Metabolic and Epigenetic Mechanisms in Hepatoblastoma: Insights into Tumor Biology and Therapeutic Targets
Abstract
1. Introduction
2. Overview of Hepatoblastoma
2.1. Epidemiology and Incidence
2.2. Etiology and Risk Factors
2.3. Pathogenesis
2.4. Clinical and Histological Classification
2.5. Diagnosis
2.6. Staging
- PRETEXT I: One section involved,
- PRETEXT II: Two sections involved,
- PRETEXT III: Three sections involved, and
- PRETEXT IV: All four sections involved.
2.7. Prognosis and Survival
2.8. Current Treatment Strategies
2.9. Challenges and Future Directions
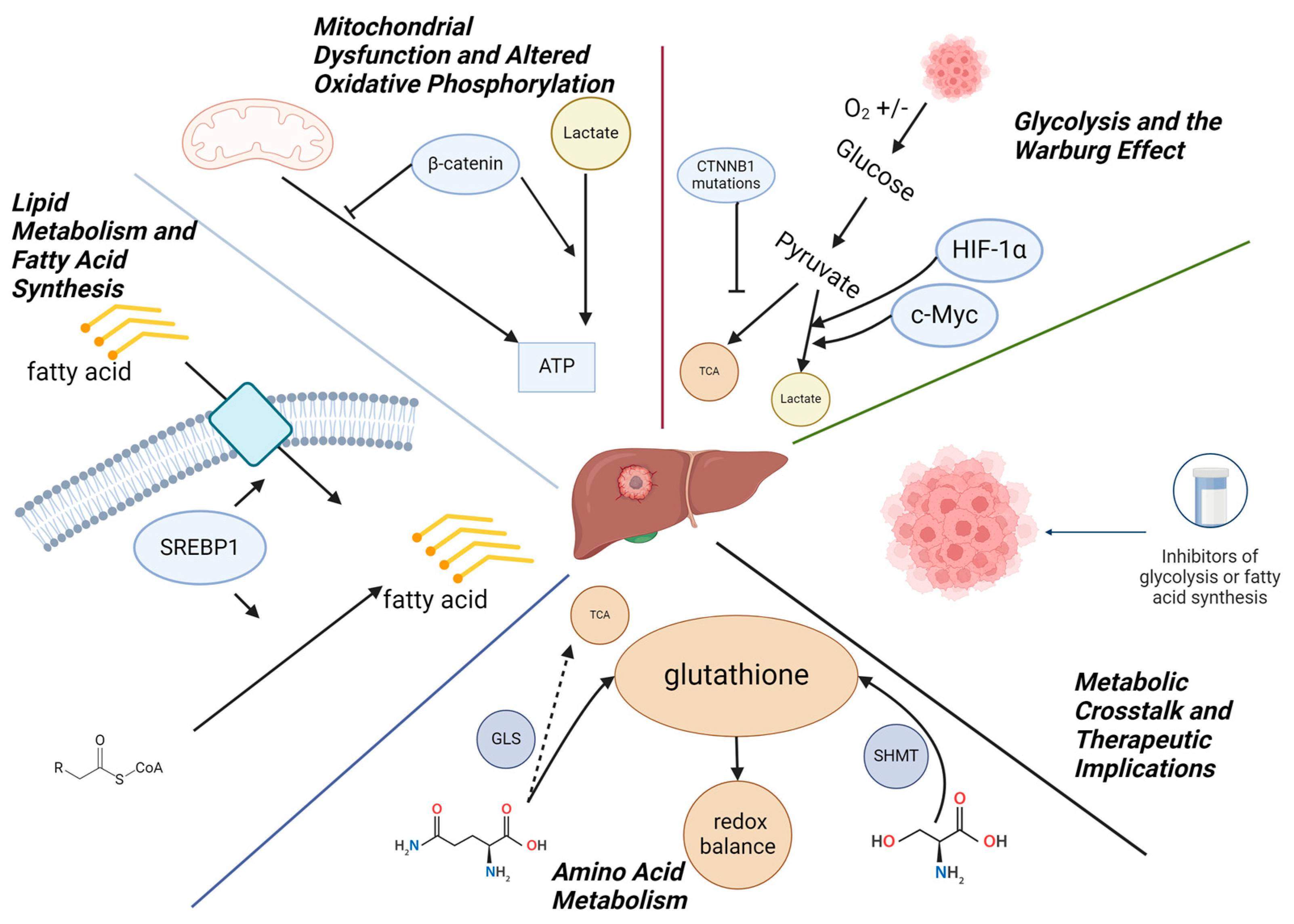
3. Metabolism in Hepatoblastoma
3.1. Glycolysis and the Warburg Effect
3.2. Mitochondrial Dysfunction and Altered Oxidative Phosphorylation
3.3. Lipid Metabolism and Fatty Acid Synthesis
3.4. Amino Acid Metabolism
3.5. Metabolic Crosstalk and Therapeutic Implications
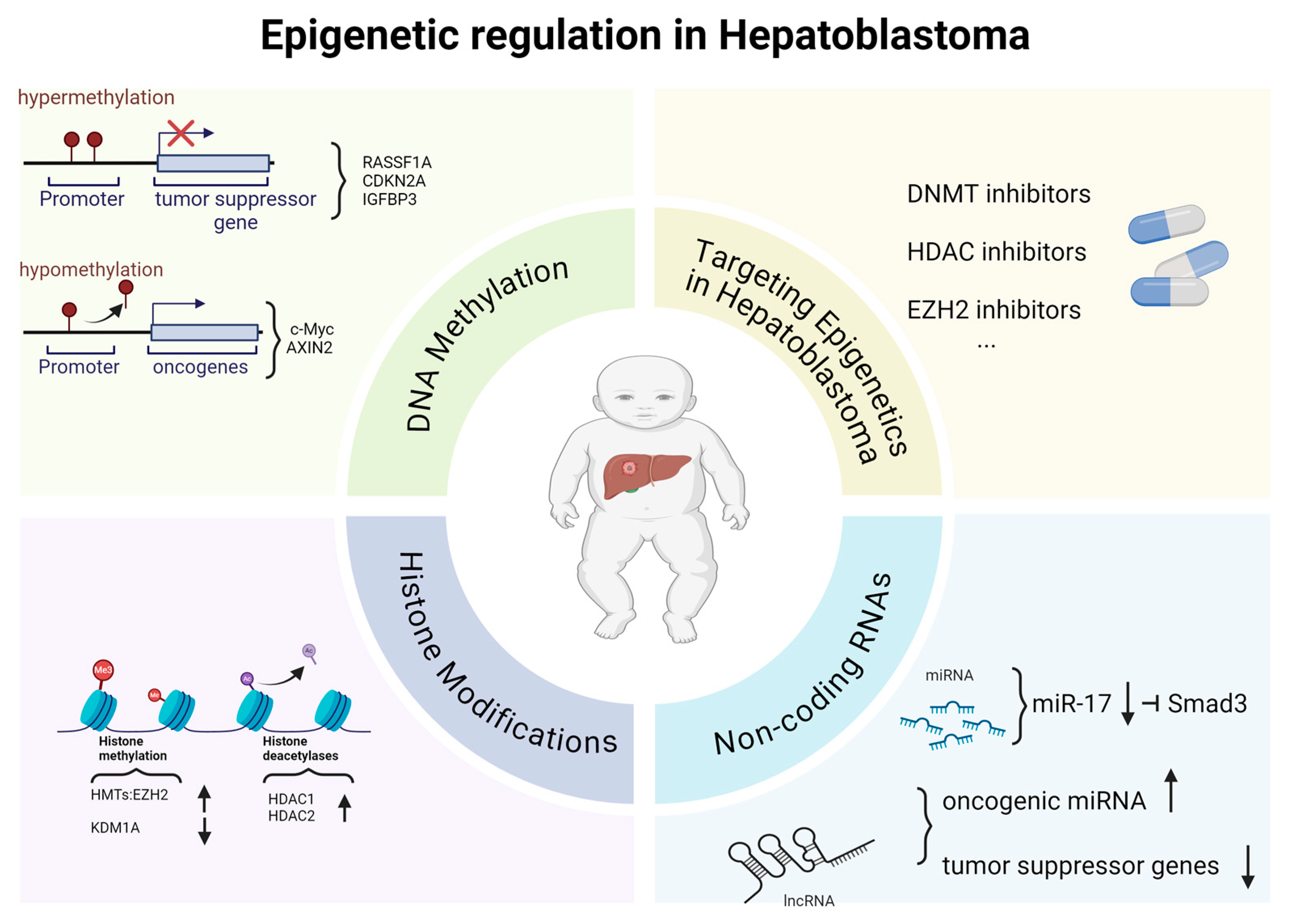
4. Epigenetic Regulation in Hepatoblastoma
4.1. DNA Methylation
4.2. Histone Modifications
4.3. Non-Coding RNAs (ncRNAs)
4.4. Therapeutic Implications of Targeting Epigenetics in Hepatoblastoma
5. Integration of Metabolism and Epigenetics in Hepatoblastoma
Metabolic Regulation of Epigenetic Modifications
6. Therapeutic Targets in Hepatoblastoma: Future Directions on Metabolic and Epigenetic Regulation
6.1. Metabolic Dysregulation in Hepatoblastoma
6.2. Epigenetic Dysregulation in Hepatoblastoma
6.3. Combination Therapies
6.4. Limitations and Future Directions: Clinical Outcomes and Ongoing Trials
| HB Biology | Drugs | Targets | Ref. |
|---|---|---|---|
| Metabolic Dysregulation in Hepatoblastoma | 2-deoxy-D-glucose | Glycolysis inhibitors | [73] |
| orlistat | FASN inhibitors | [75] | |
| TVB-2640 | Lipid synthesis | [76] | |
| Metformin | OXPHOS inhibitors | [77] | |
| Epigenetic Dysregulation in Hepatoblastoma | 5-azacytidine and decitabine | DNMTis | [79,80,81] |
| vorinostat and panobinostat | HDACis | [87] | |
| miRNA mimics or antagomiRs (anti-miRNA agents) | miRNAs | [63] | |
| Combination Therapies | / | Combination of glycolytic inhibitors with histone modifiers | [83] |
| metformin | Mitochondrial metabolism and epigenetic | [84] | |
| / | Combination FASN inhibitors with HDAC inhibitors | [85] |
7. Conclusions and Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spector, L.G.; Birch, J. The epidemiology of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Hager, J.; Sergi, C.M. Hepatoblastoma. In Liver Cancer [Internet]; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2021; Chapter 8. [Google Scholar] [PubMed]
- von Schweinitz, D. Hepatoblastoma: Recent developments in research and treatment. Semin. Pediatr. Surg. 2012, 21, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Feusner, J.; Plaschkes, J. Hepatoblastoma and low birth weight: A trend or chance observation? Med. Pediatr. Oncol. 2002, 39, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh-Lotrario, A.D.; López-Terrada, D.; Li, P.; Feusner, J.H. Hepatoblastoma in patients with molecularly proven familial adenomatous polyposis: Clinical characteristics and rationale for surveillance screening. Pediatr. Blood Cancer 2018, 65, e27103. [Google Scholar] [CrossRef] [PubMed]
