma cells by modulating p38 signaling and miR-143. Sci. Rep. 2017, 7, 41738. [Google Scholar] [CrossRef]
- Westerman, B.; Braat, A.; Taub, N.; Potman, M.; Vissers, J.; Blom, M.; Verhoeven, E.; Stoop, H.; Gillis, A.; Velds, A.; et al. A genome-wide RNAi screen in mouse embryonic stem cells identifies Mp1 as a key mediator of differentiation. J. Exp. Med. 2011, 208, 2675–2689. [Google Scholar] [CrossRef]
- Caires, K.; de Avila, J.; Cupp, A.; McLean, D. VEGFA family isoforms regulate spermatogonial stem cell homeostasis in vivo. Endocrinology 2012, 153, 887–900. [Google Scholar] [CrossRef]
- Yu, P.; Pan, G.; Yu, J.; Thomson, J. FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell 2011, 8, 326–334. [Google Scholar] [CrossRef]
- Hager, K.; Gu, W. Understanding the non-canonical pathways involved in p53-mediated tumor suppression. Carcinogenesis 2014, 35, 740–746. [Google Scholar] [CrossRef]
- Jain, A.; Allton, K.; Iacovino, M.; Mahen, E.; Milczarek, R.; Zwaka, T.; Kyba, M.; Barton, M. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 2012, 10, e1001268. [Google Scholar] [CrossRef]
- Lhee, S.; Song, E.; Kim, Y.; Han, M. SIRT1 Inhibits p53 but not NF-κB Transcriptional Activity during Differentiation of Mouse Embryonic Stem Cells into Embryoid Bodies. Int. J. Stem Cells 2012, 5, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, S.; Boussaid, I.; Floquet, C.; Raimbault, A.; Hatin, I.; Andrieu-Soler, C.; Salma, M.; Leduc, M.; Gautier, E.; Guyot, B.; et