Association Mapping of Candidate Genes Associated with Iron and Zinc Content in Rice (Oryza sativa L.) Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Field Experiment Details
2.2. Fe and Zn Content Evaluation
2.3. Genotypic Data Acquisition and Analysis
2.4. Evaluation of Linkage Disequilibrium Decay
2.5. Fe and Zn Content Data Analysis
2.6. Genome-Wide Association Analysis
2.7. Candidate Genes Identification and Gene Expression Analysis
3. Results
3.1. Fe and Zn Content Variations
3.2. Genetic Markers Distribution and Linkage Disequilibrium Analysis
3.3. Marker-Trait Associations for Fe and Zn
3.4. Candidate Genes for Fe and Zn Content and Their Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2022: Summary of Results; UN DESA/POP/2022/TR/NO. 3; United Nations Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022. [Google Scholar]
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture: Trends and Challenges; FAO: Roma, Italy, 2017. [Google Scholar]
- Elmighrabi, N.F.; Fleming, C.A.K.; Dhami, M.V.; Agho, K.E. Childhood Undernutrition in North Africa: Systematic Review and Meta-Analysis of Observational Studies. Glob. Health Action 2023, 16, 2240158. [Google Scholar] [CrossRef]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc Deficiency in Low- and Middle-income Countries: Prevalence and Approaches for Mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D. Iron Availability and Management Considerations: A 4R Approach. Crops Soils 2020, 53, 32–37. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- World Health Organization. Global Nutrition Targets 2025: Anaemia Policy Brief; World Health Organization: Geneva, Switzerland, 2014.
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and Its Importance for Human Health: An Integrative Review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Mohidem, N.A.; Hashim, N.; Shamsudin, R.; Che Man, H. Rice for Food Security: Revisiting Its Production, Diversity, Rice Milling Process and Nutrient Content. Agriculture 2022, 12, 741. [Google Scholar] [CrossRef]
- Sweeney, M.; McCouch, S. The Complex History of the Domestication of Rice. Ann. Bot. 2007, 100, 951–957. [Google Scholar] [CrossRef]
- Jauhar, A.; Wani, S.H. Rice Improvement: Physiological, Molecular Breeding and Genetic Perspectives; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Bollinedi, H.; Yadav, A.K.; Vinod, K.K.; Gopala Krishnan, S.; Bhowmick, P.K.; Nagarajan, M.; Neeraja, C.N.; Ellur, R.K.; Singh, A.K. Genome-Wide Association Study Reveals Novel Marker-Trait Associations (MTAs) Governing the Localization of Fe and Zn in the Rice Grain. Front. Genet. 2020, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Ibeanu, V.N.; Edeh, C.G.; Ani, P.N. Evidence-Based Strategy for Prevention of Hidden Hunger among Adolescents in a Suburb of Nigeria. BMC Public Health 2020, 20, 1683. [Google Scholar] [CrossRef]
- Philipo, M.; Ndakidemi, P.A.; Mbega, E.R. Environmental and Genotypes Influence on Seed Iron and Zinc Levels of Landraces and Improved Varieties of Common Bean (Phaseolus vulgaris L.) in Tanzania. Ecol. Genet. Genomics 2020, 15, 100056. [Google Scholar] [CrossRef]
- Bouis, H.E.; Graham, R.D.; Welch, R.M. The Consultative Group on International Agricultural Research (CGIAR) Micronutrients Project: Justification and Objectives. Food Nutr. Bull. 2000, 21, 374–381. [Google Scholar] [CrossRef]
- Moumin, N.A.; Angel, M.D.; Karakochuk, C.D.; Michaux, K.D.; Moursi, M.; Sawadogo, K.A.A.; Foley, J.; Hawes, M.D.; Whitfield, K.C.; Tugirimana, P.L.; et al. Micronutrient Intake and Prevalence of Micronutrient Inadequacy among Women (15-49 y) and Children (6-59 Mo) in South Kivu and Kongo Central, Democratic Republic of the Congo (DRC). PLoS ONE 2020, 15, e0223393. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; World Food Programme; International Fund for Agricultural Development. Food Loss Analysis: Causes and Solutions—Case Studies on Maize and Rice in the Democratic Republic of Congo; FAO: Rome, Italy, 2019. [Google Scholar]