- Sobel Naveh, N.S.; Traxler, E.M.; Duffy, K.A.; Kalish, J.M. Molecular networks of hepatoblastoma predisposition and oncogenesis in Beckwith-Wiedemann syndrome. Hepatol. Commun. 2022, 6, 2132–2146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, Z.H.; Lai, A.; Chen, C.K.; Chang, K.T.; Tan, A.M. Association of trisomy 18 with hepatoblastoma and its implications. Eur. J. Pediatr. 2014, 173, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Darcy, D.; Atwal, P.S.; Angell, C.; Gadi, I.; Wallerstein, R. Mosaic paternal genome-wide uniparental isodisomy with down syndrome. Am. J. Med. Genet. A 2015, 167A, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Sugiyama, M.; Terashita, Y.; Cho, Y.; Manabe, A. Hepatoblastoma with bone/bone marrow metastasis in Li-Fraumeni syndrome patient. Pediatr. Int. 2022, 64, e15135. [Google Scholar] [CrossRef] [PubMed]
- Fucic, A.; Guszak, V.; Mantovani, A. Transplacental exposure to environmental carcinogens: Association with childhood cancer risks and the role of modulating factors. Reprod. Toxicol. 2017, 72, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Ross, J.A. Smoking and hepatoblastoma: Confounding by birth weight? Br. J. Cancer 2003, 89, 602, Reply in Br. J. Cancer 2003, 89, 603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Johnson, K.J.; Williams, K.S.; Ross, J.A.; Krailo, M.D.; Tomlinson, G.E.; Malogolowkin, M.H.; Feusner, J.H.; Spector, L.G. Parental tobacco and alcohol use and risk of hepatoblastoma in offspring: A report from the children’s oncology group. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1837–1843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, D.; Subbarao, G.; Saxena, R. Hepatoblastoma. Semin. Diagn. Pathol. 2017, 34, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Solinas, A.; Cairo, S.; Evert, M.; Chen, X.; Calvisi, D.F. Molecular Mechanisms of Hepatoblastoma. Semin. Liver Dis. 2021, 41, 28–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sha, Y.L.; Liu, S.; Yan, W.W.; Dong, B. Wnt/β-catenin signaling as a useful therapeutic target in hepatoblastoma. Biosci. Rep. 2019, 39, BSR20192466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Purcell, R.; Childs, M.; Maibach, R.; Miles, C.; Turn, C.; Zimmermann, A.; Sullivan, M. HGF/c-Met related activation of β-catenin in hepatoblastoma. J. Exp. Clin. Cancer Res. 2011, 30, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, J.; Singh, S.; Cheng, C.; Natarajan, S.; Sheppard, H.; Abu-Zaid, A.; Durbin, A.D.; Lee, H.W.; Wu, Q.; Steele, J.; et al. Genome-wide mapping of cancer dependency genes and genetic modifiers of chemotherapy in high-risk hepatoblastoma. Nat. Commun. 