B Chromosome Transcriptional Inactivation in the Spermatogenesis of the Grasshopper Eyprepocnemis plorans
Abstract
1. Introduction
2. Materials and Methods
2.1. Spreading and Squashing of Seminiferous Tubules
2.2. Immunofluorescence Microscopy
2.3. Image Acquisition and Processing
2.4. RAD51 Foci Quantification
2.5. Fluorescence Quantification
2.6. Electron Microscopy
3. Results
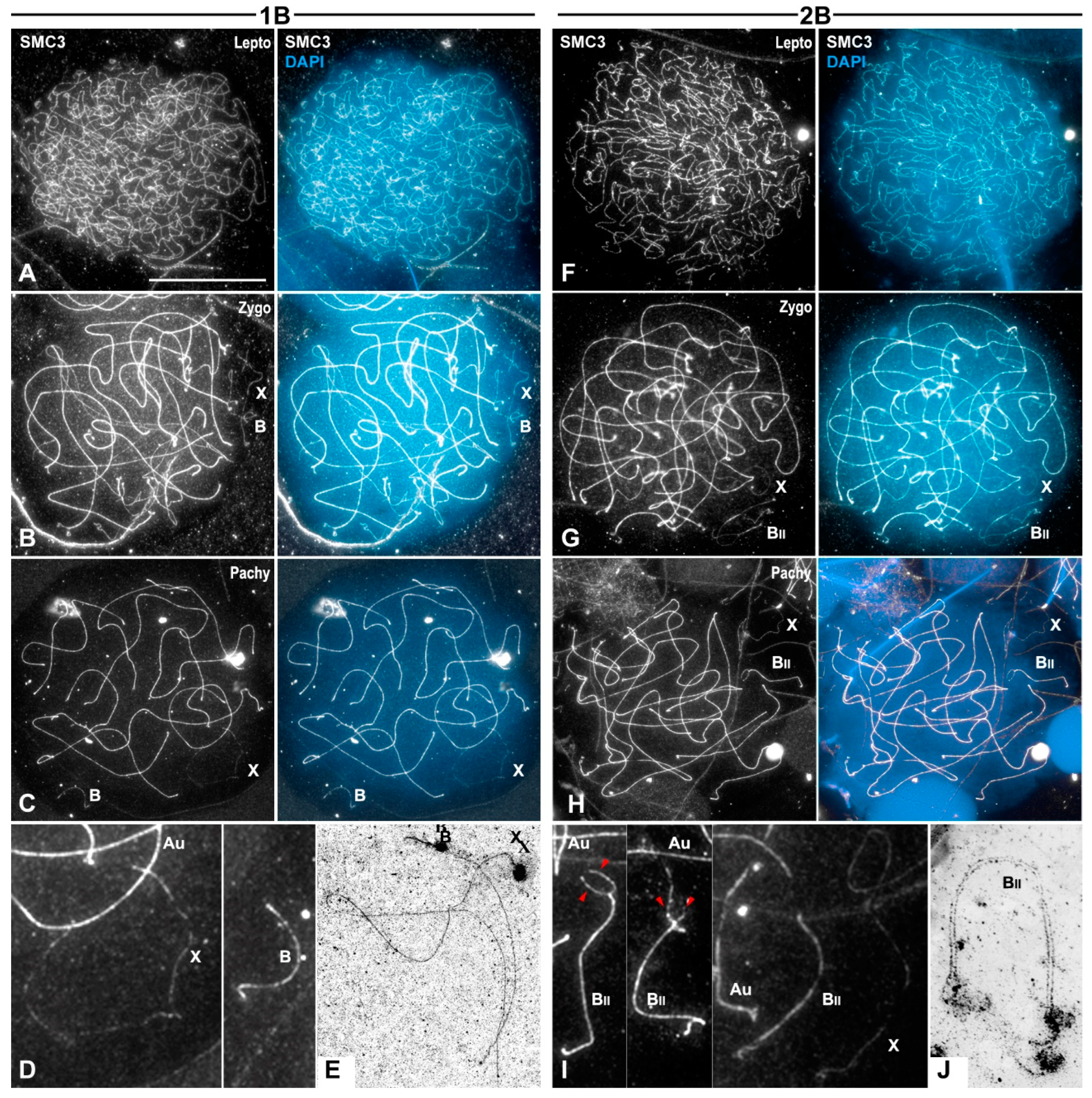
3.1. Meiotic Behavior of the B1 Chromosome
3.2. Cohesin Subunit SMC3 Immunolabeling as a Marker for Chromosome Pairing and Synapsis Throughout Prophase I Stages
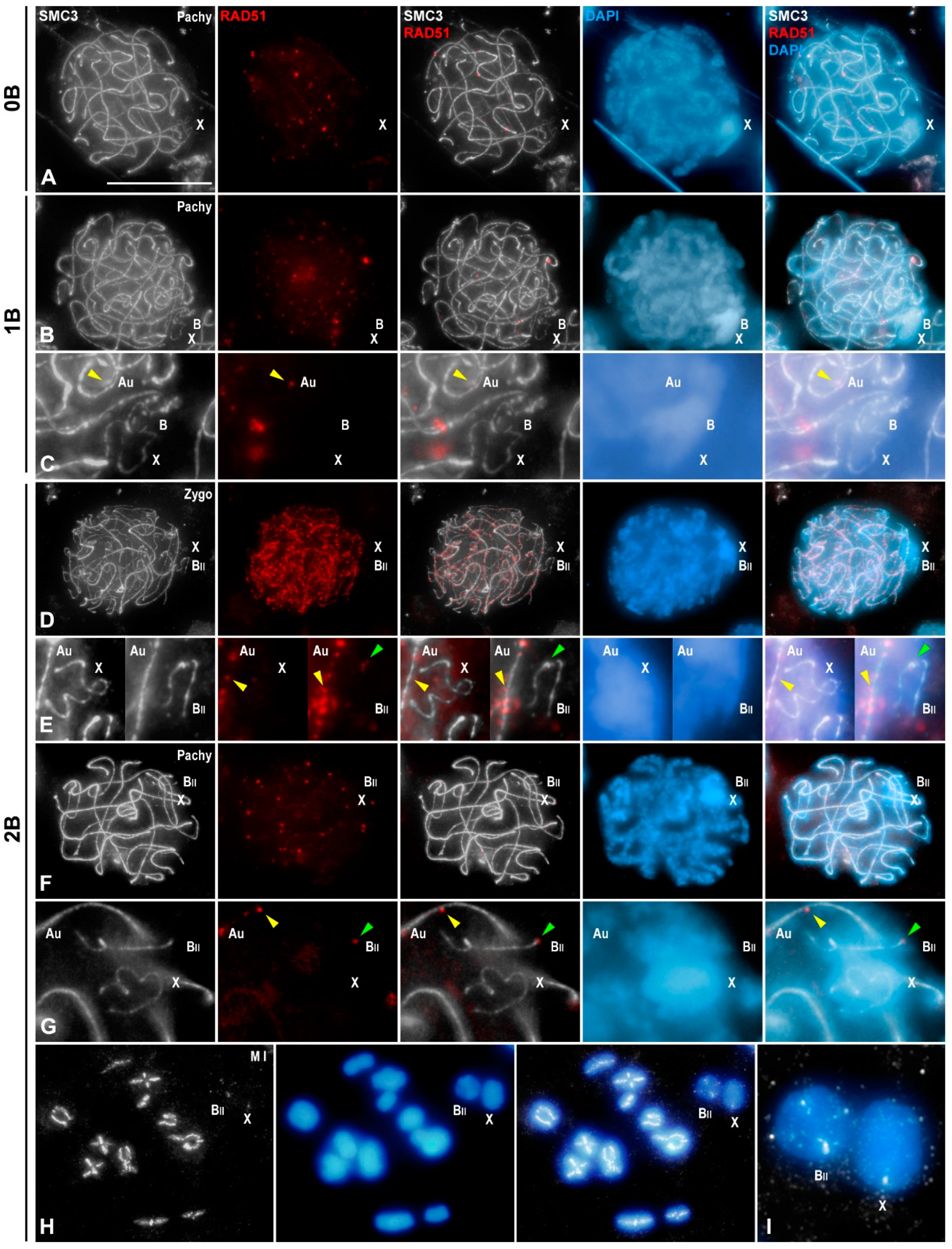
3.3. B1 Bivalent Is Chiasmatic
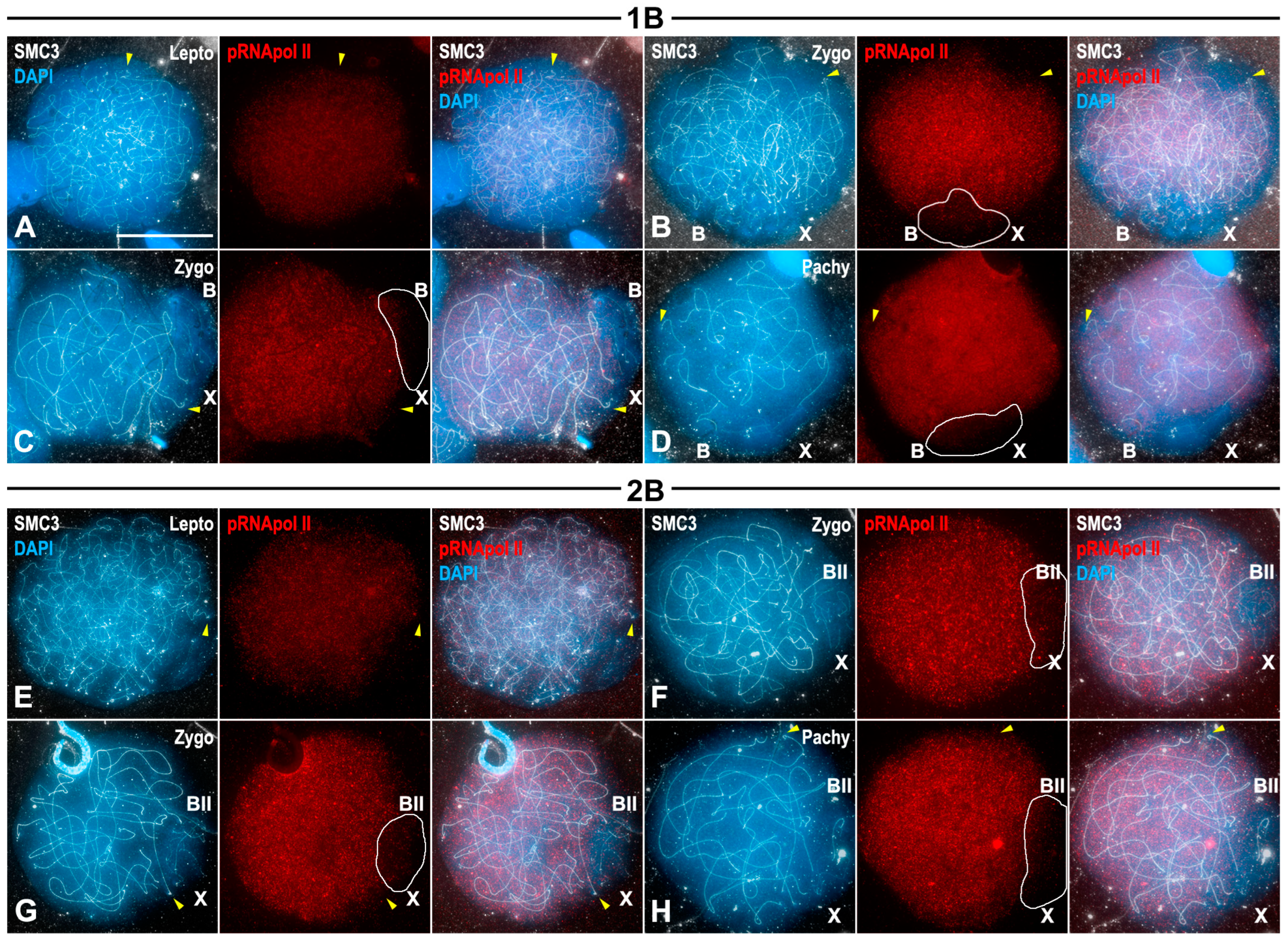
3.4. Transcriptional Activity of B1 Chromosomes Does Not Depend on Their Univalent or Bivalent Condition
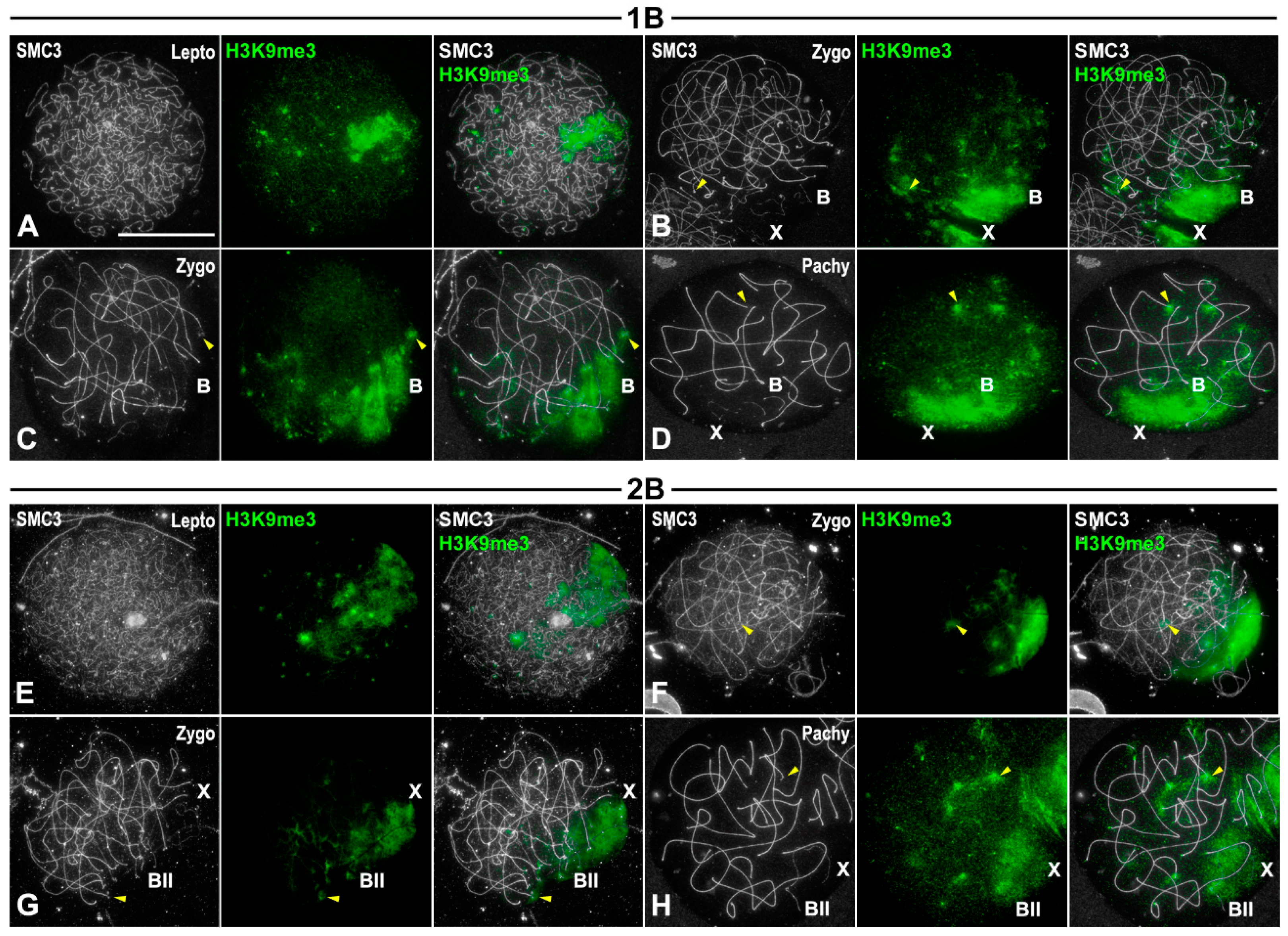
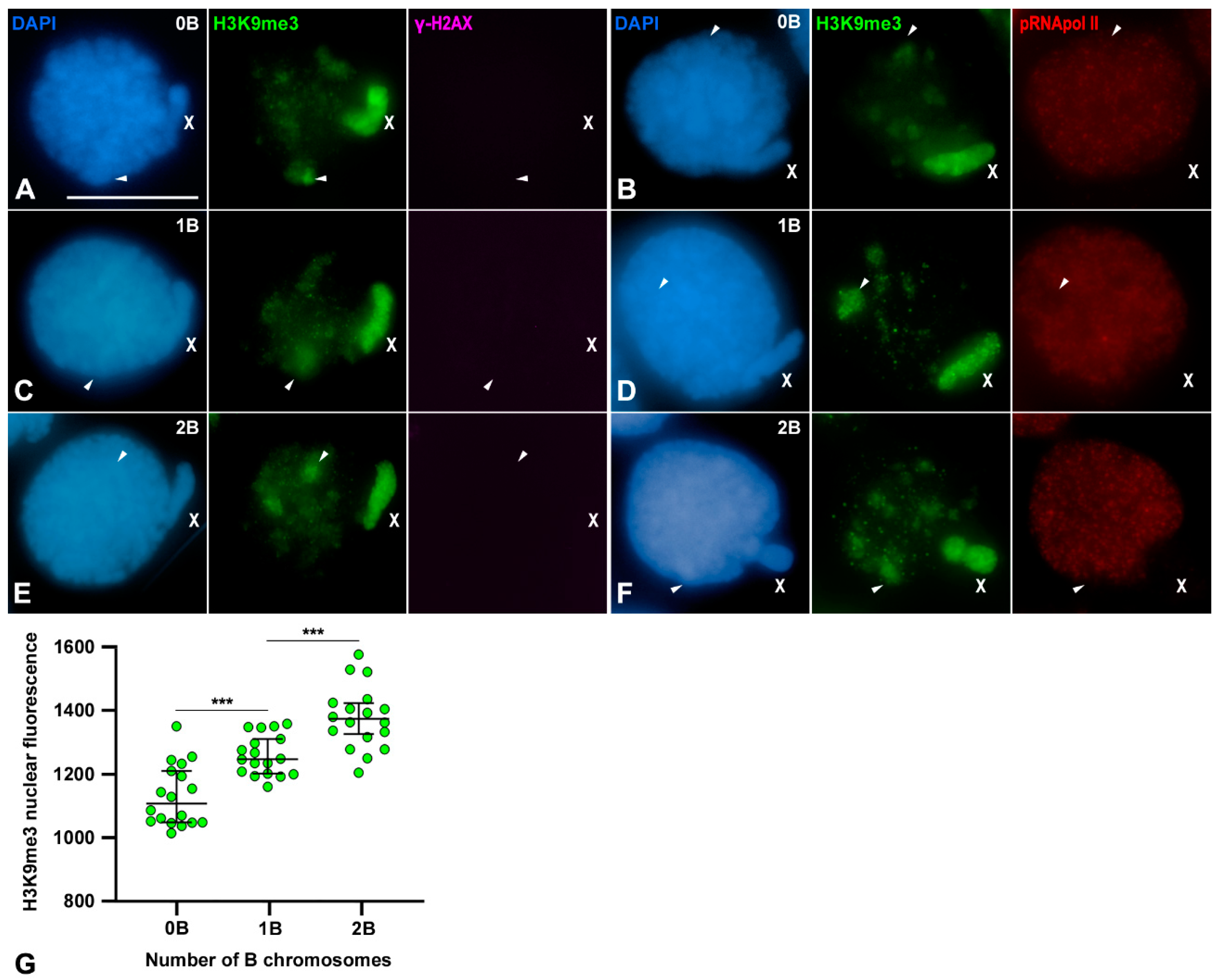
3.5. Transcriptional Inactivation of B1 Chromosome Initiates in Spermatogonia
4. Discussion
4.1. B1 Chromosomes Remain Inactive During Prophase I Regardless of Whether They Complete Synapsis or Not
4.2. X and B1 Chromosomes Enter Meiosis in a Pre-Inactivated State
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, L.; Kang, R.; Rosenberg, S.C.; Qiu, Y.; Raviram, R.; Chee, S.; Hu, R.; Ren, B.; Cole, F.; Corbett, K.D. Dynamic reorganization of the genome shapes the recombination landscape in meiotic prophase. Nat. Struct. Mol. Biol. 2019, 26, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Vara, C.; Paytuvi-Gallart, A.; Cuartero, Y.; Le Dily, F.; Garcia, F.; Salva-Castro, J.; Gomez, H.L.; Julia, E.; Moutinho, C.; Aiese Cigliano, R.; et al. Three-Dimensional Genomic Structure and Cohesin Occupancy Correlate with Transcriptional Activity during Spermatogenesis. Cell Rep. 2019, 28, 352–367.e359. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, G.W.; Derijck, A.A.; Posfai, E.; Giele, M.; Pelczar, P.; Ramos, L.; Wansink, D.G.; van der Vlag, J.; Peters, A.H.; de Boer, P. Chromosome-wide nucleosome replacement and H3.3 incorporation during mammalian meiotic sex chromosome inactivation. Nat. Genet. 2007, 39, 251–258. [Google Scholar] [CrossRef]