al. p53 activation during ribosome biogenesis regulates normal erythroid differentiation. Blood 2021, 137, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Aptullahoglu, E.; Ciardullo, C.; Wallis, J.; Marr, H.; Marshall, S.; Bown, N.; Willmore, E.; Lunec, J. Splicing Modulation Results in Aberrant Isoforms and Protein Products of p53 Pathway Genes and the Sensitization of B Cells to Non-Genotoxic MDM2 Inhibition. Int. J. Mol. Sci. 2023, 24, 2410. [Google Scholar] [CrossRef]
- Garrido-Jimenez, S.; Barrera-Lopez, J.; Diaz-Chamorro, S.; Mateos-Quiros, C.; Rodriguez-Blanco, I.; Marquez-Perez, F.; Lorenzo, M.; Centeno, F.; Roman, A.; Carvajal-Gonzalez, J. p53 regulation by MDM2 contributes to self-renewal and differentiation of basal stem cells in mouse and human airway epithelium. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21816. [Google Scholar] [CrossRef]
- El-Deiry, W. p21(WAF1) Mediates Cell-Cycle Inhibition, Relevant to Cancer Suppression and Therapy. Cancer Res. 2016, 76, 5189–5191. [Google Scholar] [CrossRef]
- Hu, L.; Liu, J.; Meng, Y.; Zheng, H.; Ding, C.; Wang, H.; Charwudzi, A.; Li, M.; Li, J.; Zhai, Z.; et al. Long non-coding RNA HOTAIR regulates myeloid differentiation through the upregulation of p21 via miR-17-5p in acute myeloid leukaemia. RNA Biol. 2021, 18, 1434–1444. [Google Scholar] [CrossRef]
- Tchakarska, G.; Sola, B. The double dealing of cyclin D1. Cell Cycle 2020, 19, 163–178. [Google Scholar] [CrossRef]
