Using High-Throughput Phenotyping Analysis to Decipher the Phenotypic Components and Genetic Architecture of Maize Seedling Salt Tolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Measurement of Morphological and Spectral Parameters
2.4. Manual Measurement of Phenotype Data
2.5. Phenotype Data Analysis
2.6. Genome-Wide Association Study
3. Result
3.1. Descriptive Statistical Analysis of Various Phenotypic Indicators
3.2. Phenotypic Variation Analysis in Maize Seeding Salt Response
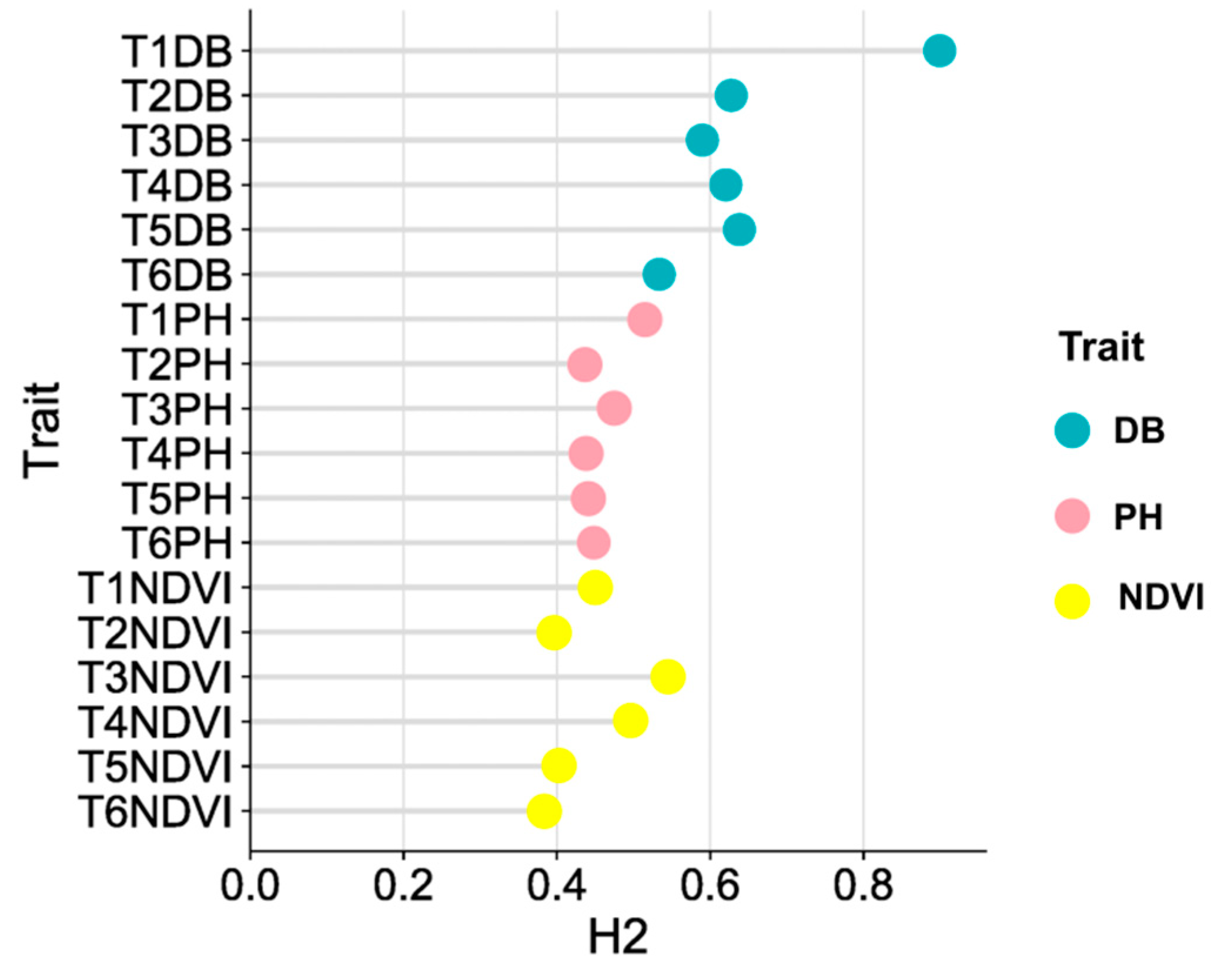
3.3. Phenotypic Variations of DB, PH and NDVI among 204 Inbred Lines
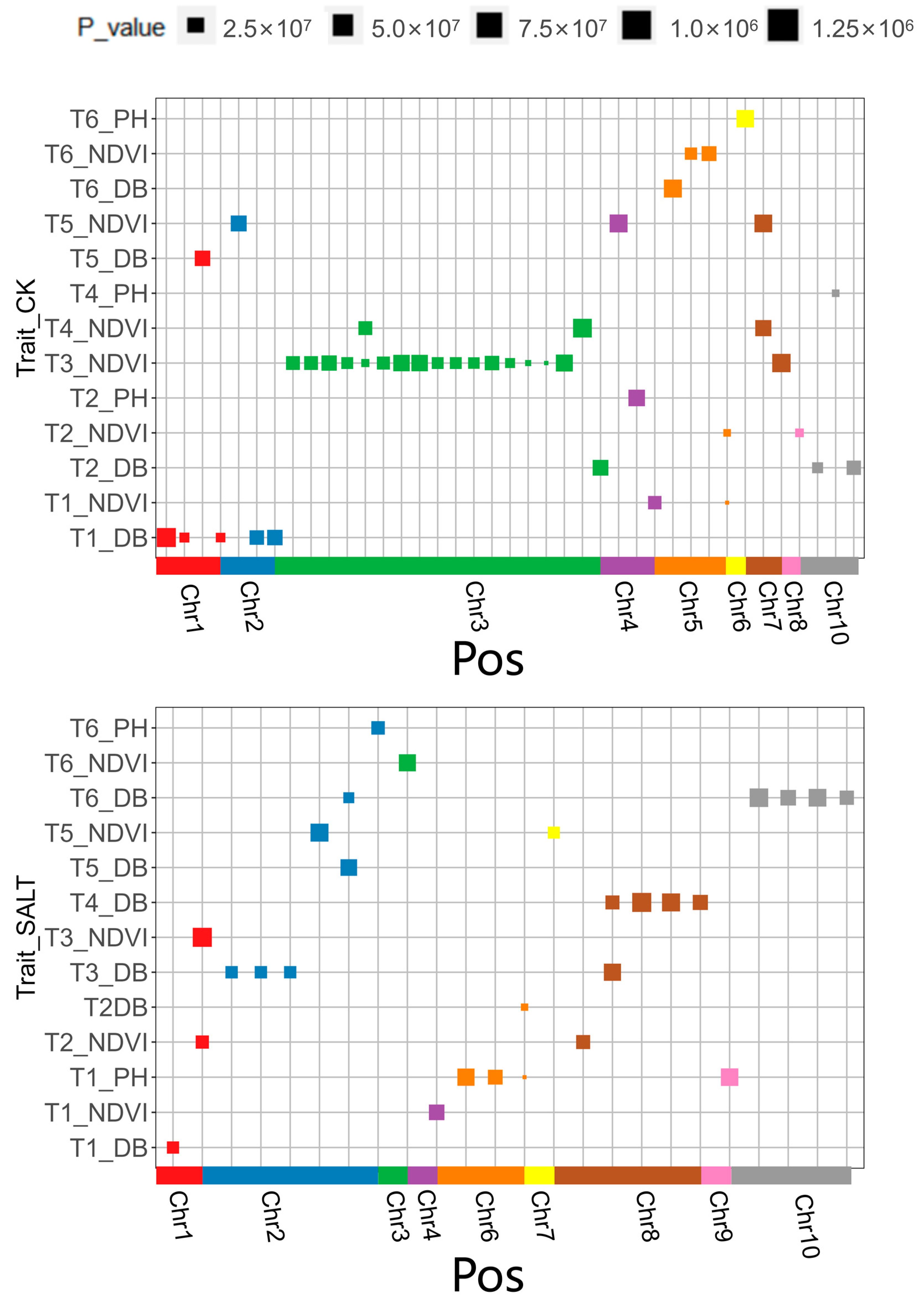
3.4. Genetic Basis of Phenotypic Traits in Maize Seedling for Salt Response
4. Discussion
4.1. Effects of Salt Stress on Maize Seedling Growth
4.2. The Differences in Response of Seedlings of Different Inbred Lines to Salt Stress in Maize-Associated Populations
4.3. Discussion on the Function of Candidate Genes Annotated by GWAS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shabala, S.; Wu, H.; Bose, J. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 2015, 241, 109–119. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Yang, Y.; Song, C.; Wang, B.; Yang, S.; Guo, Y.; Gong, Z. Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 2021, 63, 53–78. [Google Scholar] [CrossRef]
- Montanarella, L. Agricultural policy: Govern our soils. Nature 2015, 528, 32–33. [Google Scholar] [CrossRef]
