Purification and Identification of the Nematicidal Activity of S1 Family Trypsin-Like Serine Protease (PRA1) from Trichoderma longibrachiatum T6 Through Prokaryotic Expression and Biological Function Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Samples Preparation
2.3. Prokaryotic Expression of the PRA1 Gene and Protein Induction
2.4. Protein Expression Verification and Molecular Weight Determination by SDS-PAGE Analysis
2.5. Enzymatic Characterization
2.5.1. Optimal Temperature and Thermal Stability Determination
2.5.2. Optimal pH and pH Stability Determination
2.5.3. Effects of Metal Ions and Inhibitors on Recombinant Protease Activity
2.5.4. Effects of Different Substrates on Recombinant Protease Activity
2.6. Biological Function Assays
2.7. Statistical Analysis
3. Results
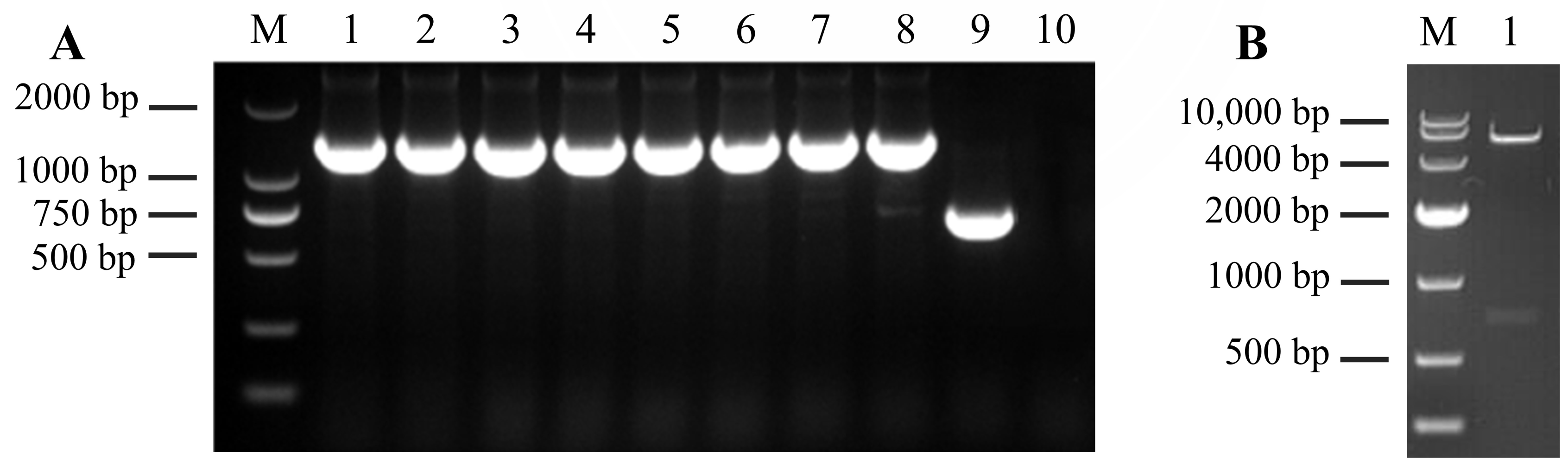
3.1. Construction of Prokaryotic Expression Vector of the PRA1 Gene
3.2. Expression and Purification of Recombinant PRA1 Protease
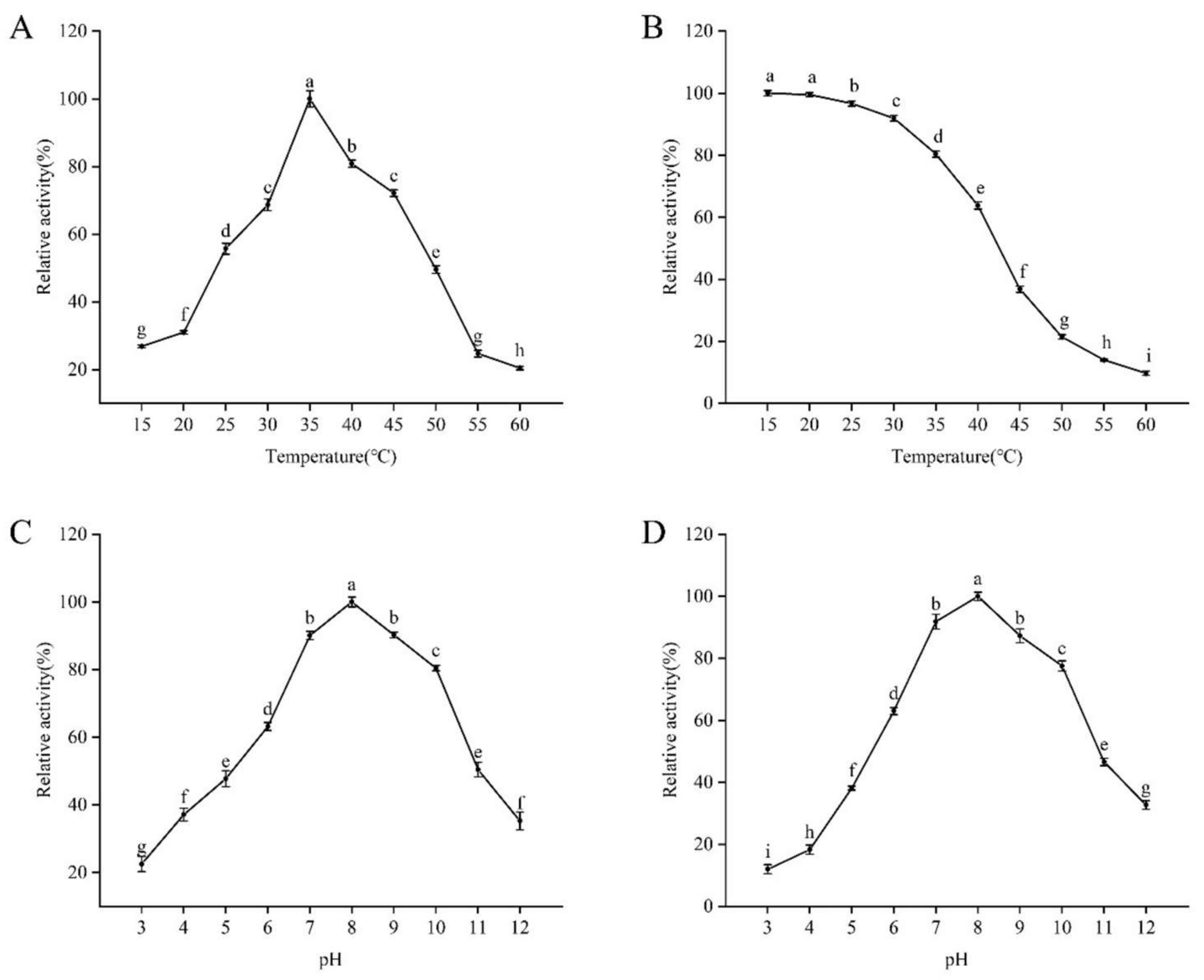
3.3. Determination of the Enzymatic Properties of Recombinant PRA1 Protease
3.4. Effects of Metal Ions and Inhibitors on Recombinant Protease Activity
3.5. Effects of Different Substrates on Recombinant Protease Activity
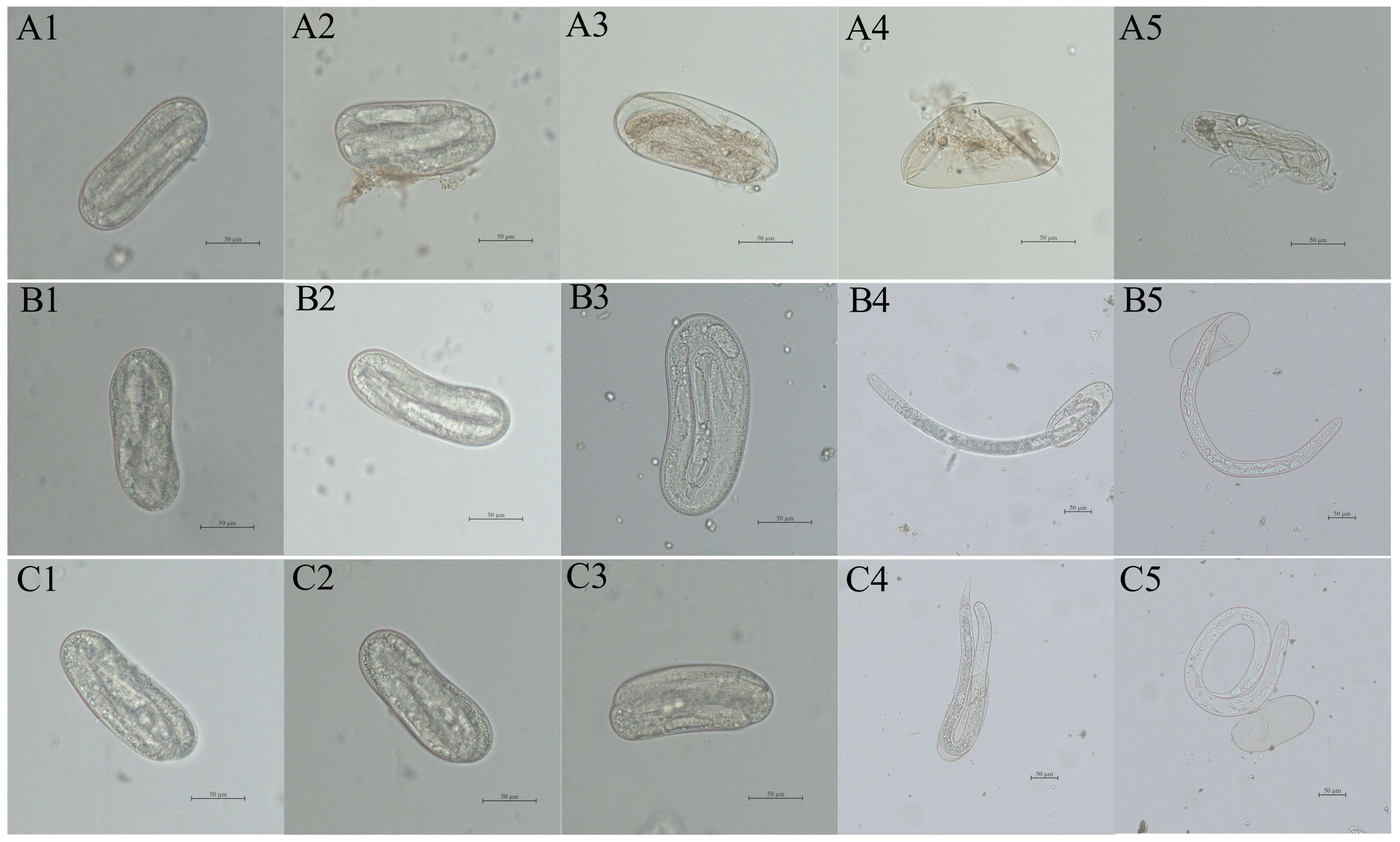
3.6. Microscopic Observation of Eggs Treated with Recombinant Protease
3.7. Inhibitory Effect of Recombinant Protein on Egg Hatching
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, M.A.; Shahzadi, M.; Zahoor, A.; Dababat, A.A.; Toktay, H.; Bakhsh, A.; Nawaz, M.A.; Li, H. Resistance to cereal cyst nematodes in wheat and barley: An emphasis on classical and modern approaches. Int. J. Mol. Sci. 2019, 20, 432. [Google Scholar] [CrossRef] [PubMed]
- Dababat, A.A.; Imren, M.; Erginbas-Orakci, G.; Ashrafi, S.; Yavuzaslanoglu, E.; Toktay, H.; Mekete, T. The importance and management strategies of cereal cyst nematodes, Heterodera spp., in Turkey. Euphytica 2015, 202, 173–188. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M. Optimizing safe approaches to manage plant-parasitic nematodes. Plants 2021, 10, 1911. [Google Scholar] [CrossRef]