2023, 14, 4003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abril-Fornaguera, J.; Torrens, L.; Andreu-Oller, C.; Carrillo-Reixach, J.; Rialdi, A.; Balaseviciute, U.; Pinyol, R.; Montironi, C.; Haber, P.K.; Del Río-Álvarez, Á.; et al. Identification of IGF2 as Genomic Driver and Actionable Therapeutic Target in Hepatoblastoma. Mol. Cancer Ther. 2023, 22, 485–498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumazin, P.; Chen, Y.; Treviño, L.R.; Sarabia, S.F.; Hampton, O.A.; Patel, K.; Mistretta, T.A.; Zorman, B.; Thompson, P.; Heczey, A.; et al. Genomic analysis of hepatoblastoma identifies distinct molecular and prognostic subgroups. Hepatology 2017, 65, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Cairo, S.; Armengol, C.; Maibach, R.; Häberle, B.; Becker, K.; Carrillo-Reixach, J.; Guettier, C.; Vokuhl, C.; Schmid, I.; Buendia, M.A.; et al. A combined clinical and biological risk classification improves prediction of outcome in hepatoblastoma patients. Eur. J. Cancer 2020, 141, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.U.; Kang, H.J. Recent updates on the classification of hepatoblastoma according to the International Pediatric Liver Tumors Consensus. J. Liver Cancer 2022, 22, 23–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marayati, R.; Julson, J.; Bownes, L.V.; Quinn, C.H.; Stafman, L.L.; Beierle, A.M.; Markert, H.R.; Hutchins, S.C.; Stewart, J.E.; Crossman, D.K.; et al. PIM3 kinase promotes tumor metastasis in hepatoblastoma by upregulating cell surface expression of chemokine receptor cxcr4. Clin. Exp. Metastasis 2022, 39, 899–912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanawa, M.; Hiyama, E.; Kawashima, K.; Hiyama, K.; Ikeda, K.; Morihara, N.; Kurihara, S.; Fukazawa, T.; Ueda, Y. Gene expression profiling in hepatoblastoma cases of the Japanese Study Group for Pediatric Liver Tumors-2 (JPLT-2) trial. Eur. J. Mol. Cancer 2018. [Google Scholar] [CrossRef]
- Baheti, A.D.; Chapman, T.; Rudzinski, E.; Albert, C.M.; Stanescu, A.L. Diagnosis, histopathologic correlation and management of hepatoblastoma: What the radiologist needs to know. Clin. Imaging 2018, 52, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, S.; Tang, H. An update on diagnosis and treatment of hepatoblastoma. Biosci. Trends 2024, 17, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.L.; Tiao, G.; de Ville de Goyet, J.; Superina, R.; Aronson, D.C. Hepatoblastoma state of the art: Pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr. Opin. Pediatr. 2014, 26, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.N.; Namgoong, J.M.; Yoon, H.M.; Cho, Y.A.; Choi, S.H.; Shin, J.; Kang, S.H.; Suh, J.K.; Kim, H.; Oh, S.H.; et al. Recent improvement in survival outcomes and reappraisal of prognostic factors in hepatoblastoma. Cancer Med. 2021, 10, 3261–3273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, H.M.; Hwang, J.; Kim, K.W.; Namgoong, J.M.; Kim, D.Y.; Koh, K.N.; Kim, H.; Cho, Y.A. Prognostic Factors for Event-Free Survival in Pediatric Patients with Hepatoblastoma Based on the 2017 PRETEXT and CHIC-HS Systems. Cancers 2019, 11, 1387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dembowska-Bagińska, B.; Więckowska, J.; Brożyna, A.; Święszkowska, E.; Ismail, H.; Broniszczak-Czyszek, D.; Stefanowicz, M.; Grajkowska, W.; Kaliciński, P. Health Status in Long-Term Survivors of Hepatoblastoma. Cancers 2019, 11, 1777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desterke, C.; Francés, R.; Monge, C.; Marchio, A.; Pineau, P.; Mata-Garrido, J. Combined Expression of DNMT3B and PFKFB4 in Hepatoblastoma Predicts Metastatic Outcome. Preprints 2024, 2024091115. [Google Scholar] [CrossRef]
