Genetic Analysis of the Peach SnRK1β3 Subunit and Its Function in Transgenic Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of the PpSnRK1β3 Subunit
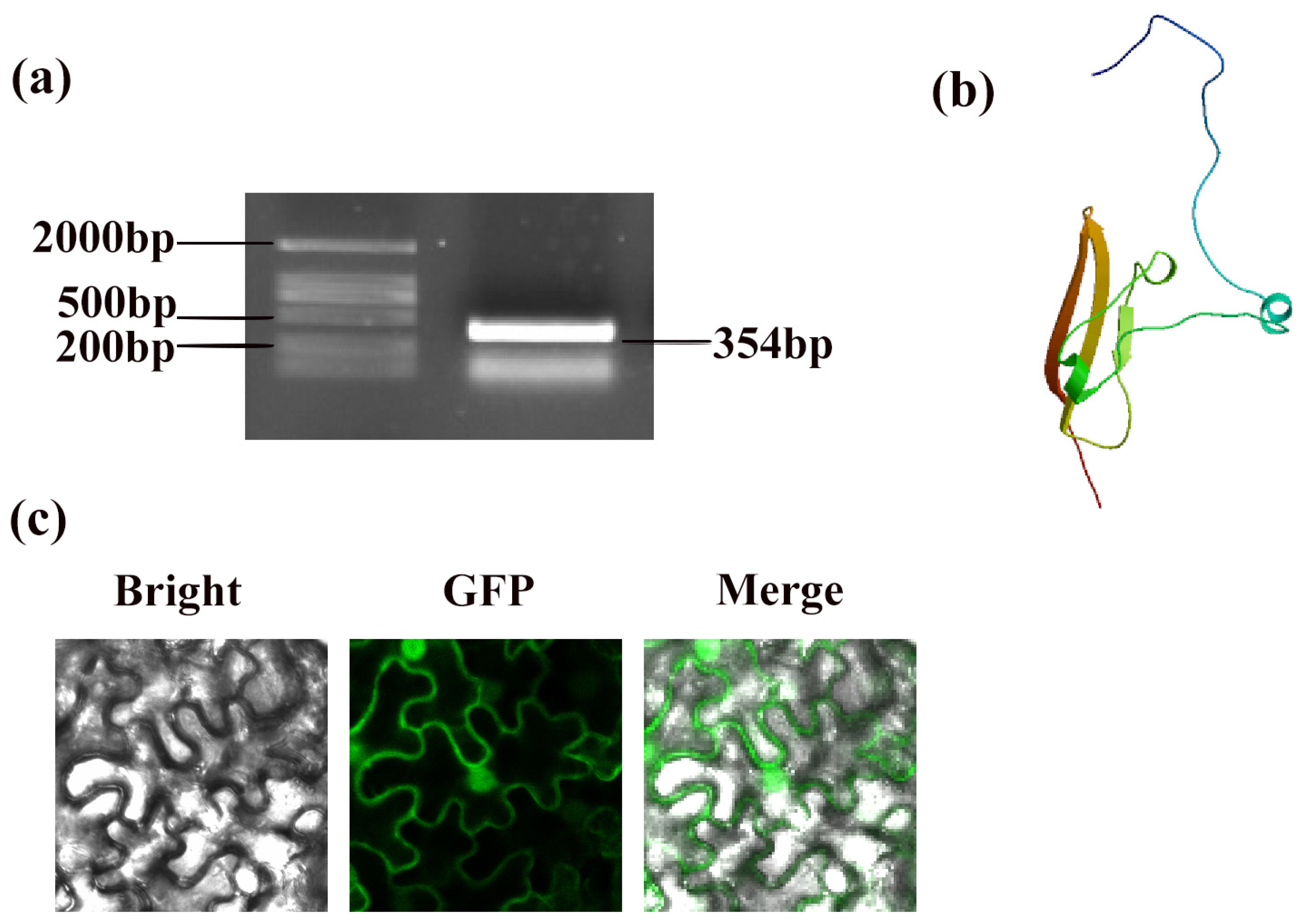
2.2. Subcellular Localization of PpSnRK1β3
2.3. Phylogenetic Tree Analysis
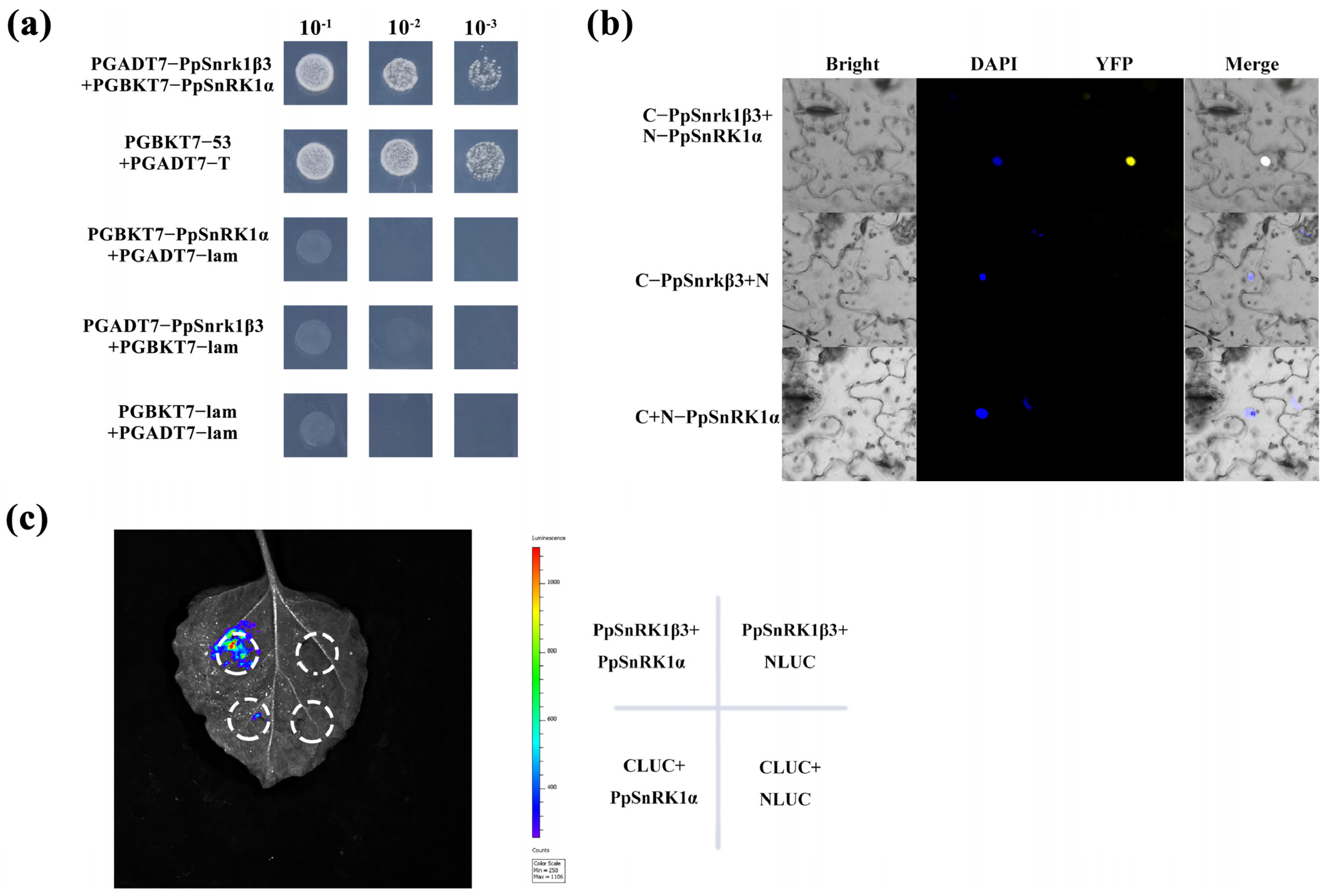
2.4. Yeast Two-Hybrid (Y2H) Assay
2.5. Bimolecular Fluorescence Complementation (BiFC) Assay
2.6. Dual Luciferase Assay
2.7. Acquisition and Experimental Treatment of Overexpressed Tomato Material
2.8. GO and KEGG Enrichment Analysis of Differentially Expressed Genes
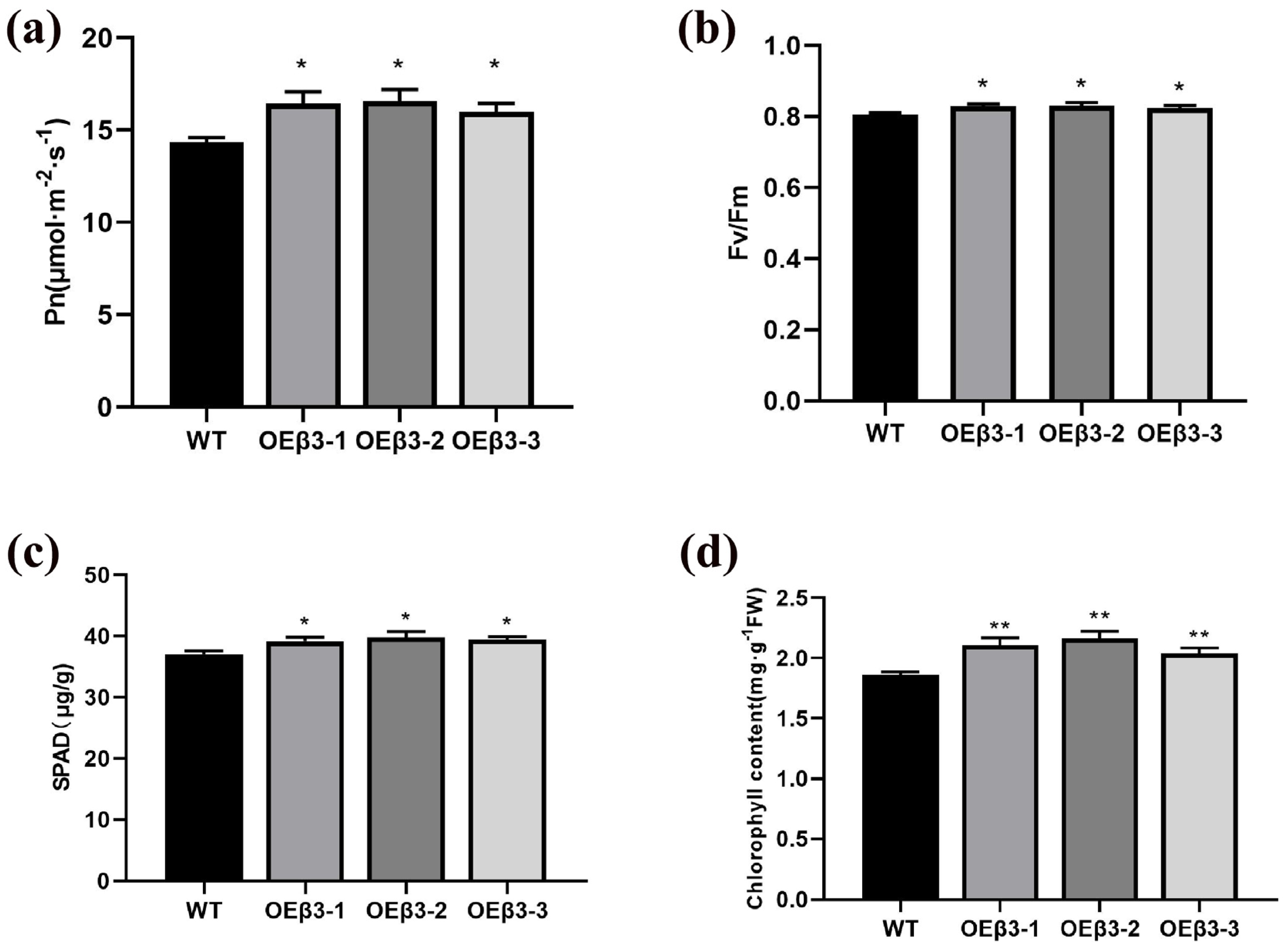
2.9. Determination of Chlorophyll Content
2.10. Determination of Photosynthetic Parameters
2.11. Measurement of MDA, H2O2, O2−, Relative Electrolyte Leakage
3. Results
3.1. Gene Length, Structure Prediction, and Subcellular Localization of PpSnRK1β3
3.2. Phylogenetic Tree Analysis of PpSnRK1β3
3.3. Relationship Between PpSnRK1β3 and the Functional Subunit PpSnRK1α
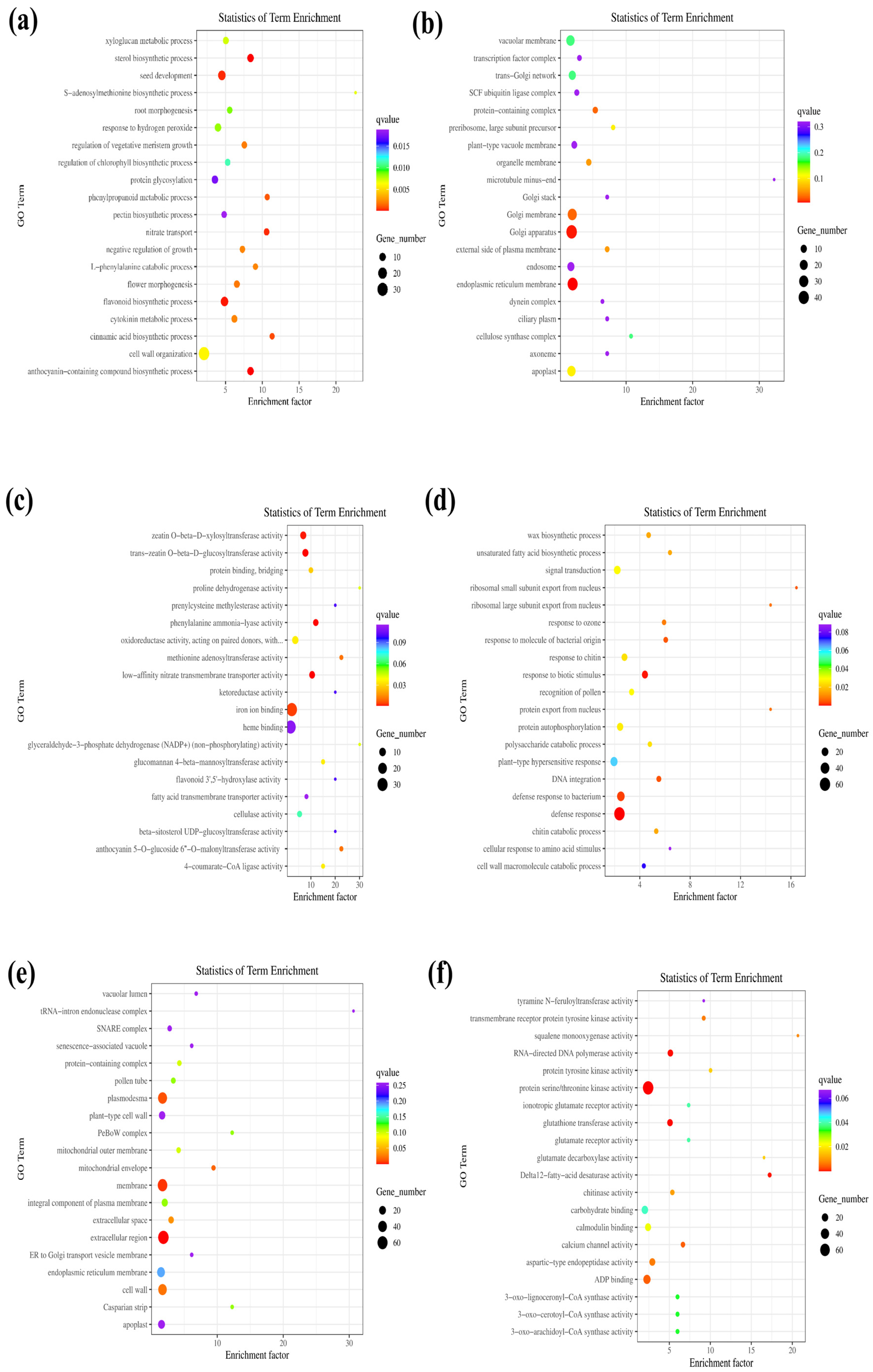
3.4. GO Functional Analysis of DEGs Comparing OEPpSnRK1β3 Tomato and Wild-Type Tomato
3.5. KEGG Enrichment Analysis of DEGs
3.6. Effect of PpSnRK1β3 Overexpression on Tomato Growth
3.7. Effect of OEPpSnRK1β3 on Photosynthesis
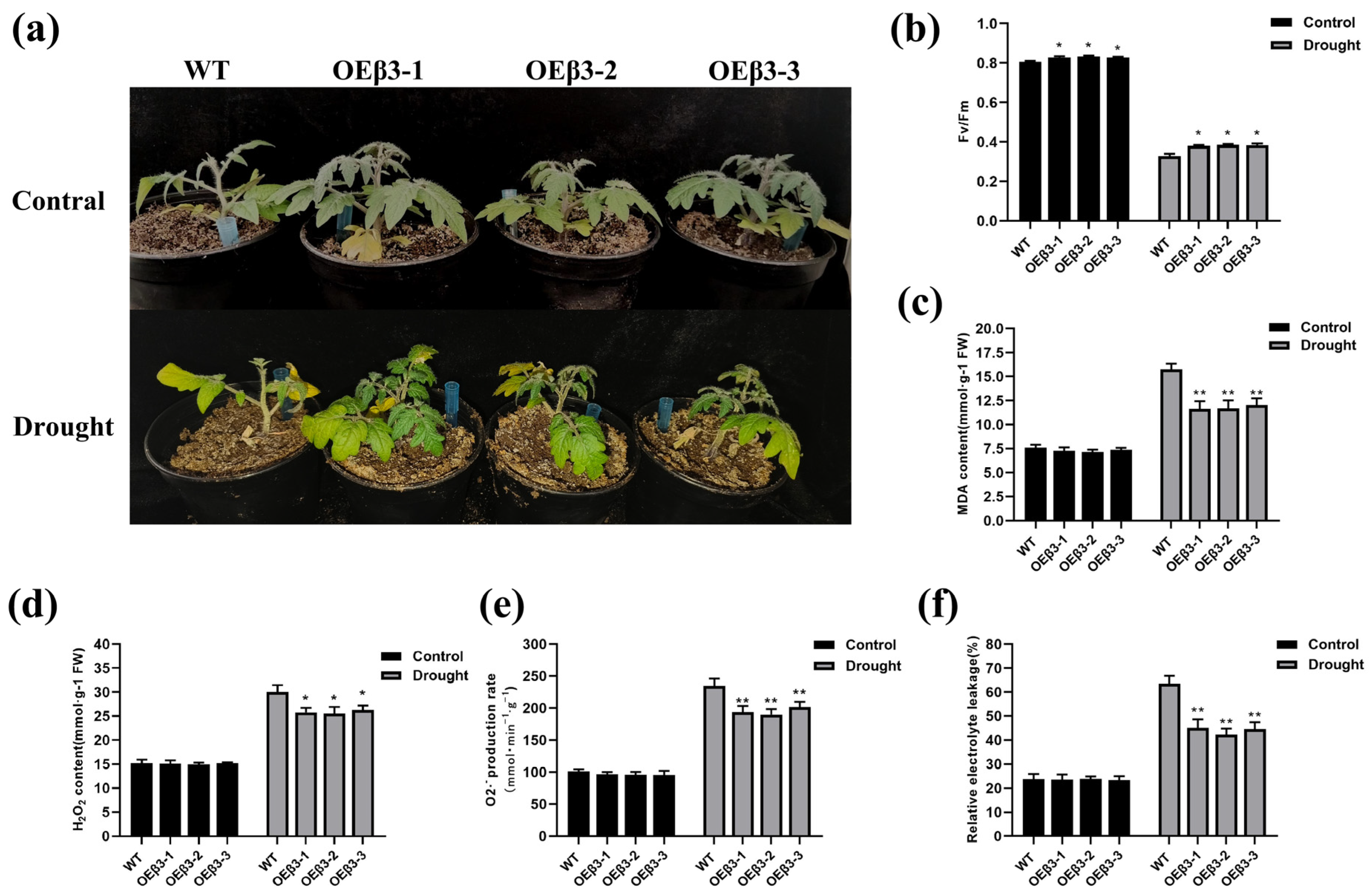
3.8. Adaptability of OEPpSnRK1β3 Tomato to Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SnRK1 | Sucrose Non-fermentation-related Kinase 1 |
| OE | Overexpression |
| Pp | Prunus persica |
| BiFC | Bimolecular Fluorescence Complementation |
| CBM | Carbohydrate-binding module |
| DEG | Differentially expressed genes |
| FLZ | FCS-like zinc |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAPK | Mitogen-activated protein kinase |
| NCBI | National Center for Biotechnology Information |
| TBA | Thiobarbituric acid |
| TCA | Trichloroacetic acid |
| WT | Wild-type |
References
- Ghillebert, R.; Swinnen, E.; Jing, W.; Vandesteene, L.; Ramon, M.; Norga, K.; Rolland, F.; Winderickx, J. The AMPK/SNF1/SnRK1 fuel gauge and energy regulator: Structure, function and regulation. FEBS J. 2011, 278, 3978–3990. [Google Scholar] [CrossRef]
- Alderson, A.; Sabelli, P.A.; Dickinson, J.R.; Cole, D.; Richardson, M.; Kreis, M.; Shewry, P.R.; Halford, N.G. Complementation of snf1, a mutation affecting global regulation of carbon metabolism in yeast, by a plant protein kinase cDNA. Proc. Natl. Acad. Sci. USA 1991, 88, 8602–8605. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, R.; Carlson, M. A family of proteins containing a conserved domain that mediates interaction with the yeast SNF1 protein kinase complex. EMBO J. 1994, 13, 5878–5886. [Google Scholar] [CrossRef] [PubMed]