- Bouis, H.E.; Welch, R.M. Biofortification-A Sustainable Agricultural Strategy for Reducing Micronutrient Malnutrition in the Global South. Crop. Sci. 2010, 50, S-20–S-32. [Google Scholar] [CrossRef]
- Tsednee, M.; Mak, Y.; Chen, Y.; Yeh, K. A Sensitive LC-ESI-Q-TOF-MS Method Reveals Novel Phytosiderophores and Phytosiderophore–Iron Complexes in Barley. New Phytol. 2012, 195, 951–961. [Google Scholar] [CrossRef]
- Gao, L.; Xiong, J. Improving Rice Grain Quality by Enhancing Accumulation of Iron and Zinc While Minimizing Cadmium and Lead. In Rice Crop—Current Developments; Shah, F., Khan, Z.H., Iqbal, A., Eds.; InTech: London, UK, 2018. [Google Scholar] [CrossRef][Green Version]
- Guerinot, M.L. The ZIP Family of Metal Transporters. Biochim. Biophys. Acta BBA Biomembr. 2000, 1465, 190–198. [Google Scholar] [CrossRef]
- Bashir, K.; Rasheed, S.; Kobayashi, T.; Seki, M.; Nishizawa, N.K. Regulating Subcellular Metal Homeostasis: The Key to Crop Improvement. Front. Plant Sci. 2016, 7, 1192. [Google Scholar] [CrossRef]
- Brachi, B.; Morris, G.P.; Borevitz, J.O. Genome-Wide Association Studies in Plants: The Missing Heritability Is in the Field. Genome Biol. 2011, 12, 232. [Google Scholar] [CrossRef]
- Islam, A.S.M.F.; Mustahsan, W.; Tabien, R.; Awika, J.M.; Septiningsih, E.M.; Thomson, M.J. Identifying the Genetic Basis of Mineral Elements in Rice Grain Using Genome-Wide Association Mapping. Genes 2022, 13, 2330. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z.; Kakar, K.U.; Li, X.; Li, S.; Zhang, B.; Shou, H.; Shu, Q. Genome-Wide Association Mapping of Quantitative Trait Loci (QTLs) for Contents of Eight Elements in Brown Rice (Oryza sativa L.). J. Agric. Food Chem. 2015, 63, 8008–8016. [Google Scholar] [CrossRef]
- Rathan, N.D.; Krishna, H.; Ellur, R.K.; Sehgal, D.; Govindan, V.; Ahlawat, A.K.; Krishnappa, G.; Jaiswal, J.P.; Singh, J.B.; Sv, S.; et al. Genome-Wide Association Study Identifies Loci and Candidate Genes for Grain Micronutrients and Quality Traits in Wheat (Triticum aestivum L.). Sci. Rep. 2022, 12, 7037. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Cheema, J.; Poland, J.; Uauy, C.; Chhuneja, P. Genome-Wide Association Mapping of Grain Micronutrients Concentration in Aegilops Tauschii. Front. Plant Sci. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Delfini, J.; Moda-Cirino, V.; Dos Santos Neto, J.; Zeffa, D.M.; Nogueira, A.F.; Ribeiro, L.A.B.; Ruas, P.M.; Gepts, P.; Gonçalves, L.S.A. Genome-Wide Association Study for Grain Mineral Content in a Brazilian Common Bean Diversity Panel. Theor. Appl. Genet. 2021, 134, 2795–2811. [Google Scholar] [CrossRef]
- Hou, X.; Amais, R.S.; Jones, B.T.; Donati, G.L. Inductively Coupled Plasma Optical Emission Spectrometry. Encycl. Anal. Chem. 2000, 2000, 9468–9485. [Google Scholar]
- Kimwemwe, P.K.; Bukomarhe, C.B.; Mamati, E.; Githiri, S.M.; Mushizi, R.C.; Mignouna, J.; Kimani, W.; Fofana, M. Population Structure and Genetic Diversity of Rice (Oryza sativa L.) Germplasm from the Democratic Republic of Congo (DRC) Using DArTseq-Derived Single Nucleotide Polymorphism (SNP). Agronomy 2023, 16, 1906. [Google Scholar] [CrossRef]
- Kilian, A.; Wenzl, P.; Huttner, E.; Carling, J.; Xia, L.; Blois, H.; Caig, V.; Heller-Uszynska, K.; Jaccoud, D.; Hopper, C.; et al. Diversity Arrays Technology: A Generic Genome Profiling Technology on Open Platforms. In Data Production and Analysis in Population Genomics; Pompanon, F., Bonin, A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 888, pp. 67–89. [Google Scholar] [CrossRef]
- Kawahara, Y.; De La Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza Sativa Nipponbare Reference Genome Using next Generation Sequence and Optical Map Data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 5 May 2023).
- Mangiafico, S.S. Summary and Analysis of Extension Program Evaluation in R, Version 1.15. 0. Rcompanion. org/Handbook. Available online: https://rcompanion.org/documents/RHandbookProgramEvaluation.pdf (accessed on 12 May 2023).