- Park, H.J.; KOM, W.-Y.; Yun, D.-J. A new insight of salt stress signaling in plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt: Tress responses. New Phytol. 2017, 217, 523–539. [Google Scholar] [CrossRef]
- Munnik, T.; Vermeer, J.E.M. Osmotic stress-induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ. 2010, 33, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, M.; Liang, X.; Li, F.; Shi, Y.; Yang, X.; Jiang, C. Natural variation of an EF-hand Ca(2+)-binding-protein coding gene confers saline-alkaline tolerance in maize. Nat. Commun. 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, G.; Zhou, S.; Ren, Y.; Wang, W. The improvement of salt tolerance in transgenic tobacco by overexpression of wheat F-box gene TaFBA1. Plant Sci. 2017, 259, 71–85. [Google Scholar] [CrossRef] [PubMed]
- James, R.A.; Munns, R.; Von Caemmerer, S.; Trejo, C.; Miller, C.; Condon, T. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl− in salt-affected barley and durum wheat. Plant Cell Environ. 2006, 29, 2185–2197. [Google Scholar] [CrossRef]
- Liang, X.; Liu, S.; Wang, T.; Li, F.; Cheng, J.; Lai, J.; Qin, F.; Li, Z.; Wang, X.; Jiang, C. Metabolomics-driven gene mining and genetic improvement of tolerance to salt-induced osmotic stress in maize. New Phytol. 2021, 230, 2355–2370. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, A.; Zhao, Y.; Fan, H.Z. Effect of salt stress on germination and seedling growth of maize. Soils Fertil. Sci. China 2007, 2, 30–34. [Google Scholar]
