Comparison of Molecularly Identified Resistant and Susceptible Johnsongrass (Sorghum halepense L.) Populations at ALS Gene, in the Absence and Presence of Field Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collections and Molecular Characterization
2.2. Investigation of Growth Rate
2.3. Johnsongrass Competition with Corn or Sunflower
3. Results
3.1. Molecular Confirmation of Mutations
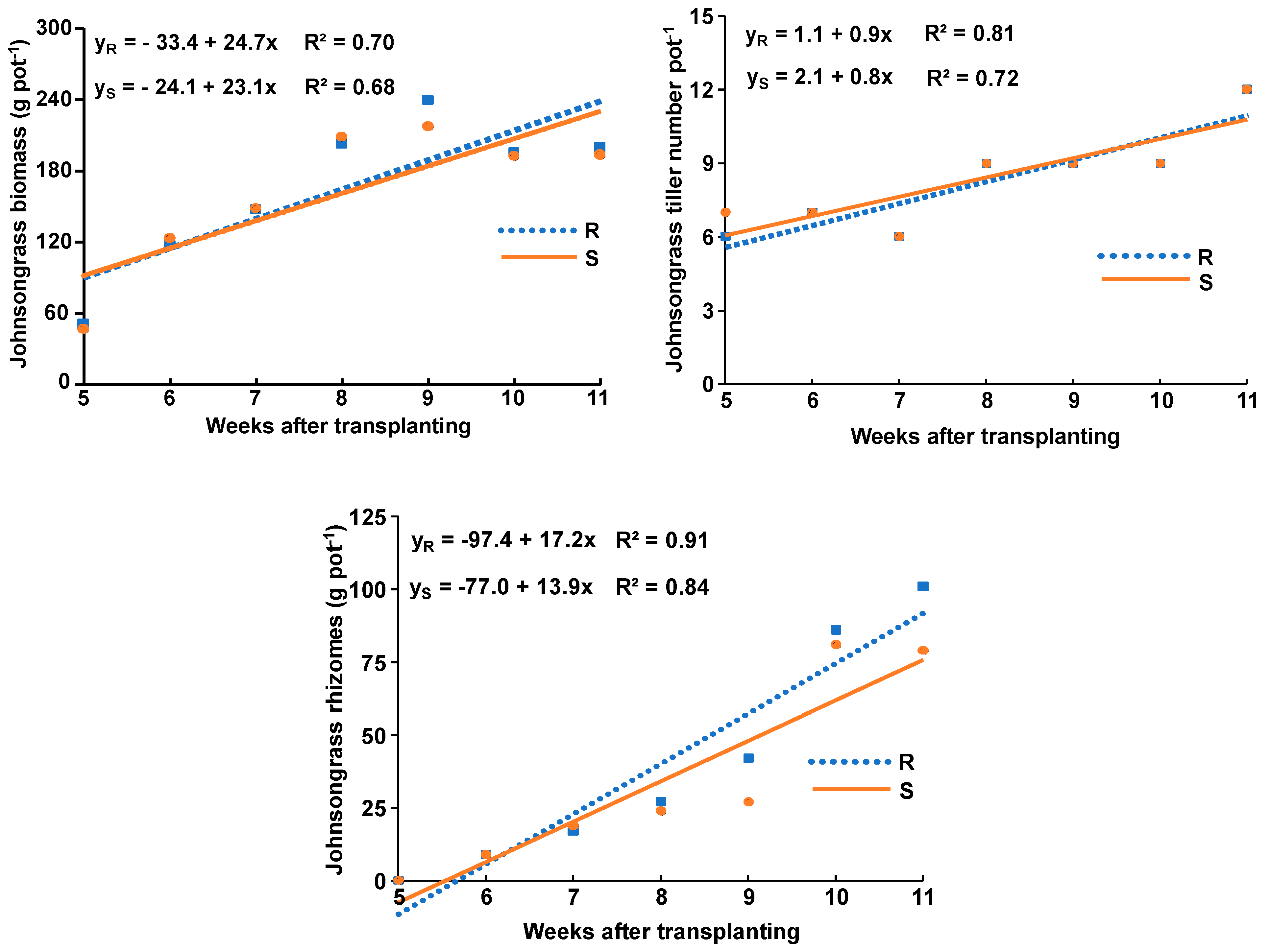
3.2. Growth Rate Results
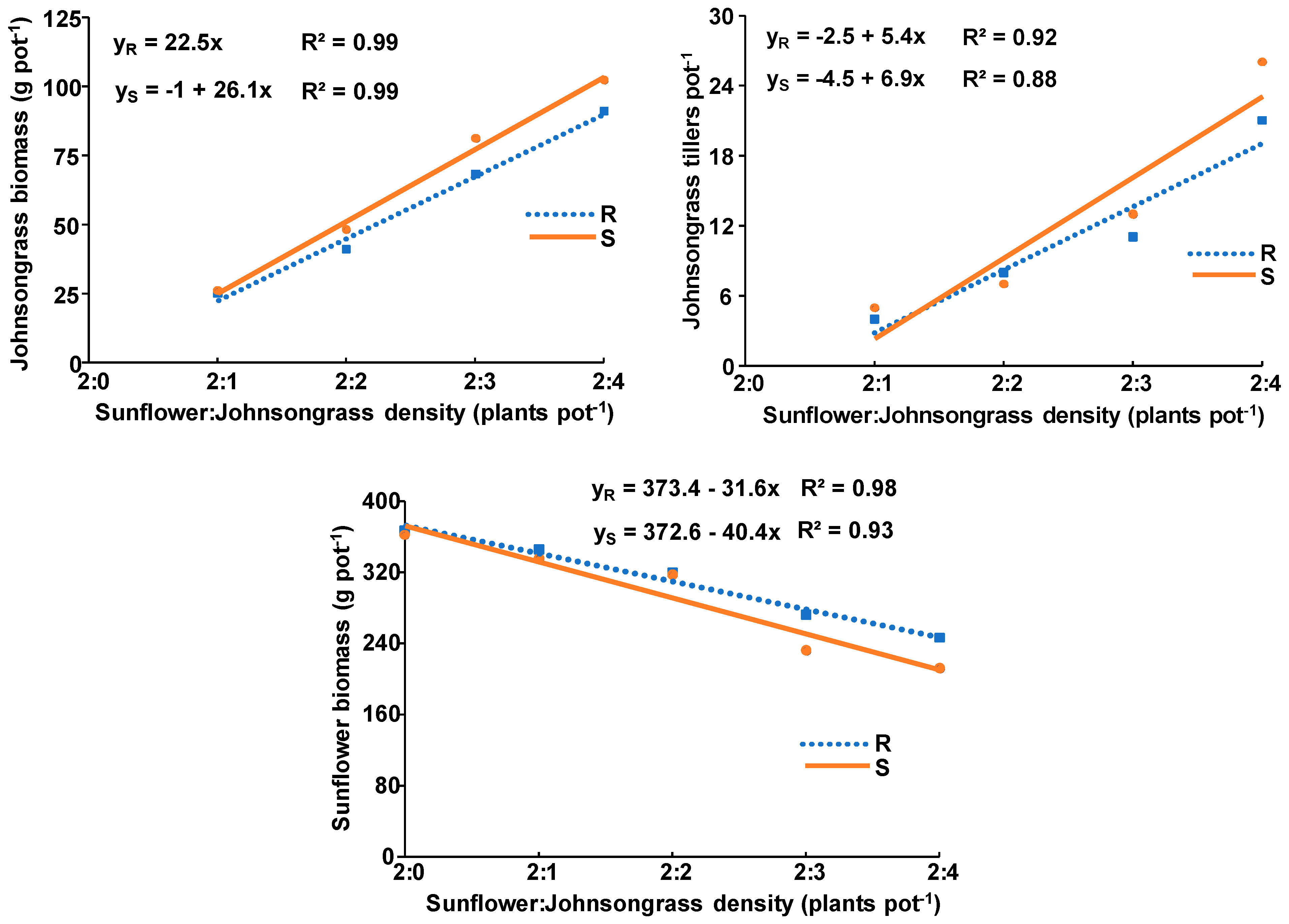
3.3. Johnsongrass Competition with Corn or Sunflower
4. Discussion
5. Conclusions
- (1)
- The S and ALS-cross R johnsongrass populations displayed similar growth rates concerning aboveground biomass and tiller number, whereas the R population displayed a slightly greater growth rate for rhizome production compared to the S population.
- (2)
- Both populations grown with corn produced more aboveground biomass than the ones grown with sunflowers.
- (3)
- The aboveground biomass of corn was reduced to a greater extent than sunflower by the presence of both johnsongrass populations, while both crops were affected more by the S than by the R population.