- Emanuelle, S.; Hossain, M.I.; Moller, I.E.; Pedersen, H.L.; Van, A.M.; Doblin, M.S.; Koay, A.; Oakhill, J.S.; Scott, J.W.; Willats, W.G. SnRK1 from Arabidopsis thaliana is an atypical AMPK. Plant J. 2015, 82, 183–192. [Google Scholar] [CrossRef]
- Margalha, L.; Valerio, C.; Baena-González, E. Plant SnRK1 Kinases: Structure, Regulation, and Function; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 403–438. [Google Scholar] [CrossRef]
- Sanagi, M.; Lu, Y.; Aoyama, S.; Morita, Y.; Mitsuda, N.; Ikeda, M.; Ohme-Takagi, M.; Sato, T.; Junji, Y. Sugar-responsive transcription factor bZIP3 affects leaf shape in Arabidopsis plants. Plant Biotechnol. 2018, 35, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.; Gazzarrini, S. Trehalose-6-phosphate and SnRK1 kinases in plant development and signaling: The emerging picture. Front. Plant Sci. 2014, 5, 119. [Google Scholar] [CrossRef]
- Wang, F.; Ye, Y.; Chen, X.; Zhou, Q. A sucrose non-fermenting-1-related protein kinase 1 gene from potato, StSnRK1, regulates carbohydrate metabolism in transgenic tobacco. Physiol. Mol. Biol. Plants 2017, 23, 933–943. [Google Scholar] [CrossRef]
- Broeckx, T.; Hulsmans, S.; Rolland, F. The plant energy sensor: Evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 2016, 67, 6215–6252. [Google Scholar] [CrossRef]
- Yu, C.; Song, L.; Song, J.; Ouyang, B.; Guo, L.; Shang, L.; Wang, T.; Li, H.; Zhang, J.; Ye, Z. ShCIGT, a Trihelix family gene, mediates cold and drought tolerance by interacting with SnRK1 in tomato. Plant Sci. 2018, 270, 140–149. [Google Scholar] [CrossRef]
- Soto-Burgos, J.; Bassham, D.C. SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS ONE 2017, 12, e0182591. [Google Scholar] [CrossRef]
- Polge, C.; Jossier, M.; Crozet, P.; Gissot, L.; Thomas, M. β-Subunits of the SnRK1 complexes share a common ancestral function together with expression and function specificities; physical interaction with nitrate reductase specifically occurs via AKIN β1-subunit. Plant Physiol. 2008, 148, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Hedbacker, K.; Townley, R.; Carlson, M. Cyclic AMP-dependent protein kinase regulates the subcellular localization of Snf1-Sip1 protein kinase. Mol. Cell. Biol. 2004, 24, 1836–1843. [Google Scholar] [CrossRef] [PubMed]