- Kota, S.K.; Feil, R. Epigenetic transitions in germ cell development and meiosis. Dev. Cell 2010, 19, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-W.G.; Brick, K.; Cheng, G.; Pratto, F.; Camerini-Otero, R.D. Cell-type-specific genomics reveals histone modification dynamics in mammalian meiosis. Nat. Commun. 2019, 10, 3821. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, R.; Pratto, F.; Hernández-Hernández, A.; Manterola, M.; López-Jiménez, P.; Gómez, R.; Viera, A.; Parra, M.T.; Kouznetsova, A.; Camerini-Otero, R.D.; et al. Epigenetic Dysregulation of Mammalian Male Meiosis Caused by Interference of Recombination and Synapsis. Cells 2021, 10, 2311. [Google Scholar] [CrossRef]
- Monesi, V. Synthetic activities during spermatogenesis in the mouse RNA and protein. Exp. Cell Res. 1965, 39, 197–224. [Google Scholar] [CrossRef]
- Almstrup, K.; Nielsen, J.E.; Hansen, M.A.; Tanaka, M.; Skakkebaek, N.E.; Leffers, H. Analysis of cell-type-specific gene expression during mouse spermatogenesis. Biol. Reprod. 2004, 70, 1751–1761. [Google Scholar] [CrossRef]
- Shima, J.E.; McLean, D.J.; McCarrey, J.R.; Griswold, M.D. The murine testicular transcriptome: Characterizing gene expression in the testis during the progression of spermatogenesis. Biol. Reprod. 2004, 71, 319–330. [Google Scholar] [CrossRef]
- Page, J.; de la Fuente, R.; Manterola, M.; Parra, M.T.; Viera, A.; Berrios, S.; Fernandez-Donoso, R.; Rufas, J.S. Inactivation or non-reactivation: What accounts better for the silence of sex chromosomes during mammalian male meiosis? Chromosoma 2012, 121, 307–326. [Google Scholar] [CrossRef]