- Liu, C.X.; Dong, R.; Liu, Q.; He, C.M.; Guan, H.Y.; Wang, J.; Liu, T.S.; Wang, L.M. Effects of salt stress on seed germination characteristics of different maize germplasm resources. Shandong Agric. Sci. 2017, 49, 27–30. [Google Scholar]
- Khan, A.A.; Rao, S.A.; Mcneilly, T. Assessment of salinity tolerance based upon seedling root growth response functions in maize (Zea mays L.). Euphytica 2003, 131, 81–89. [Google Scholar] [CrossRef]
- Wang, H.Y. Association Analysis of Salt Tolerance Traits in Maize at the Seedling Stage. Master’s Thesis, Yangzhou University, Yangzhou, China, 2016. [Google Scholar]
- Zhang, H.L.; Yuan, C.; Zhu, L.; Xu, X. Comprehensive evaluation of salt tolerance of maize at maturity using membership function value method. J. Northwest A&F Univ. 2018, 46, 47–55. [Google Scholar]
- Yang, X.H.; Gao, S.B.; Xu, S.T.; Zhang, Z.X.; Prasanna, B.M.; Li, L.; Li, J.S.; Yan, J.B. Characterization of a global germplasm collection and its potential utilization for analysis of complex quantitative traits in maize. Mol. Breed. 2011, 28, 511–526. [Google Scholar] [CrossRef]
- Yang, R.Y.; Zhang, Z.H.; Wang, H.M.; Cheng, X.P.; Hu, Q.; Gao, Z.L. Effects of rhizosphere ventilation and salt stress on growth characteristics of maize. J. Ludong Univ. 2010, 26, 35–38. [Google Scholar]