- Polge, C.; Thomas, M. SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci. 2007, 12, 20–28. [Google Scholar] [CrossRef]
- Lovas, A.; Bimbo, A.; Szabó, L.; Bánfalvi, Z. Antisense repression of StubGAL83 affects root and tuber development in potato. Plant J. 2003, 33, 139–147. [Google Scholar] [CrossRef]
- Pierre, M.; Traverso, J.A.; Boisson, B.; Domenichini, S.; Bouchez, D.; Giglione, C.; Meinnel, T. N-myristoylation regulates the SnRK1 pathway in Arabidopsis. Plant Cell 2007, 19, 2804–2821. [Google Scholar] [CrossRef]
- Henry, C.; Bledsoe, S.W.; Griffiths, C.A.; Paul, M.J.; Kollman, A.; Sakr, S.; Lagrimini, M. Differential role for trehalose metabolism in salt-stressed maize. Plant Physiol. 2015, 169, 1072–1089. [Google Scholar] [CrossRef]
- Bouly, J.P.; Gissot, L.; Lessard, P.; Kreis, M.; Thomas, M. Arabidopsis thaliana proteins related to the yeast SIP and SNF4 interact with AKINα1, an SNF1-like protein kinase. Plant J. 1999, 18, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Gissot, L.; Polge, C.; Bouly, J.P.; Lemaitre, T.; Kreis, M.; Thomas, M. AKINβ3, a plant specific SnRK1 protein, is lacking domains present in yeast and mammals non-catalytic β-subunits. Plant Mol. Biol. 2004, 56, 747–759. [Google Scholar] [CrossRef]
- Sanz, P.; Rubio, T.; Garcia-Gimeno, M.A. AMPK beta subunits: More than just a scaffold in the formation of AMPK complex. FEBS J. 2013, 280, 3723–3733. [Google Scholar] [CrossRef]
- Lumbreras, V.; Albà, M.M.; Kleinow, T.; Koncz, C.; Pagès, M. Domain fusion between SNF1-related kinase subunits during plant evolution. EMBO Rep. 2001, 2, 55–60. [Google Scholar] [CrossRef]
- Maya-Bernal, J.L.; Ávila, A.; Ruiz-Gayosso, A.; Trejo-Fregoso, R.; Pulido, N.; Sosa-Peinado, A.; Zúñiga-Sánchez, E.; Martínez-Barajas, E.; Rodríguez-Sotres, R.; Coello, P. Expression of recombinant SnRK1 in E. coli. Characterization of adenine nucleotide binding to the SnRK1. 1/AKINβγ-β3 complex. Plant Sci. 2017, 263, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gayosso, A.; Rodríguez-Sotres, R.; Martínez-Barajas, E.; Coello, P. A role for the carbohydrate-binding module (CBM) in regulatory SnRK 1 subunits: The effect of maltose on SnRK1 activity. Plant J. 2018, 96, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Beltran, E.; Crespo, J.L. Compartmentalization, a key mechanism controlling the multitasking role of the SnRK1 complex. J. Exp. Bot. 2022, 73, 7055–7067. [Google Scholar] [CrossRef]