- da Cruz, I.; Rodríguez-Casuriaga, R.; Santiñaque, F.F.; Farías, J.; Curti, G.; Capoano, C.A.; Folle, G.A.; Benavente, R.; Sotelo-Silveira, J.R.; Geisinger, A. Transcriptome analysis of highly purified mouse spermatogenic cell populations: Gene expression signatures switch from meiotic-to postmeiotic-related processes at pachytene stage. BMC Genom. 2016, 17, 294. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.; Hamra, F.K.; Garbers, D.L. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc. Natl. Acad. Sci. USA 2003, 100, 12201–12206. [Google Scholar] [CrossRef] [PubMed]
- Pang, A.L.; Johnson, W.; Ravindranath, N.; Dym, M.; Rennert, O.M.; Chan, W.Y. Expression profiling of purified male germ cells: Stage-specific expression patterns related to meiosis and postmeiotic development. Physiol. Genom. 2006, 24, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Baarends, W.M.; Wassenaar, E.; van der Laan, R.; Hoogerbrugge, J.; Sleddens-Linkels, E.; Hoeijmakers, J.H.; de Boer, P.; Grootegoed, J.A. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol. Cell. Biol. 2005, 25, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Schimenti, J. Synapsis or silence. Nat. Genet. 2005, 37, 11–13. [Google Scholar] [CrossRef]
- Turner, J.M.; Mahadevaiah, S.K.; Fernandez-Capetillo, O.; Nussenzweig, A.; Xu, X.; Deng, C.X.; Burgoyne, P.S. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 2005, 37, 41–47. [Google Scholar] [CrossRef]
- Mahadevaiah, S.K.; Turner, J.M.; Baudat, F.; Rogakou, E.P.; de Boer, P.; Blanco-Rodriguez, J.; Jasin, M.; Keeney, S.; Bonner, W.M.; Burgoyne, P.S. Recombinational DNA double-strand breaks in mice precede synapsis. Nat. Genet. 2001, 27, 271–276. [Google Scholar] [CrossRef]
- Turner, J.M.; Aprelikova, O.; Xu, X.; Wang, R.; Kim, S.; Chandramouli, G.V.; Barrett, J.C.; Burgoyne, P.S.; Deng, C.X. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 2004, 14, 2135–2142. [Google Scholar] [CrossRef]
- Turner, J.M. Meiotic sex chromosome inactivation. Development 2007, 134, 1823–1831. [Google Scholar] [CrossRef]
- Henderson, S.A. Differential Ribonucleic Acid Synthesis of X and Autosomes During Meiosis. Nature 1963, 200, 1235. [Google Scholar] [CrossRef]