- (4)
- The growth rate and competitive ability of the ALS-resistant johnsongrass population are not associated with the resistance mechanism involved, suggesting that other factors are more important for the differentiation of johnsongrass growth traits.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warwick, S.L.; Black, L.D. The biology of Canadian weeds. 61. Sorghum halepense (L.) Pers. Can. J. Plant Sci. 1983, 63, 997–1014. [Google Scholar] [CrossRef]
- Fernandez, L.; de Haro, L.A.; Distefano, A.J.; Martinez, M.C.; Lia, V.; Papa, J.C.; Olea, L.; Tosto, D.; Hopp, H.E. Population genetics structure of glyphosate-resistant Johnsongrass (Sorghum halepense L. Pers) does not support a single origin of the resistance. Ecol. Evol. 2013, 3, 3388–3400. [Google Scholar] [CrossRef]
- Follak, S.; Essl, F. Spread dynamics and agricultural impact of Sorghum halepense, an emerging invasive species in Central Europe. Weed Res. 2013, 53, 53–60. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology, 2nd ed.; Krieger Publishing Company: Malabar, FL, USA, 1977; p. 610. [Google Scholar]
- Peerzada, A.M.; Ali, H.H.; Hanif, Z.; Bajwa, A.A.; Kebaso, L.; Frimpong, D.; Iqbal, N.; Namubiru, H.; Hashim, S.; Rasool, G.; et al. Eco-biology, impact, and management of Sorghum halepense (L.) Pers. Biol. Invasions 2017, 25, 955–973. [Google Scholar] [CrossRef]
- Riar, D.S.; Norsworthy, J.K.; Johnson, D.B.; Scott, R.C.; Bagavathiannan, M. 2011. Glyphosate resistance in a Johnsongrass (Sorghum halepense) biotype from Arkansas. Weed Sci. 2011, 59, 299–304. [Google Scholar] [CrossRef]
- Paterson, A.H.; Kong, W.Q.; Johnston, R.M.; Nabukalu, P.; Wu, G.; Poehlman, W.L.; Goff, V.H.; Isaacs, K.; Lee, T.-H.; Guo, H.; et al. The evolution of an invasive plant, Sorghum halepense L. (‘Johnsongrass’). Front Genet. 2020, 11, 317. [Google Scholar] [CrossRef]
- Atwater, D.Z.; Sezen, U.U.; Goff, V.; Goff, V.; Kong, W.; Paterson, A.H.; Barney, J.N. Reconstructing changes in the genotype, phenotype, and climatic niche of an introduced species. Ecography 2015, 39, 894–903. [Google Scholar] [CrossRef]
- Atwater, D.Z.; Fletcher, R.A.; Dickinson, C.C.; Paterson, A.H.; Barney, J.N. Evidence for fine-scale habitat specialisation in an invasive weed. J. Plant Ecol. 2018, 11, 189–199. [Google Scholar] [CrossRef]
- Smith, A.L.; Atwater, D.Z.; Kim, W.; Haak, D.C.; Barney, J.N. Invasive plant rhizome production and competitiveness vary based on neighbor identity. J. Plant Ecol. 2021, 14, 638–647. [Google Scholar] [CrossRef]
- Mitskas, B.M.; Tsolis, C.E.; Eleftherohorinos, I.G.; Damalas, C.A. Interference between corn and Johnsongrass (Sorghum halepense) from seed or rhizomes. Weed Sci. 2003, 51, 540–545. [Google Scholar] [CrossRef]
- Acciaresi, H.A.; Guiamet, J.J. Below- and above-ground growth and biomass allocation in maize and Sorghum halepense in response to soil water competition. Weed Res. 2010, 50, 481–492. [Google Scholar] [CrossRef]
- Karkanis, A.; Athanasiadou, D.; Giannoulis, K.; Karanasou, K.; Zografos, S.; Souipas, S.; Bartzialis, D.; Danalatos, N. Johnsongrass (Sorghum halepense (L.) Pers) interference, control and recovery under different management practices and its effects on the grain yield and quality of maize crop. Agronomy 2020, 10, 266. [Google Scholar] [CrossRef]
- Rout, M.E.; Chrzanowski, T.H. The invasive Sorghum halepense harbors endophytic N2-fixing bacteria and alters soil biogeochemistry. Plant Soil 2009, 315, 163–172. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Eleftherohorinos, I. Allelopathic potential of bermudagrass and Johnsongrass and their interference with cotton and corn. Agron. J. 2005, 97, 303–313. [Google Scholar] [CrossRef]
- Eleftherohorinos, I.G.; Kotoula-Syka, E. Influence of herbicide application rate and timings for post-emergence control of Sorghum halepense (L.) Pers. in maize. Weed Res. 1995, 35, 99–103. [Google Scholar] [CrossRef]
- Travlos, I.S.; Montuli, J.M.; Kukorelli, G.; Malidza, G.; Dogan, M.N.; Cheimona, N.; Antonopoulos, N.; Kanatas, P.J.; Zannopoulos, S.; Peteinatos, G. Key aspects on the biology, ecology and impacts of Johnsongrass [Sorghum halepense (L.) Pers] and the role of glyphosate and non-chemical alternative practices for the management of this weed in Europe. Agronomy 2019, 9, 717. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org (accessed on 3 January 2024).