- Jamsheer, M.K.; Shukla, B.N.; Jindal, S.; Gopan, N.; Thomas, C.; Laxmi, A. The FCS-like zinc finger scaffold of the kinase SnRK1 is formed by the coordinated actions of the FLZ domain and intrinsically disordered regions. J. Biol. Chem. 2018, 293, 13134–13150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, X.; Zhang, B.; Xiao, Y.; Guo, J.; Liu, J.; Chen, Q.; Peng, F. Genome-wide identification, bioinformatics and expression analysis of HD-Zip gene family in peach. BMC Plant Biol. 2023, 23, 122. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, F.; Xiao, Y.; Wang, W.; Wu, X. Peach PpSnRK1 participates in sucrose-mediated root growth through auxin signaling. Front. Plant Sci. 2020, 11, 409. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Luo, J.; Yu, W.; Xiao, Y.; Peng, F. Peach PpSnRK1α interacts with bZIP11 and maintains trehalose balance in plants. Plant Physiol. Biochem. 2021, 160, 377–385. [Google Scholar] [CrossRef]
- Yu, W.; Peng, F.; Wang, W.; Liang, J.; Xiao, Y.; Yuan, X. SnRK1 phosphorylation of SDH positively regulates sorbitol metabolism and promotes sugar accumulation in peach fruit. Tree Physiol. 2021, 41, 1077–1086. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, H.; Wang, H.; Yu, L.; Yang, Z.; Meng, X.; Hu, P.; Fan, H.; Yu, Y.; Cui, N. SlMYC2 interacted with the SlTOR promoter and mediated JA signaling to regulate growth and fruit quality in tomato. Front. Plant Sci. 2022, 13, 1013445. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Qiao, Z.; Liu, H.; Zhao, L.; Wang, X.; Zhang, Z.; Zhang, S.; Song, L.; You, C. Genome-wide analysis of MdGeBP family and functional identification of MdGeBP3 in Malus domestica. Environ. Exp. Bot. 2023, 208, 105262. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Coin, L.; Durbin, R.; Finn, R.D.; Hollich, V.; Griffiths-Jones, S.; Khanna, A.; Marshall, M.; Moxon, S.; Sonnhammer, E.L. The Pfam protein families database. Nucleic Acids Res. 2004, 32 (Suppl. S1), D138–D141. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Roly, Z.Y.; Ruhullah, M.; Islam, Z.; Sharia, A.; Tanvir, R.Z.; Foyasal, K. Identification of accelerated evolution in the metalloproteinase domain of snake venom metalloproteinase sequences (SVMPs) through comparative analysis. Afr. J. Biotechnol. 2016, 15, 252–263. [Google Scholar] [CrossRef]
- Yang, Y.; An, X.; Rui, L.; Liu, G.; Tian, Y.; You, C.; Wang, X. MdSnRK1. 1 interacts with MdGLK1 to regulate abscisic acid-mediated chlorophyll accumulation in apple. Hortic. Res. 2024, 11, uhad288. [Google Scholar] [CrossRef]