- Pitann, B.; Schubert, S.; Muhling, K.H. Decline in leaf growth under salt stress is due to an inhibition of H′-pumpingactivity and increase in apoplastic pH of maize leaves. J. Plant Nutr. Soil Sci. 2009, 172, 535–543. [Google Scholar] [CrossRef]
- Hu, Y.M.; Guo, Y.G.; Yao, Y.; Fang, Z.M. Effects of NaCl stress on seed germination and seedling growth of maize. J. Jianghan Univ. 2017, 45, 137–143. [Google Scholar]
- Munns, R.; Tonnet, M.L.; Shenna, C.; Gardner, P.A. Effect of high external NaCl concentrations on ion transport within the shoot of Lupinus albus.I.Ions in xylem sap. Plant Cell Environ. 1988, 11, 291–300. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, Y.F.; Li, F.X.; Xiao, M.J.; Tao, Y.; Xu, W.J.; Huang, R.D. Effects of salt stress on photosynthesis and fluorescence characteristics of sorghum seedlings. Sci. Agric. Sin. 2012, 45, 3265–3272. [Google Scholar]
- Zhao, Q.; Chen, S.Y.; Wang, G.D.; Du, Y.L.; Du, J.D. Effect of saline-alkali stress on soybean seedling growth based on Planteye F500 three-dimensional quantitative study. J. Heilongjiang Bayi Agric. Univ. 2022, 34, 1–7+40. [Google Scholar]
- Guo, Q.H.; Yang, W.C.; Wu, F.F.; Pang, S.; Jin, S.; Chen, F.; Wang, X. High-throughput crop phenotype monitoring: An accelerator for breeding and precision agriculture development. Bull. Chin. Acad. Sci. 2018, 33, 940–946. [Google Scholar]