- Pulavarty, A.; Egan, A.; Karpinska, A.; Horgan, K.; Kakouli-Duarte, T. Plant parasitic nematodes: A review on their behaviour, host interaction, management approaches and their occurrence in two sites in the republic of Ireland. Plants 2021, 10, 2352. [Google Scholar] [CrossRef] [PubMed]
- Sikora, R.A.; Helder, J.; Molendijk, L.P.; Desaeger, J.; Eves-van den Akker, S.; Mahlein, A.K. Integrated nematode management in a world in transition: Constraints, policy, processes, and technologies for the future. Annu. Rev. Phytopathol. 2023, 61, 209–230. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de Los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.D.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma species: Our best fungal allies in the biocontrol of plant diseases—A review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Szabó, M.; Csepregi, K.; Gálber, M.; Virányi, F.; Fekete, C. Control plant-parasitic nematodes with Trichoderma species and nematode-trapping fungi: The role of chi18-5 and chi18-12 genes in nematode egg-parasitism. Biol. Control 2012, 63, 121–128. [Google Scholar] [CrossRef]
- Moo-Koh, F.A.; Cristóbal-Alejo, J.; Andrés, M.F.; Martín, J.; Reyes, F.; Tun-Suárez, J.M.; Gamboa-Angulo, M. In vitro assessment of organic and residual fractions of nematicidal culture filtrates from thirteen tropical Trichoderma strains and metabolic profiles of most-active. J. Fungi 2022, 8, 82. [Google Scholar] [CrossRef]
- Azeem, W.; Mukhtar, T.; Hamid, T. Evaluation of Trichoderma harzianum and Azadirachta indica in the management of Meloidogyne incognita in Tomato. Pak. J. Zool. 2021, 53, 119–125. [Google Scholar] [CrossRef]
- Sellami, S.; Benttoumi, N.; Berrahia, S.; Boureghda, H. Evaluation of antagonistic activity of Trichoderma spp. against Meloidogyne incognita. Acta Phytopathol. Entomol. Hung. 2017, 52, 177–184. [Google Scholar] [CrossRef]
- Jun, O.K.; Kim, Y.H. Aphelenchus avenae and antagonistic fungi as biological control agents of Pythium spp. Plant Pathol. J. 2004, 20, 271–276. [Google Scholar] [CrossRef]
- Mulusa, L. Biological Control of Root-Knot Nematodes Using Trichoderma spp. In Nematodes-Recent Advances, Management and New Perspectives; IntechOpen: London, UK, 2022; p. 139. [Google Scholar]