- Kerppola, T.K. Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu. Rev. Biophys. 2008, 37, 465–487. [Google Scholar] [CrossRef]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Bansal, K.C. Overexpression of osmotin gene confers tolerance to salt and drought stresses in transgenic tomato (Solanum lycopersicum L.). Protoplasma 2010, 245, 133–141. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-stimulated drought stress improves the attributes of in vitro growth, steviol glycosides production, and antioxidant activities in Stevia rebaudiana Bertoni. Plants 2020, 9, 1552. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Z.; Xiao, Y.; Liang, J.; Zhao, S.; Liu, Y.; Peng, F.; Guo, J. Genome-wide identification of trehalose-6-phosphate synthase (TPS) gene family reveals the potential role in carbohydrate metabolism in peach. Genes 2023, 15, 39. [Google Scholar] [CrossRef]
- Müller, O.A.; Grau, J.; Thieme, S.; Prochaska, H.; Adlung, N.; Sorgatz, A.; Bonas, U. Genome-wide identification and validation of reference genes in infected tomato leaves for quantitative RT-PCR analyses. PLoS ONE 2015, 10, e0136499. [Google Scholar] [CrossRef]
- Li, Y.; Qi, X. Tryptophan pretreatment adjusts transcriptome and metabolome profiles to alleviate cadmium toxicity in Arabidopsis. J. Hazard. Mater. 2023, 452, 131226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Du, H.; Yang, S.; Wu, X.; Liu, W.; Guo, J.; Xiao, Y.; Peng, F. Physiological and transcriptomic analyses of the effects of exogenous lauric acid on drought resistance in peach (Prunus persica (L.) Batsch). Plants 2023, 12, 1492. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhang, Z.; Liu, X.; Wu, Q.; Chen, Q.; Liu, Q.; van Nocker, S.; Ma, F.; Li, C. Physiological and transcriptome analyses of the effects of exogenous dopamine on drought tolerance in apple. Plant Physiol. Biochem. 2020, 148, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Gao, H.; Zou, Q. Responses of the antioxidant systems and xanthophyll cycle in Phaseolus vulgaris to the combined stress of high irradiance and high temperature. Photosynthetica 2000, 38, 205–210. [Google Scholar] [CrossRef]
- Ma, Q.; Sun, M.; Lu, J.; Liu, Y.; You, C.; Hao, Y. An apple CIPK protein kinase targets a novel residue of AREB transcription factor for ABA-dependent phosphorylation. Plant Cell Environ. 2017, 47, 2207–2219. [Google Scholar] [CrossRef]