- Henderson, S.A. RNA synthesis during male meiosis and spermiogenesis. Chromosoma 1964, 15, 345–366. [Google Scholar] [CrossRef]
- Das, N.K.; Siegel, E.P.; Alfert, M. Synthetic Activities During Spermatogenesis in the Locust. J. Cell Biol. 1965, 25, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.S.; Shakes, D.C. Spermatogenesis. Adv. Exp. Med. Biol. 2013, 757, 171–203. [Google Scholar] [CrossRef] [PubMed]
- Hennig, W.; Weyrich, A. Histone modifications in the male germ line of Drosophila. BMC Dev. Biol. 2013, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Gimenez, O.M.; Marti, D.A.; Cabral-de-Mello, D.C. Neo-sex chromosomes of Ronderosia bergi: Insight into the evolution of sex chromosomes in grasshoppers. Chromosoma 2015, 124, 353–365. [Google Scholar] [CrossRef]
- de Almeida, B.R.R.; Noronha, R.C.R.; da Costa, M.J.R.; Nagamachi, C.Y.; Pieczarka, J.C. Meiosis in the scorpion Tityus silvestris: New insights into achiasmatic chromosomes. Biol. Open 2019, 8, bio040352. [Google Scholar] [CrossRef]
- Traut, W.; Schubert, V.; Dalikova, M.; Marec, F.; Sahara, K. Activity and inactivity of moth sex chromosomes in somatic and meiotic cells. Chromosoma 2019, 128, 533–545. [Google Scholar] [CrossRef]
- Viera, A.; Parra, M.T.; Arevalo, S.; Garcia de la Vega, C.; Santos, J.L.; Page, J. X Chromosome Inactivation during Grasshopper Spermatogenesis. Genes 2021, 12, 1844. [Google Scholar] [CrossRef]
- Viera, A.; Parra, M.T.; Rufas, J.S.; Page, J. Transcription reactivation during the first meiotic prophase in bugs is not dependent on synapsis. Chromosoma 2017, 126, 179–194. [Google Scholar] [CrossRef]
- Henriques-Gil, N.; Santos, J.L.; Arana, P. Evolution of a complex B-chromosome polymorphism in the grasshopper Eyprepocnemis plorans. Chromosoma 1984, 89, 290–293. [Google Scholar] [CrossRef]