- Komsta, L.; Novomestky, F. Moments, Cumulants, Skewness, Kurtosis and Related Tests. 2015. Available online: https://www.r-project.org; http://www.komsta.net/ (accessed on 12 May 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. RMVP: A Memory-Efficient, Visualization-Enhanced, and Parallel-Accelerated Tool for Genome-Wide Association Study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef]
- VanRaden, P.M. Efficient Methods to Compute Genomic Predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef]
- Xia, L.; Zou, D.; Sang, J.; Xu, X.; Yin, H.; Li, M.; Wu, S.; Hu, S.; Hao, L.; Zhang, Z. Rice Expression Database (RED): An Integrated RNA-Seq-Derived Gene Expression Database for Rice. J. Genet. Genom. Yi Chuan Xue Bao 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Davidson, R.M.; Gowda, M.; Moghe, G.; Lin, H.; Vaillancourt, B.; Shiu, S.-H.; Jiang, N.; Robin Buell, C. Comparative Transcriptomics of Three Poaceae Species Reveals Patterns of Gene Expression Evolution: Comparative Transcriptome Analyses in Grasses. Plant J. 2012, 71, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; De Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-Wide Association Studies. Nat. Rev. Methods Primer 2021, 1, 59. [Google Scholar] [CrossRef]
- Swamy, B.P.M.; Descalsota, G.I.L.; Nha, C.T.; Amparado, A.; Inabangan-Asilo, M.A.; Manito, C.; Tesoro, F.; Reinke, R. Identification of Genomic Regions Associated with Agronomic and Biofortification Traits in DH Populations of Rice. PLoS ONE 2018, 13, e0201756. [Google Scholar] [CrossRef]
- Rakotondramanana, M.; Tanaka, R.; Pariasca-Tanaka, J.; Stangoulis, J.; Grenier, C.; Wissuwa, M. Genomic Prediction of Zinc-Biofortification Potential in Rice Gene Bank Accessions. Theor. Appl. Genet. 2022, 135, 2265–2278. [Google Scholar] [CrossRef] [PubMed]
- Descalsota, G.I.L.; Swamy, B.P.M.; Zaw, H.; Inabangan-Asilo, M.A.; Amparado, A.; Mauleon, R.; Chadha-Mohanty, P.; Arocena, E.C.; Raghavan, C.; Leung, H.; et al. Genome-Wide Association Mapping in a Rice MAGIC Plus Population Detects QTLs and Genes Useful for Biofortification. Front. Plant Sci. 2018, 9, 1347. [Google Scholar] [CrossRef]
- Bhandari, H.; Bhanu, A.N.; Srivastava, K.; Singh, M.; Shreya; Hemantaranjan, A. Assessment of Genetic Diversity in Crop Plants—An Overview. Adv. Plants Agric. Res. 2017, 7, 279–286. [Google Scholar] [CrossRef]
- Hindu, V.; Palacios-Rojas, N.; Babu, R.; Suwarno, W.B.; Rashid, Z.; Usha, R.; Saykhedkar, G.R.; Nair, S.K. Identification and Validation of Genomic Regions Influencing Kernel Zinc and Iron in Maize. Theor. Appl. Genet. 2018, 131, 1443–1457. [Google Scholar] [CrossRef]