- Hu, W.; Huang, C.; Deng, X.; Zhou, S.; Chen, L.; Li, Y.; Wang, C.; Ma, Z.; Yuan, Q.; Wang, Y. TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 2013, 36, 1449–1464. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Sun, M.; Peng, F. Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front. Plant Sci. 2020, 11, 696. [Google Scholar] [CrossRef]
- Hedbacker, K.; Carlson, M. SNF1/AMPK pathways in yeast. Front. Biosci. 2008, 13, 2408–2420. [Google Scholar] [CrossRef]
- Shen, W.; Reyes, M.I.; Hanley-Bowdoin, L. Arabidopsis protein kinases GRIK1 and GRIK2 specifically activate SnRK1 by phosphorylating its activation loop. Plant Physiol. 2009, 150, 996–1005. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Upmeyer, D.J.; Koller, H.R. Diurnal trends in net photosynthetic rate and carbohydrate levels of soybean leaves. Plant Physiol. 1973, 51, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Yu, Q. Mechanism model of stomatal conductance. Chin. J. Plant Ecol. 2009, 33, 772–782. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J.; Lv, Y.; He, J. SPAD values and nitrogen nutrition index for the evaluation of rice nitrogen status. Plant Prod. Sci. 2014, 17, 81–92. [Google Scholar] [CrossRef]
- Sommer, S.G.; Han, E.; Li, X.; Rosenqvist, E.; Liu, F. The chlorophyll fluorescence parameter Fv/Fm correlates with loss of grain yield after severe drought in three wheat genotypes grown at two CO2 concentrations. Plants 2023, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Zhao, D.; Li, Z.; Sui, X.; Han, Z.; Liu, J.; Li, Y.; Zhang, C.; Zheng, Y. Ensifer sp. GMS14 enhances soybean salt tolerance for potential application in saline soil reclamation. J. Environ. Manag. 2024, 349, 119488. [Google Scholar] [CrossRef]
- Whitlow, T.H.; Bassuk, N.L.; Ranney, T.G.; Reichert, D.L. An improved method for using electrolyte leakage to assess membrane competence in plant tissues. Plant Physiol. 1992, 98, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.L.; Ingle, R.A.; Petersen, L.N.; Denby, K.J. Basal resistance against Pseudomonas syringae in Arabidopsis involves WRKY53 and a protein with homology to a nematode resistance protein. Mol. Plant-Microbe Interact. 2007, 20, 1431–1438. [Google Scholar] [CrossRef]