- Panozzo, S.; Milani, A.; Scarabel, L.; Balogh, A.; Dancza, I.; Sattin, M. Occurrence of different resistance mechanisms to ALS inhibitors in European Sorghum halepenese. J. Agric. Food Chem. 2017, 65, 7320–7327. [Google Scholar] [CrossRef]
- Werle, R.; Begcy, K.; Yerka, M.K.; Mower, J.P.; Dweikat, I.; Jhala, A.J.; Lindquist, J.L. Independent evolution of acetolactate synthase-inhibiting herbicide resistance in weedy Sorghum populations across common geographic regions. Weed Sci. 2017, 65, 164–176. [Google Scholar] [CrossRef]
- Papapanagiotou, A.P.; Loukovitis, D.; Ntoanidou, S.; Eleftherohorinos, I.G. Target-site cross-resistance to ALS-inhibitors in Johnsongrass originating from Greek cornfields. Weed Technol. 2022, 36, 276–282. [Google Scholar] [CrossRef]
- Kaloumenos, N.S.; Eleftherohorinos, I.G. Identification of a Johnsongrass (Sorghum halepense) biotype resistant to ACCase-inhibiting herbicide in northern Greece. Weed Technol. 2009, 23, 470–476. [Google Scholar] [CrossRef]
- Scarabel, L.; Panozzo, S.; Savoia, W.; Sattin, M. Target-site ACCase-resistant Johnsongrass (Sorghum halepense) selected in summer dicot crops. Weed Technol. 2014, 28, 307–315. [Google Scholar] [CrossRef]
- Panozzo, S.; Sattin, M. Fitness costs associated to an Ile2041Asn mutation in the geophyte Sorghum halepense resistant to ACCase-inhibiting herbicides. Front Agron. 2021, 3, 711840. [Google Scholar] [CrossRef]
- Papapanagiotou, A.P.; Loukovitis, D.; Damalas, C.A.; Eleftherohorinos, I.G. Identification of an ACCase-resistant Johnsongrass (Sorghum halepense L.) population from a cotton field in northern Greece. Weed Biol. Manag. 2022, 22, 88–93. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Roux, F. A unified approach to the estimation and interpretation of resistance costs in plants. Heredity 2011, 107, 386–394. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Gundel, P.E.; Preston, C. Experimental methods for estimation of plant fitness costs associated with herbicide-resistance genes. Weed Sci. 2015, 63, 203–216. [Google Scholar] [CrossRef]
- Cousens, R.D.; Fournier-Level, A. Herbicide resistance costs: What are we actually measuring and why? Pest Manag. Sci. 2018, 74, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, E.; Abdolshahi, R.; Sasanfar, H.; Zand, E.; Beffa, R.; Dayan, F.E.; Kudsk, P. Assessing fitness costs from a herbicide-resistance management perspective: A review and insight. Weed Sci. 2019, 67, 137–148. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M. Fitness of herbicide-resistant weeds: Current knowledge and implications for management. Plants 2019, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Yanniccari, M.; Vila-Aiub, M.; Istilart, C.; Acciaresi, H.; Castro, A. Glyphosate resistance in perennial ryegrass (Lolium perenne L.) is associated with a fitness penalty. Weed Sci. 2016, 64, 71–79. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol. 2009, 184, 751–767. [Google Scholar] [CrossRef]
- Baucom, R. Evolutionary and ecological insights from herbicide-resistant weeds: What have we learned about plant adaptation and what is left to uncover? New Phytol. 2019, 223, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; p. 491. [Google Scholar]
- Ntoanidou, S.; Madesis, P.; Menexes, G.; Eleftherohorinos, I. Growth rate and genetic structure of Sinapis arvensis susceptible and herbicide resistant populations originating from Greece. Euphytica 2020, 216, 185. [Google Scholar] [CrossRef]