- Monge, C.; Francés, R.; Marchio, A.; Pineau, P.; Desterke, C.; Mata-Garrido, J. Activated Metabolic Transcriptional Program in Tumor Cells from Hepatoblastoma. Preprints 2024, 2024090699. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, N.; Liu, C.; Li, J.; Bai, Y.; Lei, S.; Huang, Q.; Sun, L.; Tang, L.; Zeng, C.; et al. BEX1 supports the stemness of hepatoblastoma by facilitating Warburg effect in a PPARγ/PDK1 dependent manner. Br. J. Cancer 2023, 129, 1477–1489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Lu, J.; Chen, X.; Schwalbe, M.; Gorka, J.E.; Mandel, J.A.; Wang, J.; Goetzman, E.S.; Ranganathan, S.; Dobrowolski, S.F.; et al. Acquired deficiency of peroxisomal dicarboxylic acid catabolism is a metabolic vulnerability in hepatoblastoma. J. Biol. Chem. 2021, 296, 100283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cosson, M.A.; Touati, G.; Lacaille, F.; Valayannnopoulos, V.; Guyot, C.; Guest, G.; Verkarre, V.; Chrétien, D.; Rabier, D.; Munnich, A.; et al. Liver hepatoblastoma and multiple OXPHOS deficiency in the follow-up of a patient with methylmalonic aciduria. Mol. Genet. Metab. 2008, 95, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.E.; Kulkarni, S.; Wang, H.; Lu, J.; Dolezal, J.M.; Bharathi, S.S.; Ranganathan, S.; Patel, M.S.; Deshpande, R.; Alencastro, F.; et al. Genetic Dissociation of Glycolysis and the TCA Cycle Affects Neither Normal nor Neoplastic Proliferation. Cancer Res. 2017, 77, 5795–5807, Erratum in Cancer Res. 2022, 82, 944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fragoulis, A.; Schenkel, J.; Schröder, N.; Brandt, E.F.; Weiand, M.; Neu, T.; Ramadori, P.; Caspers, T.; Kant, S.; Pufe, T.; et al. Nrf2 induces malignant transformation of hepatic progenitor cells by inducing β-catenin expression. Redox Biol. 2022, 57, 102453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Zai, H.; Jiang, W.; Ou, Z.; Yao, Y.; Zhu, Q. The Mutual Inhibition of FoxO1 and SREBP-1c Regulated the Progression of Hepatoblastoma by Regulating Fatty Acid Metabolism. Mediators Inflamm. 2021, 2021, 5754592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishimoto, K.; Nakamura, H.; Tachibana, K.; Yamasaki, D.; Ota, A.; Hirano, K.I.; Tanaka, T.; Hamakubo, T.; Sakai, J.; Kodama, T.; et al. Sterol-mediated regulation of human lipin 1 gene expression in hepatoblastoma cells. J. Biol. Chem. 2009, 284, 22195–22205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jadeja, R.N.; Chu, X.; Wood, C.; Bartoli, M.; Khurana, S. M3 muscarinic receptor activation reduces hepatocyte lipid accumulation via CaMKKβ/AMPK pathway. Biochem. Pharmacol. 2019, 169, 113613. [Google Scholar] [CrossRef] [PubMed]