- Kumar, J.; Pratap, A.; Kumar, S. Phenomics in Crop Plants: Trends, Options and Limitations; Springer: New Delhi, India, 2015; Volume 5. [Google Scholar]
- Li, L.; Zhang, Q.; Huang, D.F. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Cao, X.F.; Yu, K.Q.; Zhao, Y.R. Research progress of imaging spectroscopy in high-throughput analysis of plant abiotic stress phenotypes. Spectrosc. Spectral Anal. 2020, 40, 3365–3372. [Google Scholar]
- Wang, X.D.; Shi, Z.S.; Li, F.H. Changes of physiological indexes of maize varieties in different ages in North China. Maize Sci. 2011, 19, 82–86+90. [Google Scholar]
- Saijo, Y.; Kinoshita, N.; Ishiyama, K.; Hata, S.; Kyozuka, J.; Hayakawa, T.; Nakamura, T.; Shimamoto, K.; Yamaya, T.; Izui, K. A Ca2+-dependent protein kinase that endows rice plants with cold- and salt-stress tolerance functions in vascular bundles. Plant Cell Physiol. 2001, 42, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Wu, W.H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol. Biol. 2007, 65, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, R.; Sandhya, S.; Chandra, N.; Srinivasan, N. De-DUFing the DUFs: Deciphering distant evolutionary relationships of domains of unknown function using sensitive homology detection methods. Biol. Direct 2015, 10, 38. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Chen, J.Y.; Kuang, J.F.; Lu, W.J.; Shan, W. The banana fruit SINA ubiquitin ligase MaSINA1 regulates the stability of MaICE1 to be negatively involved in cold stress response. Front. Plant Sci. 2017, 8, 995. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Wang, X.; Ji, X.L.; Qiao, Z.W.; You, C.X.; Hao, Y.J. Genome-wide identification of apple ubiquitin SINA E3 ligase and functional characterization of MdSINA2. Front. Plant Sci. 2020, 11, 1109. [Google Scholar] [CrossRef]
- Guo, C.M.; Luo, C.K.; Guo, L.J.; Li, M.; Guo, X.M.; Zhang, Y.X.; Wang, L.J.; Chen, L. OsSIDP366, a DUF1644 gene, positively regulates responses to drought and salt stresses in rice. J. Integr. Plant Biol. 2016, 58, 492–502. [Google Scholar] [CrossRef]
- Tian, M.; Lou, L.; Liu, L.; Yu, F.; Zhao, Q.; Zhang, H.; Wu, Y.; Tang, S.; Xia, R.; Zhu, B.; et al. The RING finger E3 ligase STRF1 is involved in membrane trafficking and modulates salt-stress response in Arabidopsis thaliana. Plant J. 2015, 82, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Lim, S.D.; Moon, J.C.; Jang, C.S. A rice really interesting new gene H 2-type E 3 ligase, OsSIRH2-14, enhances salinity tolerance viaubiquitin/26 S proteasome-mediated degradation of salt-related proteins. Plant Cell Environ. 2019, 42, 3061–3076. [Google Scholar] [CrossRef]