- Shi, W.; Wang, L.; Yao, L.; Hao, W.; Han, C.; Fan, M.; Wang, W.; Bai, M. Spatially patterned hydrogen peroxide orchestrates stomatal development in Arabidopsis. Nat. Commun. 2022, 13, 5040. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, T.; Zhou, C.; Chen, W.; Cha, J.; Wei, X.; Xing, F.; Qian, M.; Ma, Q.; Duan, H.; et al. Characterization of subunits encoded by SnRK1 and dissection of combinations among these subunits in sorghum (Sorghum bicolor L.). J. Integr. Agric. 2023, 22, 642–649. [Google Scholar] [CrossRef]
- Crepin, N.; Rolland, F. SnRK1 activation, signaling, and networking for energy homeostasis. Curr. Opin. Plant Biol. 2019, 51, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Van Leene, J.; Eeckhout, D.; Gadeyne, A.; Matthijs, C.; Han, C.; De Winne, N.; Persiau, G.; Van De Slijke, E.; Persyn, F.; Mertens, T.; et al. Mapping of the plant SnRK1 kinase signalling network reveals a key regulatory role for the class II T6P synthase-like proteins. Nat. Plants 2022, 8, 1245–1261. [Google Scholar] [CrossRef]
- Han, X.; Zhang, L.; Zhao, L.; Xue, P.; Qi, T.; Zhang, C.; Yuan, H.; Zhou, L.; Wang, D.; Qiu, J.; et al. SnRK1 phosphorylates and destabilizes WRKY3 to enhance barley immunity to powdery mildew. Plant Commun. 2020, 1, 14. [Google Scholar] [CrossRef]
- Han, C.; Wang, H.; Shi, W.; Bai, M. The molecular associations between the SnRK1 complex and carbon/nitrogen metabolism in plants. New Crops 2023, 1, 100008. [Google Scholar] [CrossRef]
- Yang, Q.; Song, Z.; Dong, B.; Niu, L.; Cao, H.; Li, H.; Du, T.; Liu, T.; Yang, W.; Meng, D.; et al. Hyperoside regulates its own biosynthesis via MYB30 in promoting reproductive development and seed set in okra. Plant Physiol. 2021, 185, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, J.; Stracke, R.; Richter, A.S. SnRK1 acts upstream of PRODUCTION OF ANTHOCYANIN PIGMENT1, contributing to fine-tuning flavonoid biosynthesis during acclimation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Tian, C.; Wang, X.; Zhou, L.; Jiang, J.; Wang, L.; Chen, F.; Chen, S. Molecular module of CmMYB15-like-Cm4CL2 regulating lignin biosynthesis of chrysanthemum (Chrysanthemum morifolium) in response to aphid (Macrosiphoniella sanborni) feeding. New Phytol. 2023, 237, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shao, C.; Liu, L.; Wang, Y.; An, Y.; Li, H.; Ding, Y.; Jing, Y.; Li, X.; Xiao, J. PtrDJ1C, an atypical member of the DJ-1 superfamily, is essential for early chloroplast development and lignin deposition in poplar. Hortic. Plant J. 2023, 9, 1039–1054. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Q.; Dong, B.; Li, N.; Wang, M.; DU, T.; Liu, N.; Niu, L.; Jin, H.; Meng, D.; et al. Melatonin enhances plant stress tolerance by promoting flavonoid enrichment, focusing on luteolin for salt stress. J. Exp. Bot. 2022, 73, 5992–6008. [Google Scholar] [CrossRef]