- Lockman, K.A.; Baren, J.P.; Pemberton, C.J.; Baghdadi, H.; Burgess, K.E.; Plevris-Papaioannou, N.; Lee, P.; Howie, F.; Beckett, G.; Pryde, A.; et al. Oxidative stress rather than triglyceride accumulation is a determinant of mitochondrial dysfunction in in vitro models of hepatic cellular steatosis. Liver Int. 2012, 32, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhen, N.; Gu, S.; Ma, J.; Zhu, J.; Yin, M.; Xu, M.; Wang, J.; Huang, N.; Cui, Z.; Bian, Z.; et al. CircHMGCS1 Promotes Hepatoblastoma Cell Proliferation by Regulating the IGF Signaling Pathway and Glutaminolysis. Theranostics 2019, 9, 900–919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López Grueso, M.J.; Tarradas Valero, R.M.; Carmona-Hidalgo, B.; Lagal Ruiz, D.J.; Peinado, J.; McDonagh, B.; Requejo Aguilar, R.; Bárcena Ruiz, J.A.; Padilla Peña, C.A. Peroxiredoxin 6 Down-Regulation Induces Metabolic Remodeling and Cell Cycle Arrest in HepG2 Cells. Antioxidants 2019, 8, 505. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Lemire, J.; Appanna, V.D. Hepatic response to aluminum toxicity: Dyslipidemia and liver diseases. Exp. Cell Res. 2011, 317, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Hattori, Y.; Tsukada, H.; Koga, K.; Kajiwara, E.; Kawano, K.; Kobayashi, T.; Kamata, K.; Maitani, Y. Adiponectin gene therapy of streptozotocin-induced diabetic mice using hydrodynamic injection. J. Gene Med. 2007, 9, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Naniwa, Y.; Nakamura, T.; Kato, H.; Yamamoto, M.; Tanabe, H.; Inoue, K.; Imaizumi, A. A novel acetyl-CoA carboxylase inhibitor reduces de novo fatty acid synthesis in HepG2 cells and rat primary hepatocytes. Arch. Biochem. Biophys. 2007, 468, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Recillas-Targa, F. Cancer Epigenetics: An Overview. Arch. Med. Res. 2022, 53, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, G.E.; Kappler, R. Genetics and epigenetics of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.R.; Zheng, W.; Gao, Q.; Chen, T.; Pan, Z.B.; Cui, W.; Cai, M.; Fang, H. Epigenetics and genetics of hepatoblastoma: Linkage and treatment. Front. Genet. 2022, 13, 1070971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rivas, M.; Aguiar, T.; Fernandes, G.; Lemes, R.; Caires-Júnior, L.; Goulart, E.; Telles-Silva, K.; Maschietto, M.; Cypriano, M.; de Toledo, S.; et al. DNA methylation as a key epigenetic player for hepatoblastoma characterization. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101684. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, B.; Zheng, S.; Dong, K.; Dong, R. Genome-wide analysis of DNA methylation in hepatoblastoma tissues. Oncol. Lett. 2016, 12, 1529–1534. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Honda, S.; Miyagi, H.; Suzuki, H.; Minato, M.; Haruta, M.; Kaneko, Y.; Hatanaka, K.C.; Hiyama, E.; Kamijo, T.; Okada, T.; et al. RASSF1A methylation indicates a poor prognosis in hepatoblastoma patients. Pediatr. Surg. Int. 2013, 29, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Toyooka, S.; Maitra, A.; Maruyama, R.; Toyooka, K.O.; Timmons, C.F.; Tomlinson, G.E.; Mastrangelo, D.; Hay, R.J.; Minna, J.D.; et al. Aberrant promoter methylation and silencing of the RASSF1A gene in pediatric tumors and cell lines. Oncogene 2002, 21, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Regel, I.; Eichenmüller, M.; Joppien, S.; Liebl, J.; Häberle, B.; Müller-Höcker, J.; Vollmar, A.; von Schweinitz, D.; Kappler, R. IGFBP3 impedes aggressive growth of pediatric liver cancer and is epigenetically silenced in vascular invasive and metastatic tumors. Mol. Cancer 2012, 11, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clavería-Cabello, A.; Herranz, J.M.; Latasa, M.U.; Arechederra, M.; Uriarte, I.; Pineda-Lucena, A.; Prosper, F.; Berraondo, P.; Alonso, C.; Sangro, B.; et al. Identification and experimental validation of druggable epigenetic targets in hepatoblastoma. J. Hepatol. 