- Montalto, F.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Zhang, H. CCND1 silencing suppresses liver cancer stem cell differentiation through inhibiting autophagy. Hum. Cell 2020, 33, 140–147. [Google Scholar] [CrossRef]
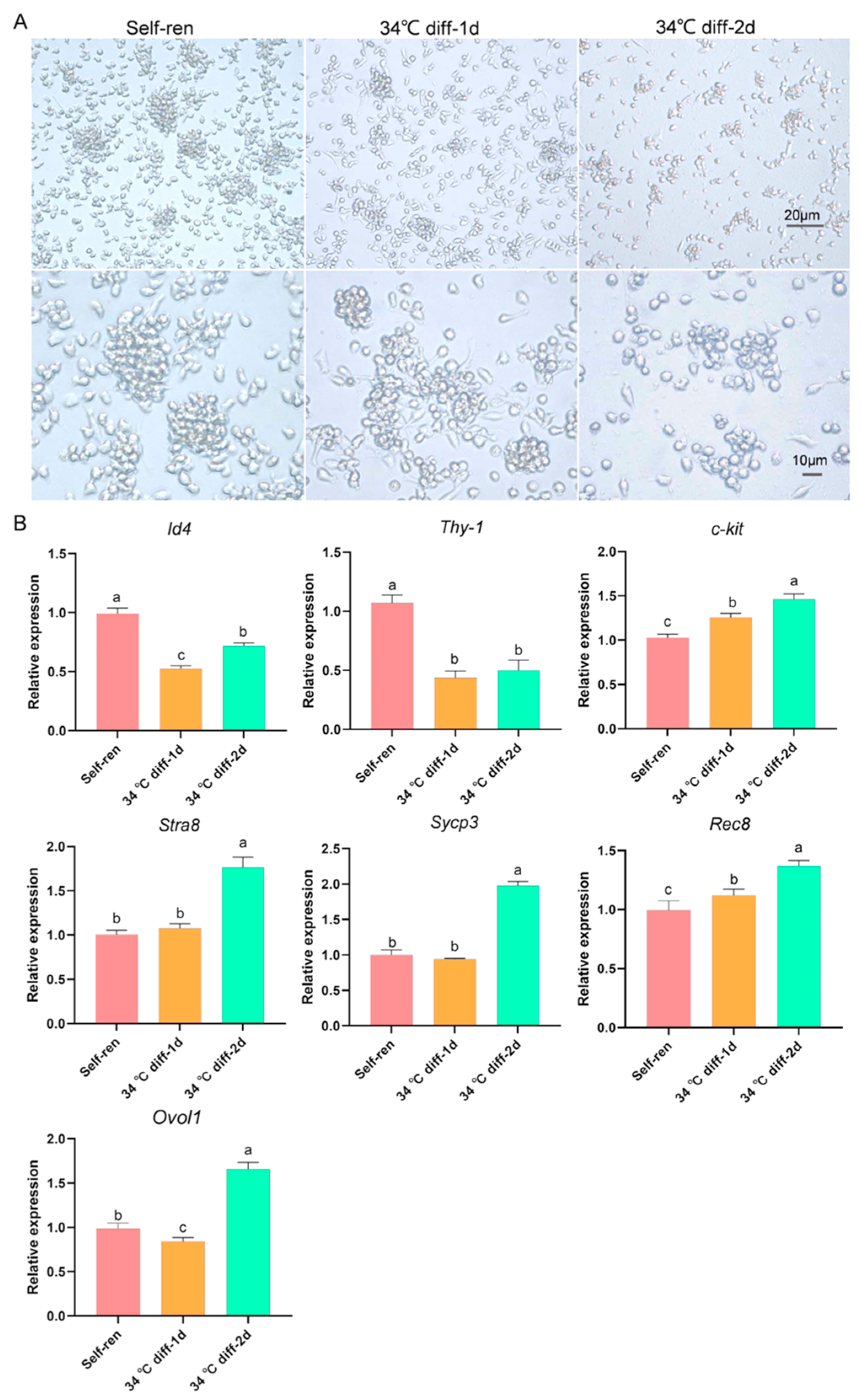
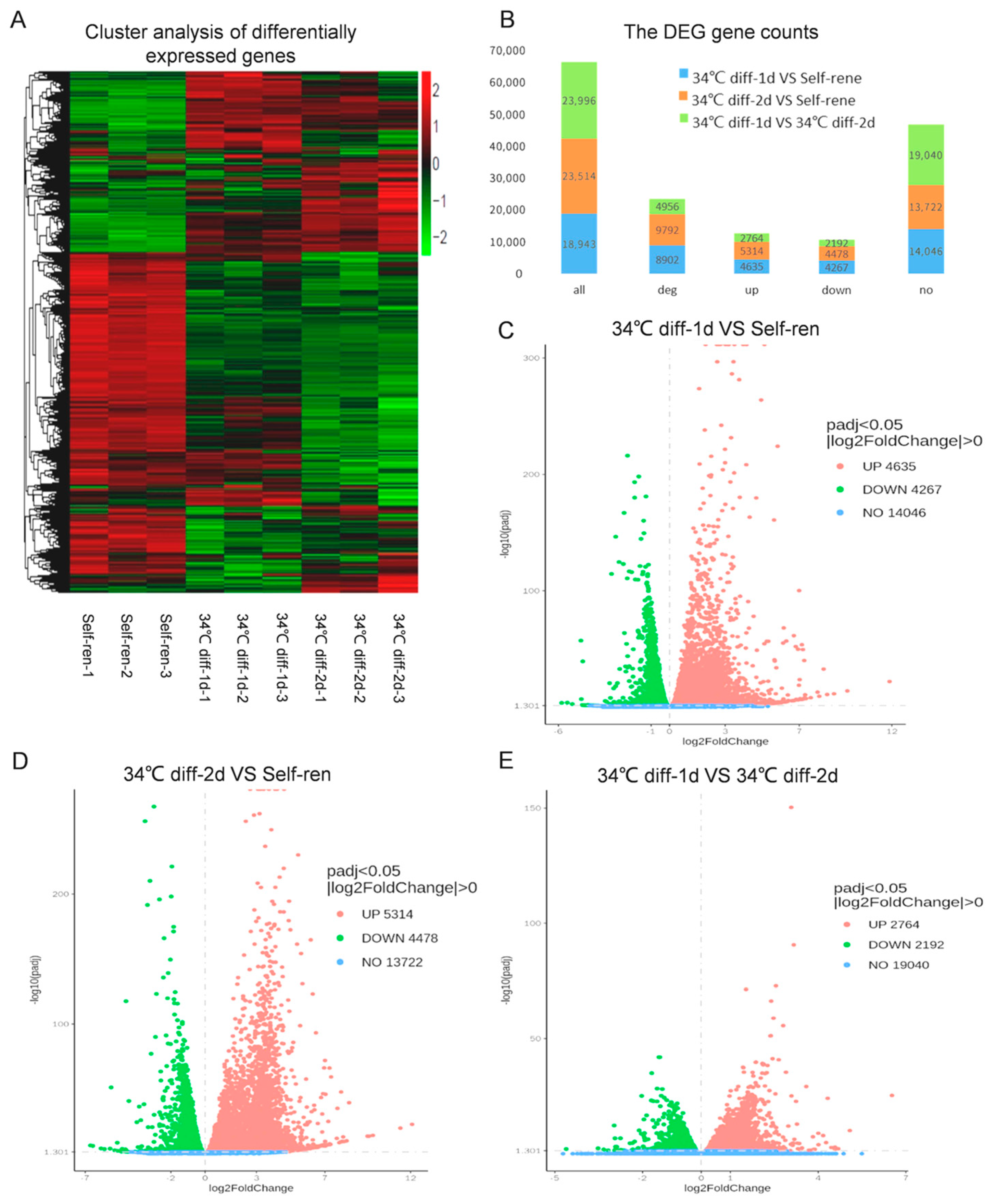
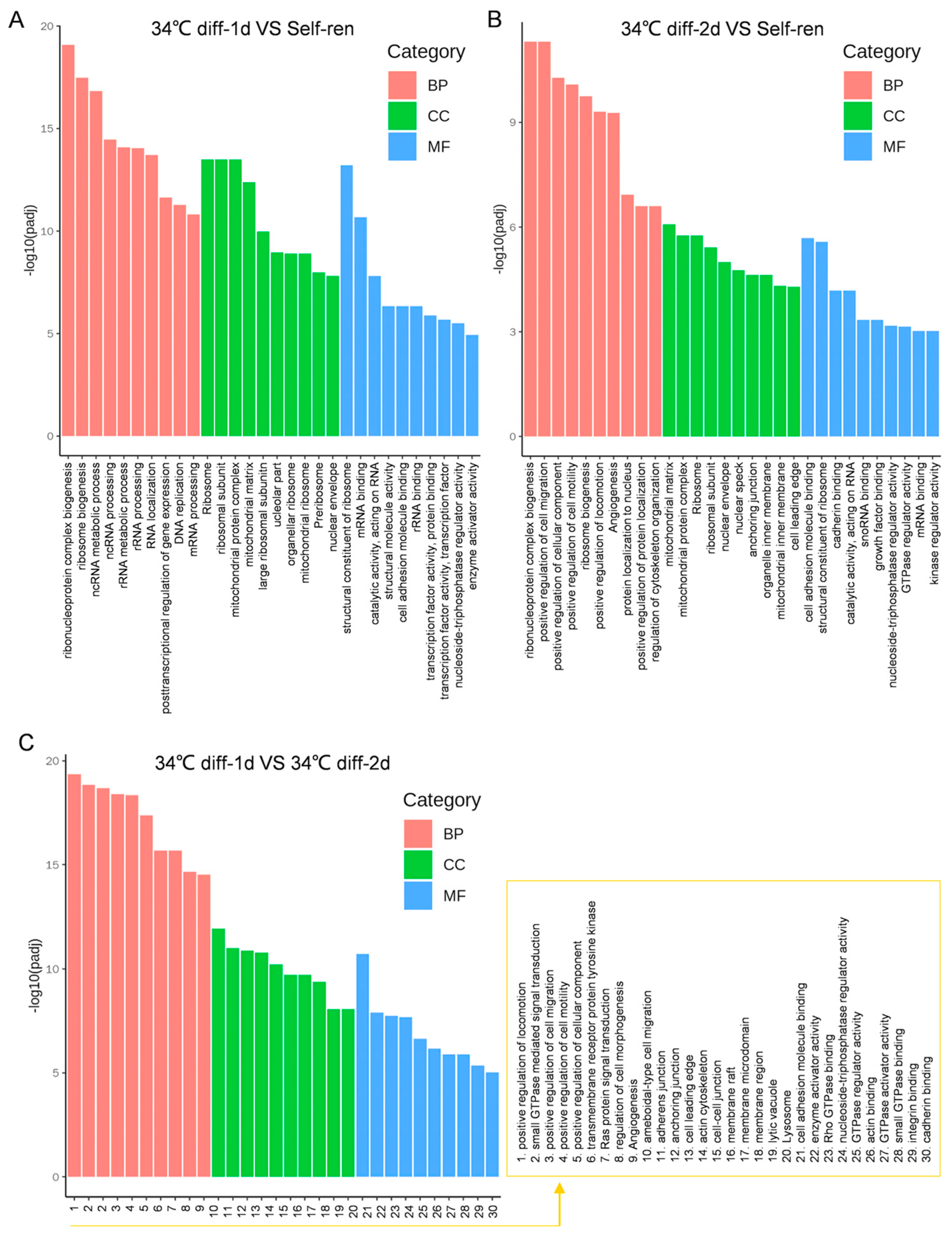
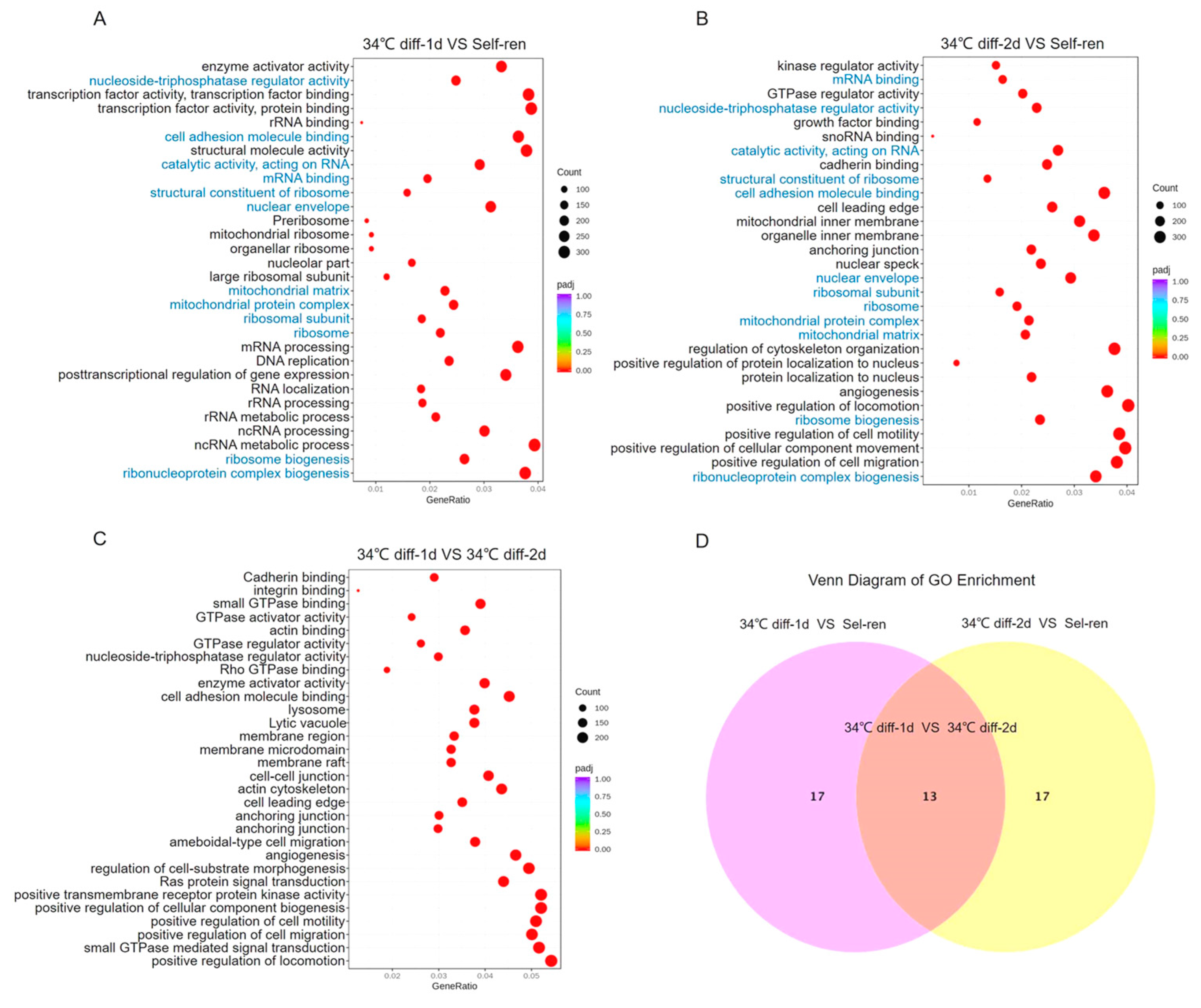
| GO-ID | GO Term | UP-Genes | DOWN-Genes | p-Value | Type |
|---|---|---|---|---|---|
| GO:0022613 | ribonucleoprotein complexbiogenesis | Cdkn2a, Mrpl10 | Gemin5, Nhp2, Mrto4, Rpl3, Rpl12, Rps23 | 1.48 × 10−23 | BP |
| GO:0042254 | Ribosome biogenesis | Rnasel, Rpsa | Rps27l, Surf6, Mrpl20, Rps2, Mrps7, Rpl7l1, Rsl24d1 | 1.17 × 10−21 | BP |
| GO:0034660 | ncRNA metabolic process | Ago4 | Snu13, Npm1, Rpl11, Rpl35a, Mrpl44 | 7.85 × 10−21 | BP |
| GO:0034470 | ncRNA processing | Nsun3, Riok3 | Rps24, Rpl10a, Rps7, Rpl14, Rpl7 | 2.40 × 10−18 | BP |
| GO:0016072 | rRNA metabolic process | Nsun4, Wdr37 | Mrps11, Rpl5, Rps21, Rps6, Rps16 | 7.24 × 10−18 | BP |
| GO:0006364 | rRNA processing | Tsr3 | Mrps9, Rps19, Rps17, Rpl26 | 9.46 × 10−18 | BP |
| GO:0003735 | structural constituent of ribosome | Rpl17, Rpl21 | Mrpl11, Rps15, Rps14, Rps8, Rpl27, Rpl34 | 5.78 × 10−17 | MF |
| GO:0005840 | ribosome | Rbm3 | Rpl10, Rps25, Rpl38, Rpl35, Rsl1d1 | 9.91 × 10−17 | CC |
| GO:0044391 | ribosomal subunit | Lcmt2, Hsd17b10 | Mrpl1, Imp3, Rpl6, Rps5, Rps18, Rpl23a, Rpl13a, Ddx3x | 9.91 × 10−17 | CC |