- Henriques-Gil, N.; Santos, J.L.; Giraldez, R. B-chromosome polymorphism and interchromosomal chiasma interference in Eyprepocnemis plorans (Acrididae; Orthoptera). Chromosoma 1982, 85, 349–359. [Google Scholar] [CrossRef]
- Camacho, J.P.M.; Carballo, A.R.; Cabrero, J. The B-chromosome system of the grasshopper Eyprepocnemis plorans subsp. plorans (Charpentier). Chromosoma 1980, 80, 163–176. [Google Scholar] [CrossRef]
- Camacho, J.P.; Sharbel, T.F.; Beukeboom, L.W. B-chromosome evolution. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2000, 355, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.H.; Plug, A.W.; van Vugt, M.J.; de Boer, P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 1997, 5, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Page, J.; Suja, J.A.; Santos, J.L.; Rufas, J.S. Squash procedure for protein immunolocalization in meiotic cells. Chromosome Res. 1998, 6, 639–642. [Google Scholar] [CrossRef]
- Viera, A.; Calvente, A.; Page, J.; Parra, M.T.; Gomez, R.; Suja, J.A.; Rufas, J.S.; Santos, J.L. X and B chromosomes display similar meiotic characteristics in male grasshoppers. Cytogenet. Genome Res. 2004, 106, 302–308. [Google Scholar] [CrossRef]
- Xiang, Y.; Tsuchiya, D.; Yu, Z.; Zhao, X.; McKinney, S.; Unruh, J.; Slaughter, B.; Lake, C.M.; Hawley, R.S. Multiple reorganizations of the lateral elements of the synaptonemal complex facilitate homolog segregation in Bombyx mori oocytes. Curr. Biol. 2024, 34, 352–360.e4. [Google Scholar] [CrossRef]
- Viera, A.; Santos, J.L.; Page, J.; Parra, M.T.; Calvente, A.; Cifuentes, M.; Gomez, R.; Lira, R.; Suja, J.A.; Rufas, J.S. DNA double-strand breaks, recombination and synapsis: The timing of meiosis differs in grasshoppers and flies. EMBO Rep. 2004, 5, 385–591. [Google Scholar] [CrossRef]
- Viera, A.; Parra, M.T.; Page, J.; Santos, J.L.; Rufas, J.S.; Suja, J.A. Dynamic relocation of telomere complexes in mouse meiotic chromosomes. Chromosome Res. 2003, 11, 797–807. [Google Scholar] [CrossRef]