2023, 79, 989–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Yin, Q.; Luo, H. Development and validation of genomic and epigenomic signatures associated with tumor immune microenvironment in hepatoblastoma. BMC Cancer 2021, 21, 1156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glaser, K.; Schepers, E.J.; Zwolshen, H.M.; Lake, C.M.; Timchenko, N.A.; Karns, R.A.; Cairo, S.; Geller, J.I.; Tiao, G.M.; Bondoc, A.J. EZH2 is a key component of hepatoblastoma tumor cell growth. Pediatr. Blood Cancer 2024, 71, e30774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, C.; Chen, L.; Yuan, M.; Xie, W.; Sheng, X.; Yin, Q. KDM1A drives hepatoblastoma progression by activating the Wnt/β-catenin pathway through inhibition of DKK3 transcription. Tissue Cell 2023, 81, 101989. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Eberherr, C.; Hagemann, M.; Cairo, S.; Häberle, B.; Vokuhl, C.; von Schweinitz, D.; Kappler, R. Connectivity map identifies HDAC inhibition as a treatment option of high-risk hepatoblastoma. Cancer Biol Ther. 2016, 17, 1168–1176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rivas, M.; Johnston, M.E., II; Gulati, R.; Kumbaji, M.; Margues Aguiar, T.F.; Timchenko, L.; Krepischi, A.; Shin, S.; Bondoc, A.; Tiao, G.; et al. HDAC1-Dependent Repression of Markers of Hepatocytes and P21 Is Involved in Development of Pediatric Liver Cancer. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Alibeg, A.A.A.; Mohammed, M.H. Design, synthesis, insilco study and biological evaluation of new isatin-sulfonamide derivatives by using mono amide linker as possible as histone deacetylase inhibitors. Pol. Merkur. Lekarski. 2024, 52, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, I.; Sanz-Álvarez, M.; Luque, M.; Caramés, C.; Rojo, F.; García-Foncillas, J. The Role of MicroRNAs in Hepatoblastoma Tumors. Cancers 2019, 11, 409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magrelli, A.; Azzalin, G.; Salvatore, M.; Viganotti, M.; Tosto, F.; Colombo, T.; Devito, R.; Di Masi, A.; Antoccia, A.; Lorenzetti, S.; et al. Altered microRNA Expression Patterns in Hepatoblastoma Patients. Transl. Oncol. 2009, 2, 157–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ecevit, Ç.Ö.; Aktaş, S.; Tosun Yildirim, H.; Demirağ, B.; Erbay, A.; Karaca, İ.; Çelik, A.; Demir, A.B.; Erçetin, A.P.; Olgun, N. MicroRNA-17, MicroRNA-19b, MicroRNA-146a, MicroRNA-302d Expressions in Hepatoblastoma and Clinical Importance. J. Pediatr. Hematol. Oncol. 