- Ibrahim, D.S.; Elderiny, M.M.; Ansari, R.A.; Rizvi, R.; Sumbul, A.; Mahmood, I. Role of Trichoderma spp. in the management of plant-parasitic nematodes infesting important crops. In Management of Phytonematodes: Recent Advances and Future Challenges; Springer: Singapore, 2020; pp. 259–278. [Google Scholar]
- Sahebani, N.; Hadavi, N. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol. Biochem. 2008, 40, 2016–2020. [Google Scholar] [CrossRef]
- Suarez, B.; Rey, M.; Castillo, P.; Monte, E.; Llobell, A. Isolation and characterization of PRA1, a trypsin-like protease from the biocontrol agent Trichoderma harzianum CECT 2413 displaying nematicidal activity. Appl. Microbiol. Biotechnol. 2004, 65, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Sharon, E.; Bar-Eyal, M.; Chet, I.; Herrera-Estrella, A.; Kleifeld, O.; Spiegel, Y. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Phytopatholopgy 2001, 91, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kredics, L.; Kocsubé, S.; Antal, Z.; Hatvani, L.; Manczinger, L.; Vágvölgyi, C. Extracellular proteases of mycoparasitic and nematophagous fungi. In Applied Mycology; CABI: Wallingford, UK, 2009; pp. 290–307. [Google Scholar] [CrossRef]
- Chen, L.L.; Liu, L.J.; Shi, M.; Song, X.Y.; Zheng, C.Y.; Chen, X.L.; Zhang, Y.Z. Characterization and gene cloning of a novel serine protease with nematicidal activity from Trichoderma pseudokoningii SMF2. FEMS Microbiol. Lett. 2009, 299, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Gan, Y.T.; Ji, W.H.; Xu, B.L.; Hou, B.H.; Liu, J. Mechanisms and characterization of Trichoderma longibrachiatum T6 in suppressing nematodes (Heterodera avenae) in wheat. Front. Plant Sci. 2017, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal. Biochem. 1979, 100, 201–220. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Zhang, S.W.; Xu, B.L. Trichoderma longibrachiatum T6: A nematocidal activity of endochitinase gene exploration and its function identification. Int. J. Biol. Macromol. 2022, 223, 1641–1652. [Google Scholar] [CrossRef]
- Yang, Z.S.; Li, G.H.; Zhao, P.J.; Zheng, X.; Luo, S.L.; Li, L.; Zhang, K.Q. Nematicidal activity of Trichoderma spp. and isolation of an active compound. World J. Microbiol. Biotechnol. 2010, 26, 2297–2302. [Google Scholar] [CrossRef]