- Mogga, M.; Sibiya, J.; Shimelis, H.; Lamo, J.; Yao, N. Diversity Analysis and Genome-Wide Association Studies of Grain Shape and Eating Quality Traits in Rice (Oryza sativa L.) Using DArT Markers. PLoS ONE 2018, 13, e0198012. [Google Scholar] [CrossRef]
- Krishnappa, G.; Khan, H.; Krishna, H.; Kumar, S.; Mishra, C.N.; Parkash, O.; Devate, N.B.; Nepolean, T.; Rathan, N.D.; Mamrutha, H.M.; et al. Genetic Dissection of Grain Iron and Zinc, and Thousand Kernel Weight in Wheat (Triticum aestivum L.) Using Genome-Wide Association Study. Sci. Rep. 2022, 12, 12444. [Google Scholar] [CrossRef]
- Mather, K.A.; Caicedo, A.L.; Polato, N.R.; Olsen, K.M.; McCouch, S.; Purugganan, M.D. The Extent of Linkage Disequilibrium in Rice (Oryza sativa L.). Genetics 2007, 177, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhan, J.; Li, J.; Lu, X.; Liu, J.; Wang, Y.; Zhao, Q.; Ye, G. Genome-Wide Association Study (GWAS) for Mesocotyl Elongation in Rice (Oryza sativa L.) under Multiple Culture Conditions. Genes 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, M.; Niu, X.; Wang, S.; Xu, Q.; Feng, Y.; Wang, C.; Deng, H.; Yuan, X.; Yu, H.; et al. Genetic Variation and Association Mapping for 12 Agronomic Traits in Indica Rice. BMC Genom. 2015, 16, 1067. [Google Scholar] [CrossRef]
- Zhang, Z.; Ersoz, E.; Lai, C.-Q.; Todhunter, R.J.; Tiwari, H.K.; Gore, M.A.; Bradbury, P.J.; Yu, J.; Arnett, D.K.; Ordovas, J.M.; et al. Mixed Linear Model Approach Adapted for Genome-Wide Association Studies. Nat. Genet. 2010, 42, 355–360. [Google Scholar] [CrossRef]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.S.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef]
- Kaler, A.S.; Gillman, J.D.; Beissinger, T.; Purcell, L.C. Comparing Different Statistical Models and Multiple Testing Corrections for Association Mapping in Soybean and Maize. Front. Plant Sci. 2020, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Swamy, B.P.M.; Marathi, B.; Ribeiro-Barros, A.I.F.; Calayugan, M.I.C.; Ricachenevsky, F.K. Iron Biofortification in Rice: An Update on Quantitative Trait Loci and Candidate Genes. Front. Plant Sci. 2021, 12, 647341. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Sun, L.; Tan, L. Progress in ZIP Transporter Gene Family in Rice. Yi Chuan Hered. 2018, 40, 33–43. [Google Scholar] [CrossRef]
- Ajeesh Krishna, T.P.; Maharajan, T.; Victor Roch, G.; Ignacimuthu, S.; Antony Ceasar, S. Structure, Function, Regulation and Phylogenetic Relationship of ZIP Family Transporters of Plants. Front. Plant Sci. 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple Functional Roles of Anthocyanins in Plant-Environment Interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