2019, 41, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, X.; Xu, Y.; Chen, M.; Chen, W.; Chen, T.; Tang, Q.; He, Z. microRNA-17 functions as an oncogene by downregulating Smad3 expression in hepatocellular carcinoma. Cell Death Dis. 2019, 10, 723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, R.; Liu, X.Q.; Zhang, B.B.; Liu, B.H.; Zheng, S.; Dong, K.R. Long non-coding RNA-CRNDE: A novel regulator of tumor growth and angiogenesis in hepatoblastoma. Oncotarget 2017, 8, 42087–42097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.J.; Yuan, M.X.; Ji, C.Y.; Zhang, Y.B.; Peng, Y.M.; Zhang, T.; Gao, H.Q.; Sheng, X.Y.; Liu, Z.Y.; Xie, W.X.; et al. Long Non-Coding RNA CRNDE Regulates Angiogenesis in Hepatoblastoma by Targeting the MiR-203/VEGFA Axis. Pathobiology 2020, 87, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, F.; Yang, F.; Liu, Y. Kockdown of OIP5-AS1 expression inhibits proliferation, metastasis and EMT progress in hepatoblastoma cells through up-regulating miR-186a-5p and down-regulating ZEB1. Biomed. Pharmacother. 2018, 101, 14–23. [Google Scholar] [CrossRef]
- Hermes, M.; Geisler, H.; Osswald, H.; Riehle, R.; Kloor, D. Alterations in S-adenosylhomocysteine metabolism decrease O6-methylguanine DNA methyltransferase gene expression without affecting promoter methylation. Biochem. Pharmacol. 2008, 75, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCabe, M.T.; Mohammad, H.P.; Barbash, O.; Kruger, R.G. Targeting Histone Methylation in Cancer. Cancer J. 2017, 23, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 2009, 19, 17–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.; et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 2015, 21, 65–80, Erratum in Cell Metab. 2015, 21, 347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranftler, C.; Meisslitzer-Ruppitsch, C.; Neumüller, J.; Ellinger, A.; Pavelka, M. Golgi apparatus dis- and reorganizations studied with the aid of 2-deoxy-D-glucose and visualized by 3D-electron tomography. Histochem. Cell Biol. 2017, 147, 415–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szczepiorkowski, Z.M.; Dickersin, G.R.; Laposata, M. Fatty acid ethyl esters decrease human hepatoblastoma cell proliferation and protein synthesis. Gastroenterology 1995, 108, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Syed-Abdul, M.M.; Parks, E.J.; Gaballah, A.H.; Bingham, K.; Hammoud, G.M.; Kemble, G.; Buckley, D.; McCulloch, W.; Manrique-Acevedo, C. Fatty Acid Synthase Inhibitor TVB-2640 Reduces Hepatic de Novo Lipogenesis in Males with Metabolic Abnormalities. Hepatology 2020, 72, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tsang, W.Y.; Fang, X.N.; Zhang, Y.; Luo, J.; Gong, L.Q.; Zhang, B.F.; Wong, C.N.; Li, Z.H.; Liu, B.L.; et al. FASN Inhibition Decreases MHC-I Degradation and Synergizes with PD-L1 Checkpoint Blockade in Hepatocellular Carcinoma. Cancer Res. 2024, 84, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; Giordani, L.; Moretti, A.; Basso, G.; Borriello, A.; Della Ragione, F. Analysis of CDKN2A, CDKN2B, CDKN2C, and cyclin Ds gene status in hepatoblastoma. Hepatology 1998, 27, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Christman, J.K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Dong, L.; Chang, Y.; Zhang, X.; Wang, C.; Chen, M.; Bo, X.; Chen, H.; Han, W.; et al. Decitabine priming increases anti-PD-1 antitumor efficacy by promoting CD8+ progenitor exhausted T cell expansion in tumor models. J. Clin. Investig. 