- Nafady, N.A.; Sultan, R.; El-Zawahry, A.M.; Mostafa, Y.S.; Alamri, S.; Mostafa, R.G.; Hashem, M.; Hassan, E.A. Effective and promising strategy in management of tomato root-knot nematodes by Trichoderma harzianum and arbuscular mycorrhizae. Agronomy 2022, 12, 315. [Google Scholar] [CrossRef]
- Smiley, R.W.; Yan, G. Discovery of Heterodera filipjevi in Washington and comparative virulence with H. avenae on wheat. Plant Dis. 2015, 99, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, N.A.; Wang, Y.; Wang, J.; Zheng, H.; Liu, Z. Subtilisin-like serine protease gene TghSS42 from Trichoderma ghanense ACCC 30153 was successfully expressed in Escherichia coli and recombinant protease rTghSS42 exhibited antifungal ability to five phytopathogens. Biocontrol Sci. 2017, 22, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Liu, Z.; Zhang, R.; Wang, N.; Dou, K.; Mijiti, G.; Wang, Z. Functional analysis of a subtilisin-like serine protease gene from biocontrol fungus Trichoderma harzianum. J. Microbiol. 2014, 52, 129–138. [Google Scholar] [CrossRef]
- Grinyer, J.; McKay, M.; Nevalainen, H.; Herbert, B.R. Fungal proteomics: Initial mapping of biological control strain Trichoderma harzianum. Curr. Genet. 2004, 45, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Convey, P.; Gonzalez, M.; Smykla, J.; Alias, S.A. Effects of temperature on extracellular hydrolase enzymes from soil micro-fungi. Polar Biol. 2018, 41, 537–551. [Google Scholar] [CrossRef]
- Jomova, K.; Baros, S.; Valko, M. Redox active metal-induced oxidative stress in biological systems. Transit. Met. Chem. 2012, 37, 127–134. [Google Scholar] [CrossRef]
- Dupureur, C.M. An integrated look at metallonuclease mechanism. Curr. Chem. Biol. 2008, 2, 159–173. [Google Scholar]
- Perry, M.; Ghosal, G. Mechanisms and regulation of DNA-protein crosslink repair during DNA replication by SPRTN protease. Front. Mol. Biosci. 2022, 9, 916697. [Google Scholar] [CrossRef]
- Xue, Y.; Yan, Q.; Li, X.; Jiang, Z. Characterization of a novel aspartic protease from Trichoderma asperellum for the preparation of duck blood peptides. Appl. Microbiol. Biotechnol. 2024, 108, 131. [Google Scholar] [CrossRef]