| GO-ID | GO Term | UP-Genes | DOWN-Genes | p-Value | Type |
|---|---|---|---|---|---|
| GO:0042254 | ribosome biogenesis | Cdkn2a, Riok3, Rnasel | Npm1, C1qbp, Nop53 | 1.54 × 10−13 | BP |
| GO:0022613 | ribonucleoprotein complex biogenesis | Eif2a, Nmd3 | Hsp90ab1, Dicer1 | 1.21 × 10−15 | BP |
| GO:0030335 | positive regulation of cell migration | Fn1, Fgfr1, Thbs1, Anxa1 | Tert, Mapk14, Akt1 | 1.71 × 10−15 | BP |
| GO:0001667 | ameboidal-type cell migration | Epha2, Ilk, Jup | Pdcd6, Itgb3, Prkd2 | 8.48 × 10−10 | BP |
| GO:0034504 | Protein localization to nucleus | Lrrk2, Ptgs2, Lif, BMP4, Cd2ap | Cdk5rap3, Tmem173, Park7 | 1.65 × 10−10 | BP |
| GO:1900182 | positive regulation of protein localization to nucleus | Smo, Src, Cdkn2a | Jak2, Sesn2, Hyal2, Stk11 | 4.64 × 10−10 | BP |
| GO:0010608 | posttranscriptional regulation of gene expression | Ep300, Parp9 | Tgfb1, Mapk1, Tpr | 8.42 × 10−10 | BP |
| GO:0050839 | cell adhesion molecule binding | Fn1, Cttn, Timp2, Fgfr1 | Pdlim1, Twf2 | 2.06 × 10−6 | MF |
| GO:0051098 | regulation of binding | Jak2, Nmd3 Tgfb2 | Parp1 | 4.78 × 10−10 | BP |
| GO:0001525 | angiogenesis | Card10, Ngfr, Eif2ak3, Tek | Gpld1 | 5.36 × 10−10 | BP |
| GO-ID | GO Term | UP-Genes | p-Value | Type |
|---|---|---|---|---|
| GO:0001525 | angiogenesis | Ptgs2, Plau | 4.05 × 10−30 | BP |
| GO:0040017 | positive regulation of locomotion | Prl2c2, Hbegf | 2.67 × 10−28 | BP |