- Yu, Q.; Han, H.; Vila-Aiub, M.M.; Powles, S.B. AHAS herbicide resistance endowing mutations: Effect on AHAS functionality and plant growth. J. Exp. Bot. 2010, 61, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, R.B.; Pandolfo, C.E.; Cantamutto, M.; Presotto, A. Interference of feral radish (Raphanus sativus) resistant to AHAS-inhibiting herbicides with oilseed rape, wheat, and sunflower. Intern. J. Pest Manag. 2024, 70, 111–120. Available online: https://www.tandfonline.com/doi/abs/10.1080/09670874.2021.1959081 (accessed on 10 June 2024). [CrossRef]
- Zhao, N.; Yan, Y.; Du, L.; Zhang, X.; Liu, W.; Wang, J. Unravelling the effect of two herbicide resistance mutations on acetolactate synthase kinetics and growth traits. J. Exp. Bot. 2020, 71, 3535–3542. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Q.; Han, H.; Vila-Aiub, M.; Powles, S.B. ALS herbicide resistance mutations in Raphanus raphanistrum: Evaluation of pleiotropic effects on vegetative growth and ALS activity. Pest Manag. Sci. 2013, 69, 689–695. [Google Scholar] [CrossRef]
- Wang, R.; Han, Y.; Sun, Y.; Huang, H.; Wei, S.; Huang, Z. Growth and competitiveness of ALS-inhibiting herbicide-resistant Amaranthus retroflexus L. Plants 2022, 11, 2639. [Google Scholar] [CrossRef]
- Tseng, T.-M.; Shrestha, S.; McCurdy, J.D.; Wilson, E.; Sharma, G. Target-site mutation and fitness cost of acetolactate synthase inhibitor-resistant annual bluegrass. Hortscience 2019, 54, 701–705. [Google Scholar] [CrossRef]
- Vercellino, R.B.; Hernández, F.; Pandolfo, C.E.; Cantamutto, M.; Presotto, A. Ecological fitness cost associated with the AHAS Trp574Leu mutation in feral Raphanus sativus. Weed Res. 2021, 61, 210–220. [Google Scholar] [CrossRef]
- Leon, R.G.; Dunne, J.B.; Gould, F. The role of population and quantitative genetics and modern sequencing technologies to understand evolved herbicide resistance and weed fitness. Pest Manag. Sci. 2021, 77, 12–21. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapanagiotou, A.P.; Anthimidou, E.A.; Eleftherohorinos, I.G.; Giantsis, I.A. Comparison of Molecularly Identified Resistant and Susceptible Johnsongrass (Sorghum halepense L.) Populations at ALS Gene, in the Absence and Presence of Field Crops. Genes 2024, 15, 1415. https://doi.org/10.3390/genes15111415
Papapanagiotou AP, Anthimidou EA, Eleftherohorinos IG, Giantsis IA. Comparison of Molecularly Identified Resistant and Susceptible Johnsongrass (Sorghum halepense L.) Populations at ALS Gene, in the Absence and Presence of Field Crops. Genes. 2024; 15(11):1415. https://doi.org/10.3390/genes15111415
Chicago/Turabian StylePapapanagiotou, Aristeidis P., Eleni A. Anthimidou, Ilias G. Eleftherohorinos, and Ioannis A. Giantsis. 2024. "Comparison of Molecularly Identified Resistant and Susceptible Johnsongrass (Sorghum halepense L.) Populations at ALS Gene, in the Absence and Presence of Field Crops" Genes 15, no. 11: 1415. https://doi.org/10.3390/genes15111415
APA StylePapapanagiotou, A. P., Anthimidou, E. A., Eleftherohorinos, I. G., & Giantsis, I. A. (2024). Comparison of Molecularly Identified Resistant and Susceptible Johnsongrass (Sorghum halepense L.) Populations at ALS Gene, in the Absence and Presence of Field Crops. Genes, 15(11), 1415. https://doi.org/10.3390/genes15111415