- Dube, B.; Smart, G.C. Biological control of Meloidogyne incognita by Paecilomyces lilacinus and Pasteuria penetrans. J. Nematol. 1987, 19, 222. [Google Scholar]
- Escudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biol. 2016, 120, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Liang, L.; Mi, Q.; Yang, J.; Lou, Z.; Sun, Y.; Zhang, K. Preliminary crystallographic study of two cuticle-degrading proteases from the nematophagous fungi Lecanicillium psalliotae and Paecilomyces lilacinus. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Hajjaji, A.; Ibnchekh, G.; Chouati, T.; Rached, B.; Ali, M.A.; Rhallabi, N.; Mhand, R.A.; Mellouki, F. Nematicidal potential of enzymatic extracts of Pseudomonas aeruginosa and Bacillus cereus on different stages of potato cyst nematode Globodera pallida. J. Crop Health 2024, 76, 1063–1072. [Google Scholar] [CrossRef]
- Meyer, S.L.; Massoud, S.I.; Chitwood, D.J.; Roberts, D.P. Evaluation of Trichoderma virens and Burkholderia cepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita. Nematology 2000, 2, 871–879. [Google Scholar]
- Szabó, M.; Urbán, P.; Virányi, F.; Kredics, L.; Fekete, C. Comparative gene expression profiles of Trichoderma harzianum proteases during in vitro nematode egg-parasitism. Biol. Control 2013, 67, 337–343. [Google Scholar] [CrossRef]
- Ganeshan, S.; Settu, V.; Mannu, J.; Annaiyan, S.; Muthusamy, G.; Arun, A.; Somasundaram, P.; Ramkumar, H.; Krithika, V. Genomic analysis of Bacillus subtilis GEB5 reveals its genetic assets for nematicidal and plant growth promoting mechanisms. Rhizosphere 2024, 31, 100953. [Google Scholar] [CrossRef]
- Geng, C.; Nie, X.; Tang, Z.; Zhang, Y.; Lin, J.; Sun, M.; Peng, D. A novel serine protease, Sep1, from Bacillus firmus DS-1 has nematicidal activity and degrades multiple intestinal-associated nematode proteins. Sci. Rep. 2016, 6, 25012. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence (5′ to 3′) |
|---|---|
| YF | ACGCGGATCCATCCAGCCCCGTGGCGCCGA |
| YR | ACCGCTCGAGTGCGAGGTTCTGGTTGATGTAGTTG |
| T7 | TAATACGACTCACTATAGGG |
| T7ter | GCTAGTTATTGCTCAGCGG |
| Reagents | Concentration (mM) | Relative Activity (%) |
|---|---|---|
| Control | - | 100.00 ± 2.18 c |
| Fe2+ | 5 | 41.80 ± 1.43 g |
| Fe3+ | 5 | 39.70 ± 0.69 g |
| Cu2+ | 5 | 62.56 ± 0.66 f |