| GO:0001667 | ameboidal-type cell migration | Grem1, Vegfa, Ccbe1 | 2.76 × 10−27 | BP |
| GO:0030335 | positive regulation of cell migration | Spry2, Jun, Sphk1 | 5.15 × 10−27 | BP |
| GO:2000147 | positive regulation of cell motility | Fn1, Thbs1 | 6.66 × 10−27 | BP |
| GO:0071363 | cellular response to growth factor stimulus | Adgra2, Ccl2 | 8.38 × 10−27 | BP |
| GO:0051272 | positive regulation of cellular component movement | Amotl1, Itgb1 | 1.10 × 10−26 | BP |
| GO:0070848 | response to growth factor | Ets1, Fgf2 | 1.38 × 10−26 | BP |
| GO:0005912 | adherens junction | Jag1, Afap1, Itgb1, Itgav Tns3 | 1.12 × 10−19 | CC |
| GO:0050839 | cell adhesion molecule binding | Fn1, Cd9, Fgf2, Itgb1, Epha2, Ctgf | 2.88 × 10−16 | MF |
| KWGG-ID | KEGG Pathway | UP-Genes | DOWN-Genes | p-Value |
|---|---|---|---|---|
| mmu03040 | Spliceosome | Ncbp1, Hspa2, Hspa1a | Ddx39b, Ncbp2, Alyref, Snu13 | 2.65 × 10−9 |
| mmu04110 | Cell cycle | Cdkn2a, Gadd45b | Ccne1, Ccnd2, Ccnb1, Cdk1 | 4.87 × 10−9 |
| mmu03010 | Ribosome | Mrpl10, Rpsa, Rpl21, Rpl17, Rpl34-ps1 | Mrpl12, Rpl35a | 1.06 × 10−7 |
| mmu03013 | RNA transport | Magohb, Lig1, Nxt2 | Pop5, Pop4, Pop1, Nxt1, Nxf1, Nvl | 1.53 × 10−7 |
| mmu03030 | DNA replication | Rfc2, Pold4 | Pola1, Pole4, Prim2, Pola2, Pole | 3.50 × 10−7 |
| mmu04142 | Lysosome | Smo, Src, Cdkn2a | Manba, Atp6v1h, Gga2, Ap1g2 | 2.12 × 10−6 |
| mmu03008 | Ribosome biogenesis in eukaryotes | Xrn1, Nxt2 | Ran, Rpp25, Xpo1 | 6.79 × 10−6 |