- Cerro, A.L.; Santos, J.L. Synapsis in grasshopper bivalents heterozygous for centric shifts. Genome 1995, 38, 616–622. [Google Scholar] [CrossRef]
- Calvente, A.; Viera, A.; Parra, M.T.; de la Fuente, R.; Suja, J.A.; Page, J.; Santos, J.L.; de la Vega, C.G.; Barbero, J.L.; Rufas, J.S. Dynamics of cohesin subunits in grasshopper meiotic divisions. Chromosoma 2013, 122, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, A.; Ogawa, H.; Ogawa, T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 1992, 69, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Cowell, I.G.; Aucott, R.; Mahadevaiah, S.K.; Burgoyne, P.S.; Huskisson, N.; Bongiorni, S.; Prantera, G.; Fanti, L.; Pimpinelli, S.; Wu, R.; et al. Heterochromatin, HP1 and methylation at lysine 9 of histone H3 in animals. Chromosoma 2002, 111, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Church, K. The grasshopper X chromosome. I. States of condensation and the nuclear envelope at G1, S and G2 of premeiotic interphase and at early meiotic prophase. Chromosoma 1979, 71, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M. Meiotic Silencing in Mammals. Annu. Rev. Genet. 2015, 49, 395–412. [Google Scholar] [CrossRef]
- Abe, H.; Yeh, Y.H.; Munakata, Y.; Ishiguro, K.I.; Andreassen, P.R.; Namekawa, S.H. Active DNA damage response signaling initiates and maintains meiotic sex chromosome inactivation. Nat. Commun. 2022, 13, 7212. [Google Scholar] [CrossRef]
- Guioli, S.; Lovell-Badge, R.; Turner, J.M. Error-prone ZW pairing and no evidence for meiotic sex chromosome inactivation in the chicken germ line. PLoS Genet. 2012, 8, e1002560. [Google Scholar] [CrossRef]
- Solari, A.J. Equalization of Z and W axes in chicken and quail oocytes. Cytogenet. Cell Genet. 1992, 59, 52–56. [Google Scholar] [CrossRef]
- Pigozzi, M.I. The Chromosomes of Birds during Meiosis. Cytogenet. Genome Res. 2016, 150, 128–138. [Google Scholar] [CrossRef]