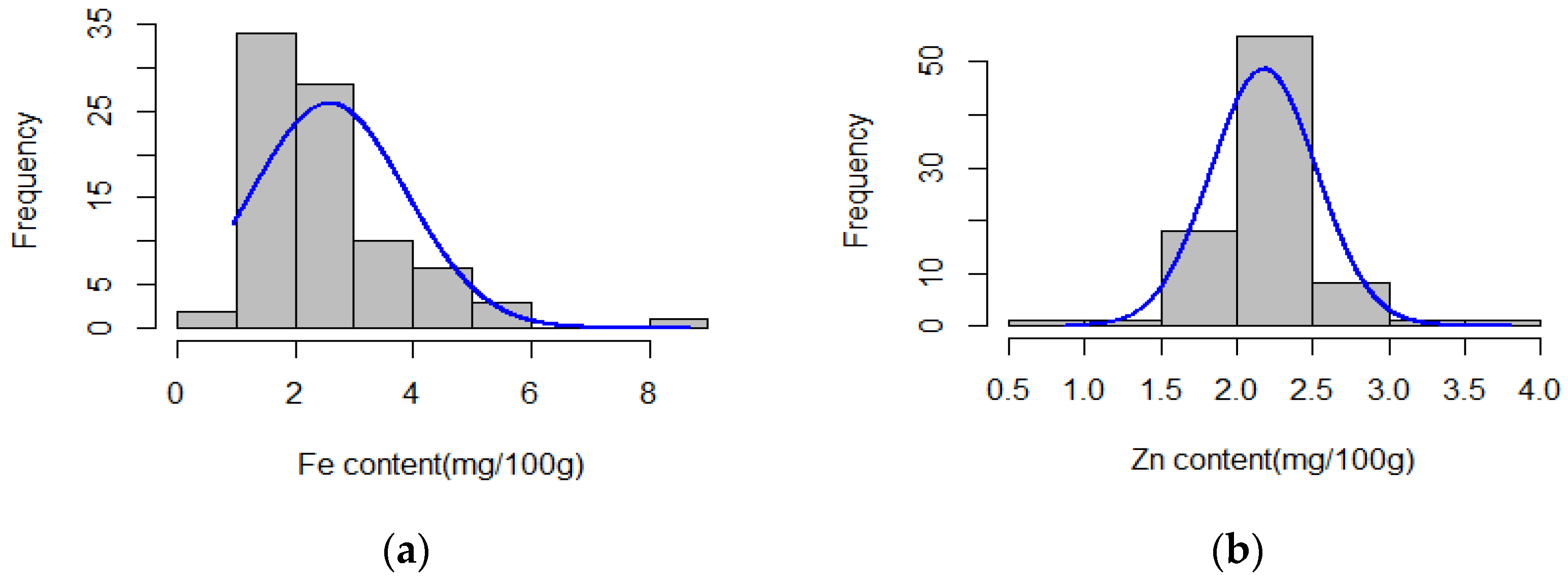



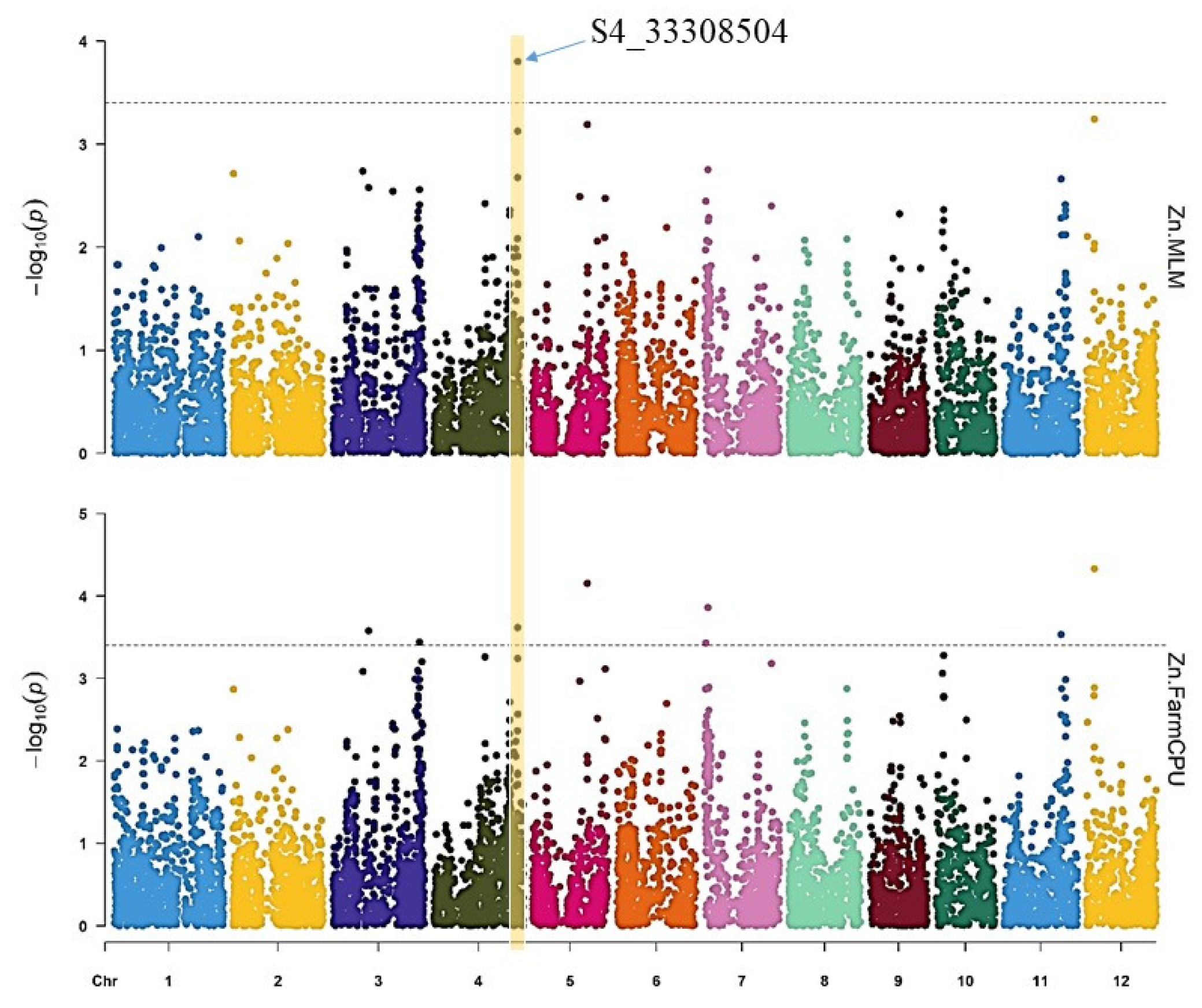
| Trait | Model | SNP | Chr | Position (bp) | p-Value |
|---|---|---|---|---|---|
| Fe | FarmCPU | S1_34232231 | 1 | 34,232,231 | 3.56 × 10−4 |
| Fe | FarmCPU | S11_2567279 | 11 | 2,567,279 | 4.01 × 10−5 |
| Fe | MLM | S11_2567279 | 11 | 2,567,279 | 4.01 × 10−5 |
| Zn | FarmCPU | S3_14236041 | 3 | 14,236,041 | 2.63 × 10−4 |
| Zn | FarmCPU | S3_34414350 | 3 | 34,414,350 | 3.62 × 10−4 |
| Zn | FarmCPU | S4_33308504 | 4 | 33,308,504 | 2.41 × 10−4 |
| Zn | FarmCPU | S5_21747243 | 5 | 21,747,243 | 7.01 × 10−5 |
| Zn | FarmCPU | S7_308113 | 7 | 308,113 | 3.72 × 10−4 |
| Zn | FarmCPU | S7_1159472 | 7 | 1,159,472 | 1.37 × 10−4 |
| Zn | FarmCPU | S11_22639501 | 11 | 22,639,501 | 2.92 × 10−4 |
| Zn | FarmCPU | S12_3069954 | 12 | 3,069,954 | 4.66 × 10−5 |
| Zn | MLM | S4_33308504 | 4 | 33,308,504 | 2.41 × 10−4 |
| Trait | SNP | Chromosome Position | Number of Candidate Gene Identified | ||||
|---|---|---|---|---|---|---|---|
| Total | Low Expressed | Intermediate Expressed | High Expressed | Unknown | |||
| Fe | S1_34232231 | 1 | 127 | 62 | 33 | 29 | 3 |
| S11_2567279 | 11 | 120 | 57 | 37 | 22 | 4 | |
| Zn | S3_14236041 | 3 | 22 | 11 | 7 | 3 | 1 |
| S3_34414350 | 3 | 144 | 54 | 40 | 47 | 3 | |
| S4_33308504 | 4 | 118 | 57 | 24 | 32 | 5 | |
| S5_21747243 | 5 | 127 | 65 | 36 | 20 | 6 | |
| S7_308113 | 7 | 117 | 56 | 33 | 23 | 5 | |
| S7_1159472 | 7 | 141 | 87 | 33 | 19 | 2 | |
| S11_22639501 | 11 | 107 | 72 | 18 | 17 | 0 | |
| S12_3069954 | 12 | 121 | 66 | 34 | 18 | 3 | |
| Trait | SNP | Chr | Candidate Gene | Distance (kb) | Annotation | ||
|---|---|---|---|---|---|---|---|
| Gene ID | Start_Pos | End_Pos | |||||
| Fe | S1_34232231 | 1 | LOC_Os01g58760 | 33,962,625 | 33,963,552 | 268.679 | bZIP transcription factor domain containing protein |
| 1 | LOC_Os01g59350 | 34,306,514 | 34,313,319 | −74.283 | transcription factor, TGA5, putative, expressed | ||
| 1 | LOC_Os01g59660 | 34,508,454 | 34,512,781 | −276.223 | MYB family transcription factor | ||
| 1 | LOC_Os01g59760 | 34,565,292 | 34,568,290 | −333.061 | bZIP transcription factor, putative, expressed | ||
| S11_2567279 | 11 | LOC_Os11g05390 | 2,413,596 | 2,417,533 | 149.746 | transporter, major facilitator family | |
| 11 | LOC_Os11g05480 | 2,462,254 | 2,468,733 | 98.546 | transcription factor | ||
| 11 | LOC_Os11g05640 | 2,560,816 | 2,563,634 | 3.645 | bZIP transcription factor domain containing protein | ||
| 11 | LOC_Os11g05700 | 2,605,745 | 2,610,544 | −38.466 | ABC transporter family protein, putative | ||
| 11 | LOC_Os11g06170 | 2,939,742 | 2,942,834 | −372.463 | bZIP transcriptional activator RSG | ||