| Mn2+ | 5 | 69.17 ± 1.34 e |
| Ca2+ | 5 | 133.83 ± 0.99 a |
| Mg2+ | 5 | 123.16 ± 1.45 b |
| Na+ | 5 | 100.30 ± 0.91 c |
| K+ | 5 | 101.05 ± 1.04 c |
| Li+ | 5 | 101.95 ± 0.94 c |
| Zn2+ | 5 | 102.71 ± 0.40 c |
| EDTA | 5 | 96.39 ± 1.73 d |
| PMSF | 1 | 25.26 ± 0.69 h |
| Substrate | Concentration (%) | Relative Activity (%) |
|---|---|---|
| Casein | 1 | 100.00 ± 1.88 a |
| Skimmed milk | 1 | 78.94 ± 1.15 b |
| Bovine serum albumin | 1 | 25.77 ± 1.66 c |
| Gelatin | 1 | 23.12 ± 1.45 c |
| Nematode eggshell | 1 | 26.07 ± 1.79 c |
| Sample | Inhibition Rate of Eggs Hatching (%) | Relative Inhibition Rate of Eggs Hatching (%) |
|---|---|---|
| PRA1 protease | 70.60 ± 3.57 a | 66.58 ± 4.06 |
| E. coli BL21 (DE3) solution of pET-32a empty vector (Positive control) | 10.88 ± 0.67 b | - |
| Sterile water (Negative control) | 12.04 ± 1.64 b | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, N.; Lv, H.; Boamah, S.; Zhang, S.; Xu, B. Purification and Identification of the Nematicidal Activity of S1 Family Trypsin-Like Serine Protease (PRA1) from Trichoderma longibrachiatum T6 Through Prokaryotic Expression and Biological Function Assays. Genes 2024, 15, 1437. https://doi.org/10.3390/genes15111437
Ma N, Lv H, Boamah S, Zhang S, Xu B. Purification and Identification of the Nematicidal Activity of S1 Family Trypsin-Like Serine Protease (PRA1) from Trichoderma longibrachiatum T6 Through Prokaryotic Expression and Biological Function Assays. Genes. 2024; 15(11):1437. https://doi.org/10.3390/genes15111437
Chicago/Turabian StyleMa, Nan, Hang Lv, Solomon Boamah, Shuwu Zhang, and Bingliang Xu. 2024. "Purification and Identification of the Nematicidal Activity of S1 Family Trypsin-Like Serine Protease (PRA1) from Trichoderma longibrachiatum T6 Through Prokaryotic Expression and Biological Function Assays" Genes 15, no. 11: 1437. https://doi.org/10.3390/genes15111437
APA StyleMa, N., Lv, H., Boamah, S., Zhang, S., & Xu, B. (2024). Purification and Identification of the Nematicidal Activity of S1 Family Trypsin-Like Serine Protease (PRA1) from Trichoderma longibrachiatum T6 Through Prokaryotic Expression and Biological Function Assays. Genes, 15(11), 1437. https://doi.org/10.3390/genes15111437