- Gaginskaya, E.; Kulikova, T.; Krasikova, A. Avian lampbrush chromosomes: A powerful tool for exploration of genome expression. Cytogenet. Genome Res. 2009, 124, 251–267. [Google Scholar] [CrossRef]
- Weith, A.; Traut, W. Synaptic adjustment, non-homologous pairing, and non-pairing of homologous segments in sex chromosome mutants of Ephestia kuehniella (Insecta, Lepidoptera). Chromosoma 1986, 94, 125–131. [Google Scholar] [CrossRef]
- Marec, F.; Traut, W. Synaptonemal complexes in female and male meiotic prophase of Ephestia kuehniella (Lepidoptera). Heredity 1993, 71, 394–404. [Google Scholar] [CrossRef][Green Version]
- Cabrero, J.; Teruel, M.; Carmona, F.D.; Jimenez, R.; Camacho, J.P. Histone H3 lysine 9 acetylation pattern suggests that X and B chromosomes are silenced during entire male meiosis in a grasshopper. Cytogenet. Genome Res. 2007, 119, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, M.D.; Neves, N.; Schwarzacher, T.; Heslop-Harrison, J.S.; Hewitt, G.M.; Camacho, J.P. Possible origin of a B chromosome deduced from its DNA composition using double FISH technique. Chromosome Res. 1994, 2, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Cabrero, J.; Lopez-Leon, M.D.; Bakkali, M.; Camacho, J.P. Common origin of B chromosome variants in the grasshopper eyprepocnemis plorans. Heredity 1999, 83, 435–439. [Google Scholar] [CrossRef]
- Cabrero, J.; Perfectti, F.; Gomez, R.; Camacho, J.P.; Lopez-Leon, M.D. Population variation in the A chromosome distribution of satellite DNA and ribosomal DNA in the grasshopper Eyprepocnemis plorans. Chromosome Res. 2003, 11, 375–381. [Google Scholar] [CrossRef]
- Navarro-Dominguez, B.; Ruiz-Ruano, F.J.; Cabrero, J.; Corral, J.M.; Lopez-Leon, M.D.; Sharbel, T.F.; Camacho, J.P. Protein-coding genes in B chromosomes of the grasshopper Eyprepocnemis plorans. Sci. Rep. 2017, 7, 45200. [Google Scholar] [CrossRef]
- Fox, D.P.; Hewitt, G.M.; Hall, D.J. DNA replication and RNA transcription of euchromatic and heterochromatic chromosome regions during grasshopper meiosis. Chromosoma 1974, 45, 43–62. [Google Scholar] [CrossRef]