| Zn | S3_14236041 | 3 | LOC_Os03g25430 | 14,538,130 | 14,539,609 | −302.089 | transcription regulator, putative, expressed |
| 3 | LOC_Os03g25470 | 14,552,198 | 14,552,749 | −316.157 | ctr copper transporter family protein | ||
| S3_34414350 | 3 | LOC_Os03g60130 | 34,194,882 | 34197992 | 216.358 | transcription elongation factor protein | |
| 3 | LOC_Os03g60820 | 34,554,642 | 34,560,824 | −140.292 | transporter, major facilitator superfamily domain containing protein | ||
| 3 | LOC_Os03g60850 | 34,574,307 | 34,576,973 | −159.957 | peptide transporter PTR2, putative, expressed | ||
| 3 | LOC_Os03g61030 | 34,671,286 | 34,674,494 | −256.936 | transcription termination factor nusG family protein | ||
| 3 | LOC_Os03g61100 | 34,708,403 | 34,711,045 | −294.053 | GDP-mannose transporter, putative | ||
| S4_33308504 | 4 | LOC_Os04g55970 | 33,341,978 | 33,346,562 | −33.474 | AP2-like ethylene-responsive transcription factor AINTEGUMENTA, putative, expressed | |
| 4 | LOC_Os04g56330 | 33,580,318 | 33,582,347 | −271.814 | ABC transporter, ATP-binding protein | ||
| 4 | LOC_Os04g56470 | 33,661,879 | 33,664,246 | −353.375 | amino acid transporter | ||
| S5_21747243 | 5 | LOC_Os05g37040 | 21,646,920 | 21,647,702 | 99.541 | MYB family transcription factor | |
| 5 | LOC_Os05g37050 | 21,650,252 | 21,651,057 | 96.186 | MYB family transcription factor | ||
| 5 | LOC_Os05g37060 | 21,654,182 | 21,655,380 | 91.863 | MYB family transcription factor | ||
| 5 | LOC_Os05g37170 | 21,720,654 | 21,724,490 | 22.753 | transcription factor | ||
| 5 | LOC_Os05g37470 | 21,926,799 | 21,931,594 | −179.556 | transmembrane amino acid transporter protein | ||
| 5 | LOC_Os05g37730 | 22,081,343 | 22,083,544 | −334.1 | MYB family transcription factor | ||
| S7_308113 | 7 | LOC_Os07g01070 | 42,657 | 44,577 | 263.536 | peptide transporter | |
| 7 | LOC_Os07g01560 | 348,475 | 350,594 | −40.362 | transporter family protein | ||
| S7_1159472 | 7 | LOC_Os07g02800 | 1,046,017 | 1,048,052 | 111.42 | MYB family transcription factor | |
| 7 | LOC_Os07g03220 | 1,267,975 | 1,268,550 | −108.503 | bZIP transcription factor domain containing | ||
| 7 | LOC_Os07g03250 | 1,299,598 | 1,304,299 | −140.126 | AP2-like ethylene-responsive transcription factor PLETHORA 2 | ||
| S11_22639501 | 11 | LOC_Os11g38160 | 22,625,530 | 22,627,579 | 11.922 | transporter family protein | |
| S12_3069954 | 12 | LOC_Os12g05830 | 2,684,760 | 2,688,178 | 381.776 | transporter-related | |
| 12 | LOC_Os12g06200 | 2,939,684 | 2,945,048 | 124.906 | E2F family transcription factor protein | ||
| 12 | LOC_Os12g06340 | 3,029,596 | 3,034,778 | 35.176 | BEL1-like homeodomain transcription factor | ||