- Desaki, Y.; Takahashi, S.; Sato, K.; Maeda, K.; Matsui, S.; Yoshimi, I.; Miura, T.; Jumonji, J.-I.; Takeda, J.; Yashima, K.; et al. PUB4, a CERK1-Interacting Ubiquitin Ligase, Positively Regulates MAMP-Triggered Immunity in Arabidopsis. Plant Cell Physiol. 2019, 60, 2573–2583. [Google Scholar] [CrossRef]
- Knip, M.; de Pater, S.; Hooykaas, P.J.J. The SLEEPER genes: A transposase-derived angiosperm-specific gene family. BMC Plant Biol. 2012, 12, 192. [Google Scholar]
- Wang, Z.M.; Li, H.X.; He, Y.; Zeng, H.L. Advances in Plant Pyruvate, Orthophosphate Dikinase. Plant Physiol. J. 2012, 48, 949–957. [Google Scholar]
- Ogg, S.C.; Poritz, M.A.; Walter, P. Signal recognition particle receptor is important for cell growth and protein secretion in Saccharomyces cerevisiae. Mol. Biol. Cell 1992, 3, 895–911. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ji, S.L.; Siegel, A.; Shan, S.O.; Grimm, B.; Wang, P. Chloroplast SRP43 autonomously protects chlorophyll biosynthesis proteins against heat shock. Nat. Plants 2021, 7, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Susanne, F.; Takahiro, N.; Nicole, B.; Gadi, S. HCF152, an Arabidopsis RNA Binding Pentatricopeptide Repeat Protein Involved in the Processing of Chloroplast psbB-psbT-psbH-petB-petD RNAs. Plant Cell 2003, 15, 1480–1495. [Google Scholar]
- Ravi, V.M.; Sun, G.; Grzybowski, M.; Tross, M.; Jin, H.; Smith, C.; Newton, L.; Andorf, C.; Woodhouse, M.; Thompson, A.; et al. Association mapping across a multitude of traits collected in diverse environments in maize. GigaScience 2022, 11, giac080. [Google Scholar] [CrossRef]