- Su, L.; Lv, A.; Wen, W.; Fan, N.; Liu, J.; Gao, L.; Zhou, P.; An, Y. MsMYB741 is involved in alfalfa resistance to aluminum stress by regulating flavonoid biosynthesis. Plant J. 2022, 112, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Sadeghnezhad, E.; Wang, J.; Yu, W.; Zheng, J.; Zhong, R.; Xu, Y.; Zhang, Y.; Dong, T.; Fang, J.; et al. VvSnRK1-VvSS3 regulates sugar accumulation during grape berry ripening in response to abscisic acid. Sci. Hortic. 2023, 320, 112208. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Li, G. Dynamic epigenetic modifications in plant sugar signal transduction. Trends Plant Sci. 2022, 27, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, F.; Li, M.; Yang, L.; Li, G. Expression of a heterologous SnRK1 in tomato increases carbon assimilation, nitrogen uptake and modifies fruit development. J. Plant Physiol. 2012, 169, 1173–1182. [Google Scholar] [CrossRef]
- Ramon, M.; Dang, T.V.T.; Broeckx, T.; Hulsmans, S.; Crepin, N.; Sheen, J.; Rolland, F.A. Default activation and nuclear translocation of the plant cellular energy sensor SnRK1 regulate metabolic stress responses and development. Plant Cell 2019, 31, 1614–1632. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Wu, X.; Liang, J.; Wang, Z.; Fan, S.; Du, H.; Yu, H.; Xiao, Y.; Peng, F. Genetic Analysis of the Peach SnRK1β3 Subunit and Its Function in Transgenic Tomato Plants. Genes 2024, 15, 1574. https://doi.org/10.3390/genes15121574
Zhao S, Wu X, Liang J, Wang Z, Fan S, Du H, Yu H, Xiao Y, Peng F. Genetic Analysis of the Peach SnRK1β3 Subunit and Its Function in Transgenic Tomato Plants. Genes. 2024; 15(12):1574. https://doi.org/10.3390/genes15121574
Chicago/Turabian StyleZhao, Shilong, Xuelian Wu, Jiahui Liang, Zhe Wang, Shihao Fan, Hao Du, Haixiang Yu, Yuansong Xiao, and Futian Peng. 2024. "Genetic Analysis of the Peach SnRK1β3 Subunit and Its Function in Transgenic Tomato Plants" Genes 15, no. 12: 1574. https://doi.org/10.3390/genes15121574
APA StyleZhao, S., Wu, X., Liang, J., Wang, Z., Fan, S., Du, H., Yu, H., Xiao, Y., & Peng, F. (2024). Genetic Analysis of the Peach SnRK1β3 Subunit and Its Function in Transgenic Tomato Plants. Genes, 15(12), 1574. https://doi.org/10.3390/genes15121574