| 12 | LOC_Os12g06520 | 3,153,015 | 3,156,795 | −83.061 | bZIP transcription factor domain containing protein | ||
| 12 | LOC_Os12g06850 | 3,326,815 | 3,329,114 | −256.861 | transcription elongation factor protein | ||
| Gene ID | Annotation | Expression (FPKM) | ||||
|---|---|---|---|---|---|---|
| Aleurone | Anther | Panicle | Pistil | Seed | ||
| LOC_Os01g58760 | bZIP transcription factor domain containing protein | 0.06 | 0.94 | 0.07 | 2.61 | 0.62 |
| LOC_Os01g59350 | transcription factor, TGA5 | 24.05 | 11.10 | 6.22 | 3.62 | 0.78 |
| LOC_Os01g59660 | MYB family transcription factor | 161.80 | 37.04 | 2.68 | 4.80 | 0.94 |
| LOC_Os01g59760 | bZIP transcription factor | 0.21 | 6.76 | 5.20 | 4.46 | 0.59 |
| LOC_Os11g05390 | transporter, major facilitator family | 0.29 | 0.32 | 0.02 | 0.30 | 0.99 |
| LOC_Os11g05480 | transcription factor | 0.99 | 67.84 | 0.63 | 1.32 | 0.85 |
| LOC_Os11g05640 | bZIP transcription factor domain containing protein | 19.56 | 0.61 | 0.14 | 0.11 | 0.89 |
| LOC_Os11g05700 | ABC transporter family protein | 26.76 | 2.48 | 0.81 | 8.05 | 0.74 |
| LOC_Os11g06170 | bZIP transcriptional activator RSG | 6.15 | 11.27 | 8.74 | 29.41 | 0.54 |
| LOC_Os03g25430 | transcription regulator | 3.46 | 6.64 | 15.32 | 0.94 | 0.78 |
| LOC_Os03g25470 | ctr copper transporter family protein | 1.45 | 3.55 | 2.77 | 0.43 | 0.60 |
| LOC_Os03g60130 | transcription elongation factor protein | 54.84 | 28.37 | 28.78 | 32.92 | 0.76 |
| LOC_Os03g60820 | transporter, major facilitator superfamily domain containing protein | 3.23 | 16.14 | 29.83 | 22.14 | 0.94 |
| LOC_Os03g60850 | peptide transporter PTR2 | 32.95 | 0.38 | 6.49 | 4.85 | 0.84 |
| LOC_Os03g61030 | transcription termination factor nusG family protein | 0.73 | 2.45 | 0.64 | 28.07 | 0.87 |
| LOC_Os03g61100 | GDP-mannose transporter | 0.13 | 0.57 | 0.03 | 0.01 | 0.89 |
| LOC_Os04g55970 | AP2-like ethylene-responsive transcription factor AINTEGUMENTA | 52.83 | 4.09 | 0.95 | 0.17 | 0.93 |
| LOC_Os04g56330 | ABC transporter, ATP-binding protein | 0.00 | 0.01 | 0.00 | 0.00 | 0.78 |
| LOC_Os04g56470 | amino acid transporter | 18.79 | 0.42 | 0.34 | 1.00 | 0.96 |
| LOC_Os05g37040 | MYB family transcription factor | 0.00 | 0.00 | 0.00 | 0.00 | 0.74 |
| LOC_Os05g37050 | MYB family transcription factor | 0.02 | 0.51 | 0.29 | 0.53 | 0.94 |
| LOC_Os05g37060 | MYB family transcription factor | 0.15 | 0.10 | 0.03 | 0.08 | 0.91 |
| LOC_Os05g37170 | transcription factor | 4.62 | 8.86 | 1.47 | 5.29 | 0.61 |
| LOC_Os05g37470 | transmembrane amino acid transporter protein | 0.10 | 1.10 | 0.72 | 3.10 | 0.77 |