| mmu04115 | p53 signaling pathway | Thbs1, Rrm2b, Ccnd1, Mdm2, Fas | Mcm5, Mcm2, Mcm6, Bub1b, Cdk4, Cdk6, Bax, Cdk2 | 5.27 × 10−5 |
| mmu01200 | Carbon metabolism | Idh1, Ogdhl | Shmt2, Psat1, Pfkl, Eno1b | 8.96 × 10−5 |
| mmu05205 | Proteoglycans in cancer | Mdm2, Ctsl, Cd63 | Casp3, Myc | 6.44 × 10−4 |
| KWGG-ID | KEGG Pathway | UP-Genes | DOWN-Genes | p-Value |
|---|---|---|---|---|
| mmu05206 | MicroRNAs in cancer | Cdkn1a, Hmga2, Thbs1, Cdkn2a | E2f2, Ccne1 | 1.06 × 10−5 |
| mmu04210 | Apoptosis | Ctsd, Ctsb, Gzmb, Tnfrsf1a | Hras, Ptpn13, Map3k14 | 4.28 × 10−5 |
| mmu03030 | DNA replication | Lig1, Pold4 | Mcm2, Mcm5, Pold1, Mcm6 | 1.15 × 10−4 |
| mmu03040 | Spliceosome | Magohb, Slu7, Ddx5, Prpf40a | U2af2, Srsf7, Hnrnpc, Hspa8 | 1.68 × 10−4 |
| mmu03008 | Ribosome biogenesis in eukaryotes | Riok2, Riok1 | Heatr1, Nat10, Emg1, Rcl1 | 3.15 × 10−4 |
| mmu04110 | Cell cycle | Cdkn2a, Ep300, Tgfb2 | Mcm2, Mcm5 | 3.62 × 10−4 |
| mmu01230 | Biosynthesis of amino acids | Idh1, Eno2 | Mcm4, Mcm6 | 4.36 × 10−4 |
| mmu00531 | Glycosaminoglycan degradation | Hexb, Sgsh | GusbHyal2, Hyal3, Idua | 5.37 × 10−4 |
| mmu04142 | Lysosome | Ctsd, Ctsb, Ctsl | Hyal2, Hyal3, Idua, Gusb | 7.53 × 10−4 |
| KWGG-ID | KEGG Pathway | UP-Genes | p-Value |
|---|---|---|---|
| mmu05205 | Proteoglycans in cancer | Cdkn1a, Fgf2 | 1.21 × 10−14 |
| mmu04510 | Focal adhesion | Src, Thbs1, Itga5 | 2.21 × 10−13 |