- Ishak, B.; Jaafar, H.; Maetz, J.L.; Rumpler, Y. Absence of transcriptional activity of the B-chromosomes of Apodemus peninsulae during pachytene. Chromosoma 1991, 100, 278–281. [Google Scholar] [CrossRef]
- Collombet, S.; Rall, I.; Dugast-Darzacq, C.; Heckert, A.; Halavatyi, A.; Le Saux, A.; Dailey, G.; Darzacq, X.; Heard, E. RNA polymerase II depletion from the inactive X chromosome territory is not mediated by physical compartmentalization. Nat. Struct. Mol. Biol. 2023, 30, 1216–1223. [Google Scholar] [CrossRef]
- Loda, A.; Collombet, S.; Heard, E. Gene regulation in time and space during X-chromosome inactivation. Nat. Rev. Mol. Cell Biol. 2022, 23, 231–249. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.L.; Parra, M.T.; Arévalo, S.; Guajardo-Grence, A.; Page, J.; Suja, J.Á.; García de la Vega, C.; Viera, A. B Chromosome Transcriptional Inactivation in the Spermatogenesis of the Grasshopper Eyprepocnemis plorans. Genes 2024, 15, 1512. https://doi.org/10.3390/genes15121512
Santos JL, Parra MT, Arévalo S, Guajardo-Grence A, Page J, Suja JÁ, García de la Vega C, Viera A. B Chromosome Transcriptional Inactivation in the Spermatogenesis of the Grasshopper Eyprepocnemis plorans. Genes. 2024; 15(12):1512. https://doi.org/10.3390/genes15121512
Chicago/Turabian StyleSantos, Juan Luis, María Teresa Parra, Sara Arévalo, Andrea Guajardo-Grence, Jesús Page, José Ángel Suja, Carlos García de la Vega, and Alberto Viera. 2024. "B Chromosome Transcriptional Inactivation in the Spermatogenesis of the Grasshopper Eyprepocnemis plorans" Genes 15, no. 12: 1512. https://doi.org/10.3390/genes15121512
APA StyleSantos, J. L., Parra, M. T., Arévalo, S., Guajardo-Grence, A., Page, J., Suja, J. Á., García de la Vega, C., & Viera, A. (2024). B Chromosome Transcriptional Inactivation in the Spermatogenesis of the Grasshopper Eyprepocnemis plorans. Genes, 15(12), 1512. https://doi.org/10.3390/genes15121512