| LOC_Os05g37730 | MYB family transcription factor | 11.14 | 4.40 | 14.46 | 9.07 | 0.59 |
| LOC_Os07g01070 | peptide transporter | 0.74 | 0.28 | 0.26 | 0.32 | 0.72 |
| LOC_Os07g01560 | transporter family protein | 68.32 | 3.04 | 0.41 | 16.62 | 0.81 |
| LOC_Os07g02800 | MYB family transcription factor | 10.58 | 4.90 | 15.90 | 30.67 | 18.77 |
| LOC_Os07g03220 | bZIP transcription factor domain containing protein | 0.08 | 0.13 | 0.00 | 0.11 | 0.18 |
| LOC_Os07g03250 | AP2-like ethylene-responsive transcription factor PLETHORA 2 | 0.00 | 0.06 | 0.05 | 0.05 | 0.08 |
| LOC_Os11g38160 | transporter family protein | 6.35 | 7.59 | 0.02 | 0.03 | 0.81 |
| LOC_Os12g05830 | transporter-related | 12.63 | 17.54 | 2.35 | 9.77 | 0.54 |
| LOC_Os12g06200 | E2F family transcription factor protein | 1.12 | 13.01 | 1.65 | 4.16 | 0.75 |
| LOC_Os12g06340 | BEL1-like homeodomain transcription factor | 0.02 | 0.76 | 8.60 | 1.44 | 0.67 |
| LOC_Os12g06520 | bZIP transcription factor domain containing protein | 9.15 | 18.58 | 18.24 | 14.45 | 0.28 |
| LOC_Os12g06850 | transcription elongation factor protein | 14.57 | 12.17 | 11.82 | 29.74 | 0.56 |
| LOC_Os01g58760 | bZIP transcription factor domain containing protein | 0.06 | 0.94 | 0.07 | 2.61 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukomarhe, C.B.; Kimwemwe, P.K.; Githiri, S.M.; Mamati, E.G.; Kimani, W.; Mutai, C.; Nganga, F.; Nguezet, P.-M.D.; Mignouna, J.; Civava, R.M.; et al. Association Mapping of Candidate Genes Associated with Iron and Zinc Content in Rice (Oryza sativa L.) Grains. Genes 2023, 14, 1815. https://doi.org/10.3390/genes14091815
Bukomarhe CB, Kimwemwe PK, Githiri SM, Mamati EG, Kimani W, Mutai C, Nganga F, Nguezet P-MD, Mignouna J, Civava RM, et al. Association Mapping of Candidate Genes Associated with Iron and Zinc Content in Rice (Oryza sativa L.) Grains. Genes. 2023; 14(9):1815. https://doi.org/10.3390/genes14091815
Chicago/Turabian StyleBukomarhe, Chance Bahati, Paul Kitenge Kimwemwe, Stephen Mwangi Githiri, Edward George Mamati, Wilson Kimani, Collins Mutai, Fredrick Nganga, Paul-Martin Dontsop Nguezet, Jacob Mignouna, René Mushizi Civava, and et al. 2023. "Association Mapping of Candidate Genes Associated with Iron and Zinc Content in Rice (Oryza sativa L.) Grains" Genes 14, no. 9: 1815. https://doi.org/10.3390/genes14091815
APA StyleBukomarhe, C. B., Kimwemwe, P. K., Githiri, S. M., Mamati, E. G., Kimani, W., Mutai, C., Nganga, F., Nguezet, P.-M. D., Mignouna, J., Civava, R. M., & Fofana, M. (2023). Association Mapping of Candidate Genes Associated with Iron and Zinc Content in Rice (Oryza sativa L.) Grains. Genes, 14(9), 1815. https://doi.org/10.3390/genes14091815