2023, 133, e165673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, L.M.; Zhang, J.H. Histone Deacetylase Inhibitors in Tumor Immunotherapy. Curr. Med. Chem. 2019, 26, 2990–3008. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Si, W.; Xia, L.; Yin, D.; Wei, T.; Tao, M.; Cui, X.; Yang, J.; Hong, T.; Wei, R. Positive feedback regulation between glycolysis and histone lactylation drives oncogenesis in pancreatic ductal adenocarcinoma. Mol. Cancer 2024, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its potential anti-cancer and anti-aging effects. Adv. Hyg. Exp. Med. 2017, 71, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.D.; Scopigno, T.; Pediconi, N.; Levrero, M.; Hagman, H.; Kiskis, J.; Enejder, A. The histone deacetylase inhibiting drug Entinostat induces lipid accumulation in differentiated HepaRG cells. Sci. Rep. 2016, 6, 28025, Erratum in Sci. Rep. 2016, 6, 37204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, T.C.S.; Chan, A.H.Y.; Ganesan, A. Thirty Years of HDAC Inhibitors: 2020 Insight and Hindsight. J. Med. Chem. 2020, 63, 12460–12484. [Google Scholar] [CrossRef] [PubMed]
- Afifi, S.; Michael, A.; Azimi, M.; Rodriguez, M.; Lendvai, N.; Landgren, O. Role of Histone Deacetylase Inhibitors in Relapsed Refractory Multiple Myeloma: A Focus on Vorinostat and Panobinostat. Pharmacotherapy 2015, 35, 1173–1188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Factors | In Details | Reference |
|---|---|---|
| Prematurity and Low Birth Weight | Children born preterm or with a birth weight under 1500 g | [4] |
| Familial Adenomatous Polyposis | Mutations in the APC gene | [5] |
| Beckwith–Wiedemann Syndrome | This overgrowth disorder is frequently linked to the abnormal regulation of the imprinted IGF2 gene. | [6] |
| Trisomy 18 and Trisomy 21 | Children with these chromosomal abnormalities have an increased predisposition to liver tumors | [7,8] |
| Li–Fraumeni Syndrome | TP53 mutations | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Francés, R.; Monge, C.; Desterke, C.; Marchio, A.; Pineau, P.; Chang-Marchand, Y.; Mata-Garrido, J. Metabolic and Epigenetic Mechanisms in Hepatoblastoma: Insights into Tumor Biology and Therapeutic Targets. Genes 2024, 15, 1358. https://doi.org/10.3390/genes15111358
Fu Y, Francés R, Monge C, Desterke C, Marchio A, Pineau P, Chang-Marchand Y, Mata-Garrido J. Metabolic and Epigenetic Mechanisms in Hepatoblastoma: Insights into Tumor Biology and Therapeutic Targets. Genes. 2024; 15(11):1358. https://doi.org/10.3390/genes15111358
Chicago/Turabian StyleFu, Yuanji, Raquel Francés, Claudia Monge, Christophe Desterke, Agnès Marchio, Pascal Pineau, Yunhua Chang-Marchand, and Jorge Mata-Garrido. 2024. "Metabolic and Epigenetic Mechanisms in Hepatoblastoma: Insights into Tumor Biology and Therapeutic Targets" Genes 15, no. 11: 1358. https://doi.org/10.3390/genes15111358
APA StyleFu, Y., Francés, R., Monge, C., Desterke, C., Marchio, A., Pineau, P., Chang-Marchand, Y., & Mata-Garrido, J. (2024). Metabolic and Epigenetic Mechanisms in Hepatoblastoma: Insights into Tumor Biology and Therapeutic Targets. Genes, 15(11), 1358. https://doi.org/10.3390/genes15111358