| mmu04360 | Axon guidance | Met, Ptk2, Rhoa | 1.46 × 10−10 |
| mmu05418 | Fluid shear stress and atherosclerosis | Egfr, Pdgfa | 4.24 × 10−10 |
| mmu04151 | PI3K-Akt signaling pathway | Akt3, Jak1, Osm | 1.90 × 10−9 |
| mmu05206 | MicroRNAs in cancer | Vegfa, Cdkn1a | 6.37 × 10−9 |
| mmu04015 | Rap1 signaling pathway | Itgb1, Igf1r, Pik3cb, Rap1b | 1.59 × 10−8 |
| mmu04010 | MAPK signaling pathway | Map2k1, Kras, Pak1 | 3.44 × 10−8 |
| mmu04390 | Hippo signaling pathway | Ctnnb1, Ccnd1 | 4.35 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Yin, P.; Li, H.; Gao, W.; Jia, H.; Ma, W. Transcriptome Analysis of Key Genes Involved in the Initiation of Spermatogonial Stem Cell Differentiation. Genes 2024, 15, 141. https://doi.org/10.3390/genes15020141
Lu X, Yin P, Li H, Gao W, Jia H, Ma W. Transcriptome Analysis of Key Genes Involved in the Initiation of Spermatogonial Stem Cell Differentiation. Genes. 2024; 15(2):141. https://doi.org/10.3390/genes15020141
Chicago/Turabian StyleLu, Xinran, Pengluo Yin, Huixia Li, Weijun Gao, Hua Jia, and Wenzhi Ma. 2024. "Transcriptome Analysis of Key Genes Involved in the Initiation of Spermatogonial Stem Cell Differentiation" Genes 15, no. 2: 141. https://doi.org/10.3390/genes15020141
APA StyleLu, X., Yin, P., Li, H., Gao, W., Jia, H., & Ma, W. (2024). Transcriptome Analysis of Key Genes Involved in the Initiation of Spermatogonial Stem Cell Differentiation. Genes, 15(2), 141. https://doi.org/10.3390/genes15020141