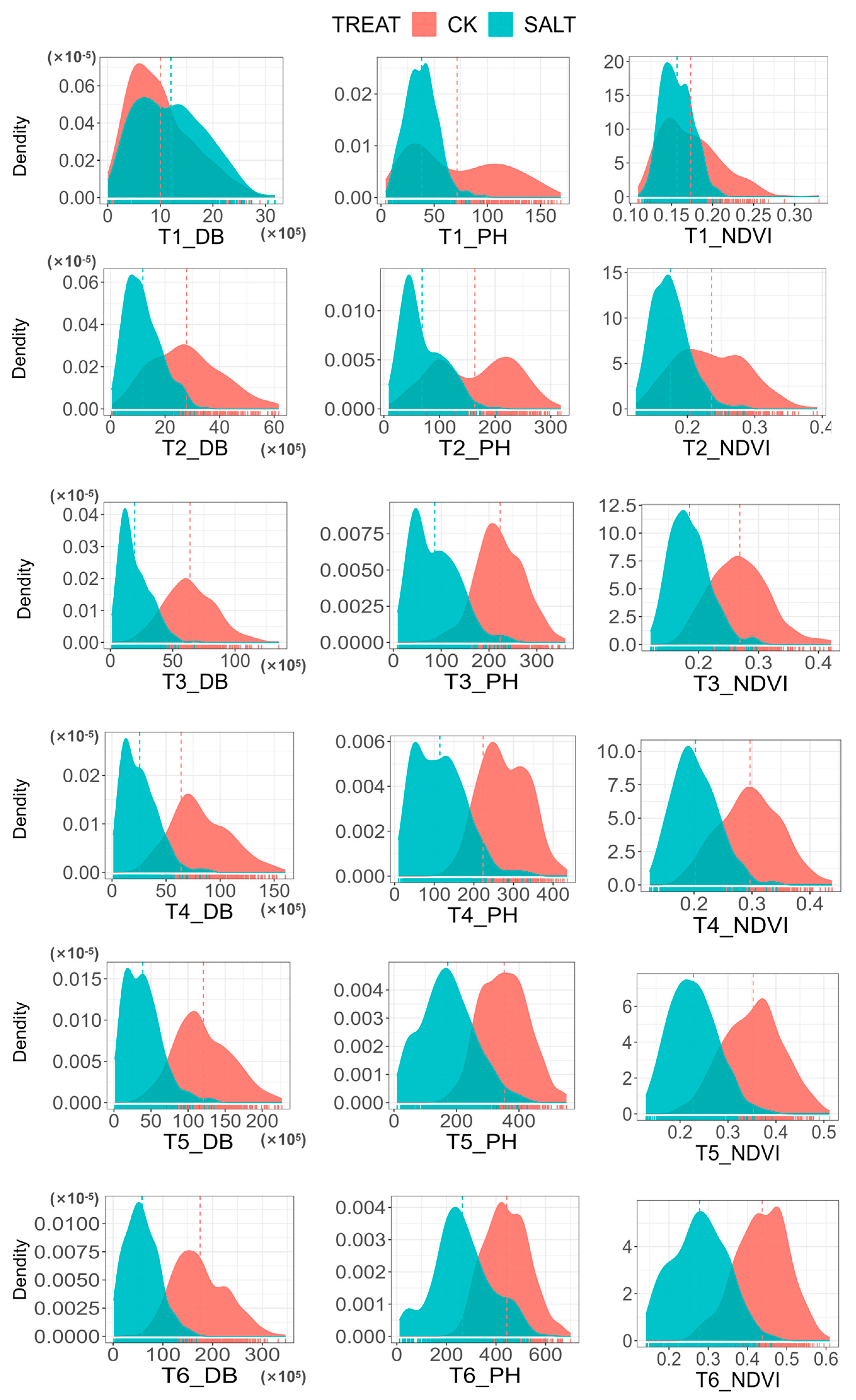
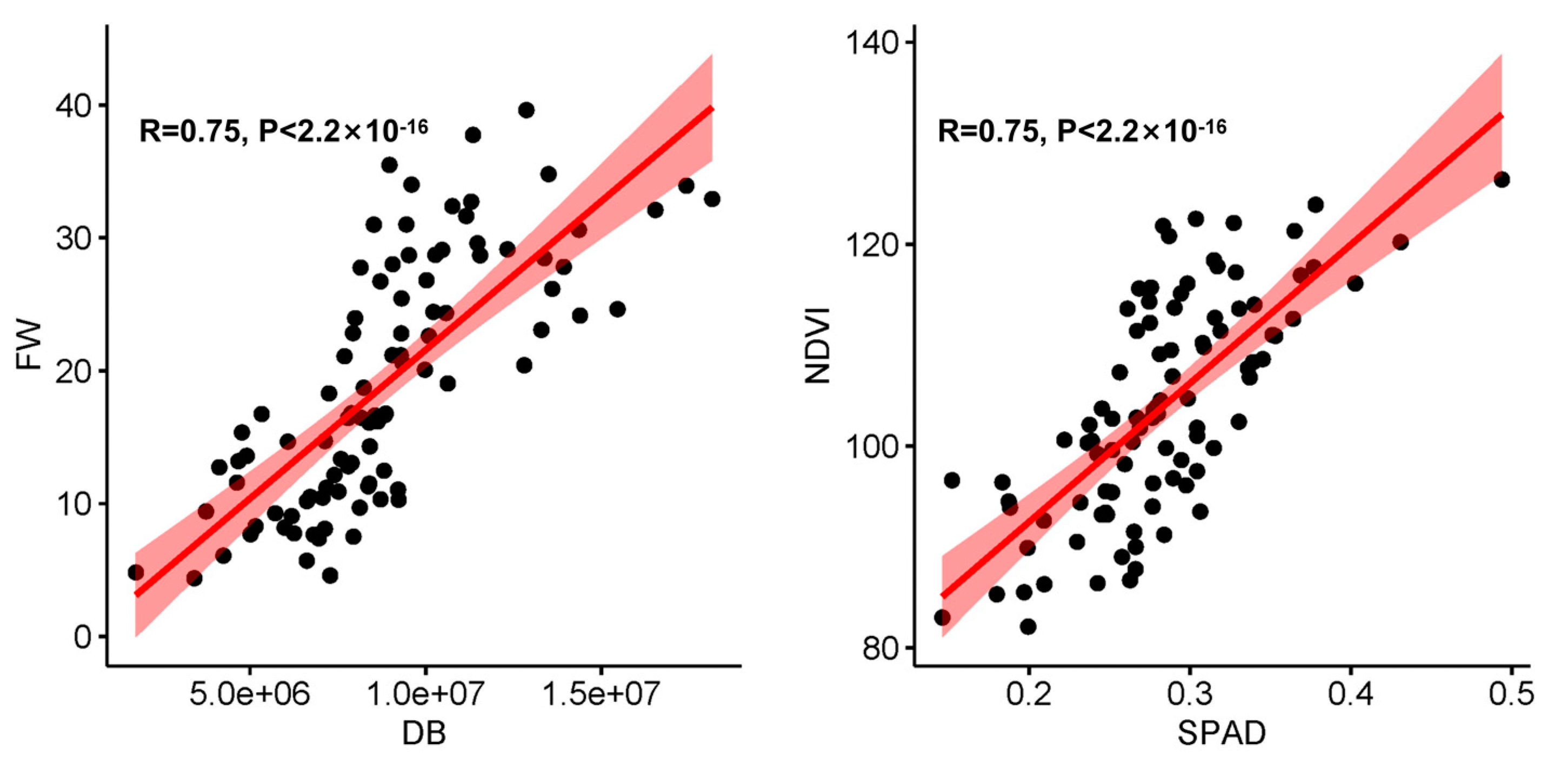

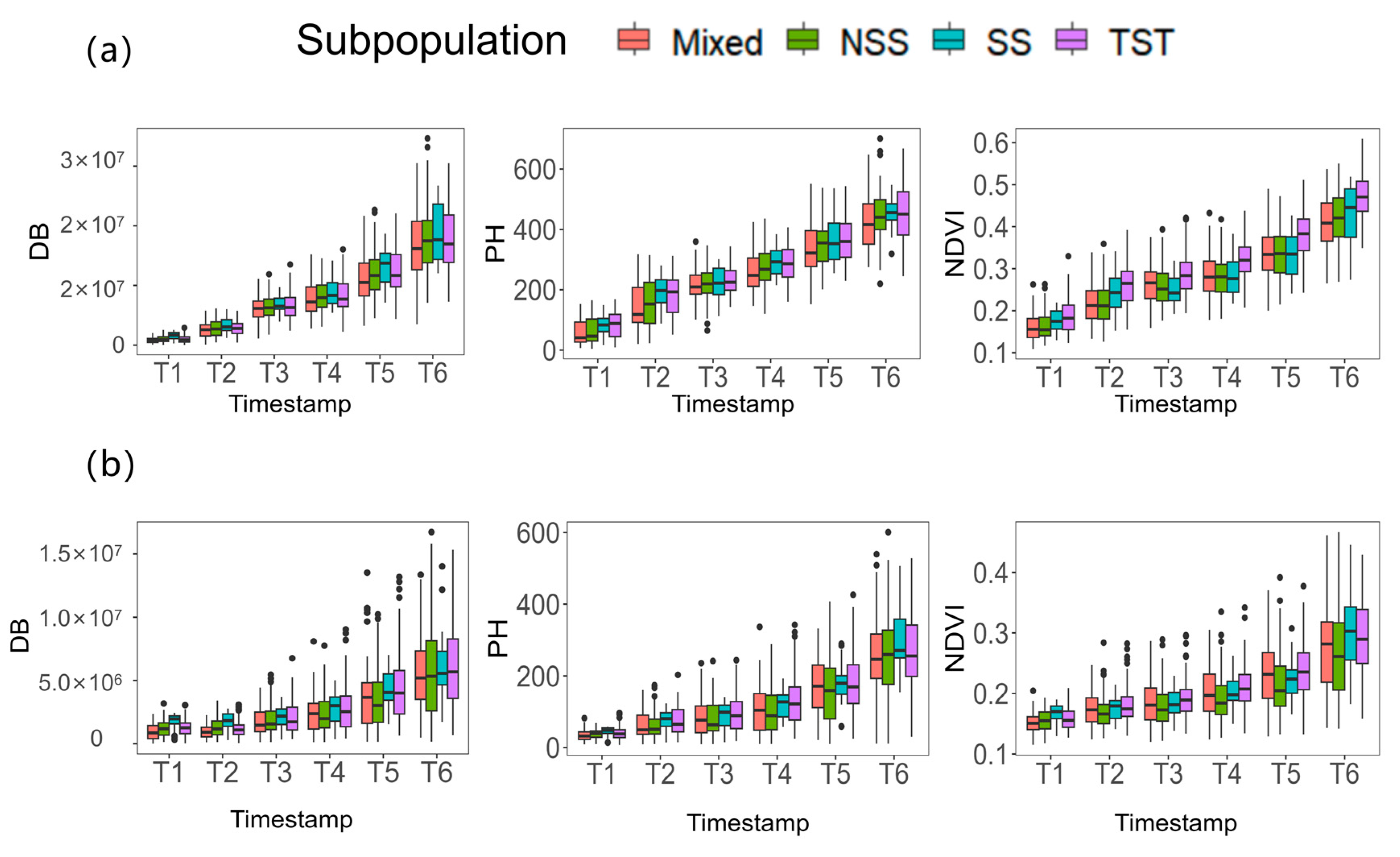
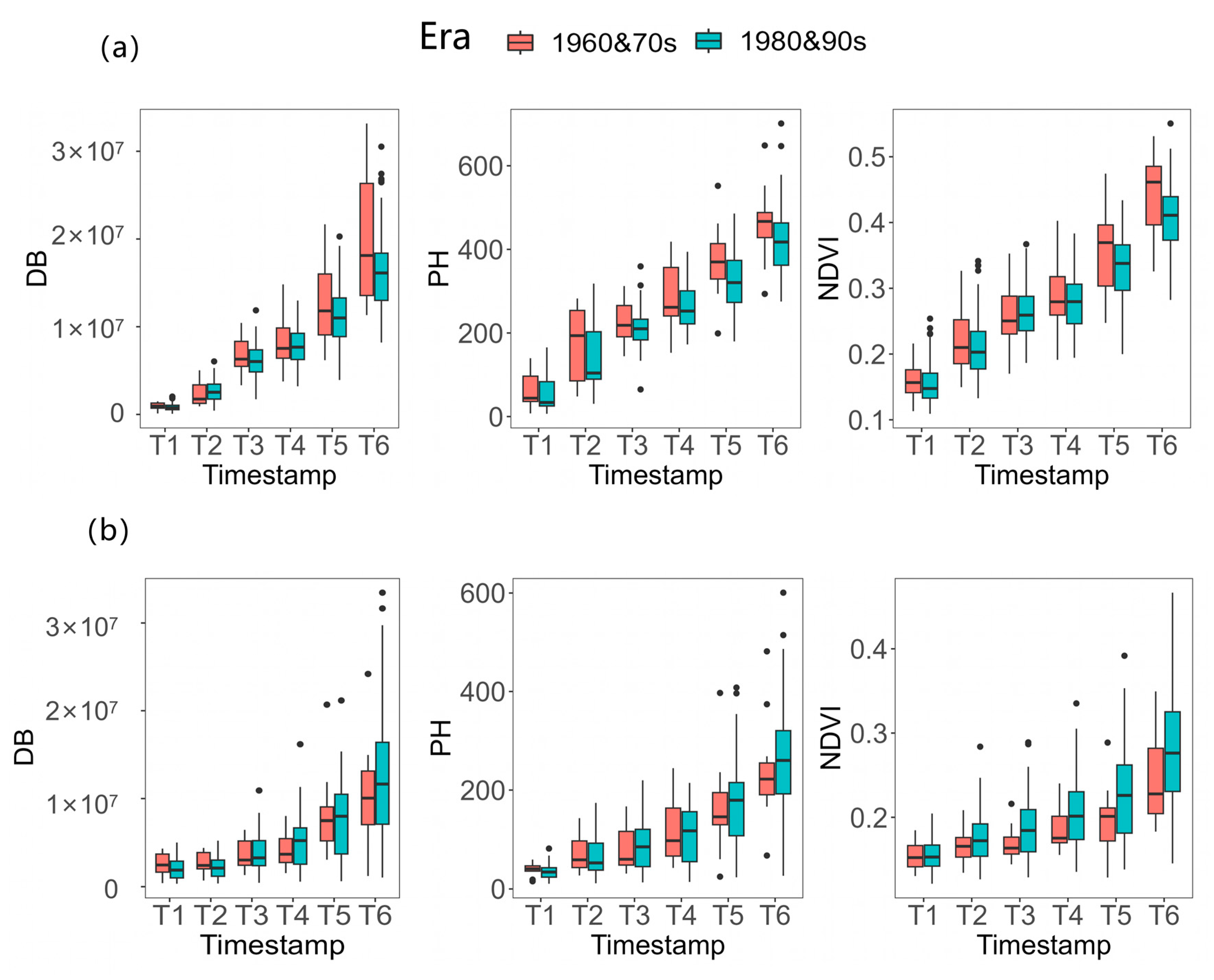
| Treatment | Trait | Time | Mean | Standard Deviation (SD) | Maximum | Minimum | Coefficient of Variation (CV) |
|---|---|---|---|---|---|---|---|
| CK | Digital Biomass (DB) | T1 | 1,003,810.824 | 598,971.384 | 2,894,770 | 34,594.4 | 59.67% |
| T2 | 2,789,052.783 | 1,270,686.545 | 6,166,630 | 89,783.4 | 45.56% | ||
| T3 | 6,389,575.784 | 2,021,845.248 | 13,513,400 | 1,085,700 | 31.64% | ||
| T4 | 8,195,848.799 | 2,679,092.923 | 16,033,700 | 2,233,340 | 32.69% | ||
| T5 | 12,074,676.89 | 3,776,405.623 | 22,666,800 | 3,226,160 | 31.28% | ||
| T6 | 17,486,558.09 | 5,313,994.885 | 34,648,400 | 3,504,570 | 30.39% | ||
| Plant Height (PH) | T1 | 71.53755637 | 43.37708332 | 169.07 | 4.435 | 60.64% | |
| T2 | 163.5589779 | 70.85797429 | 318.159 | 20.157 | 43.32% | ||
| T3 | 223.3098873 | 48.90702459 | 359.377 | 64.917 | 21.90% | ||
| T4 | 276.8431838 | 60.74844002 | 435.668 | 119.777 | 21.94% | ||
| T5 | 352.7212475 | 74.76313441 | 552.051 | 152.431 | 21.20% | ||
| T6 | 443.250299 | 87.54977091 | 701.195 | 219.865 | 19.75% | ||
| Normalized Difference Vegetation Index (NDVI) | T1 | 0.173306299 | 0.036154175 | 0.32981 | 0.10914 | 20.86% | |
| T2 | 0.235952132 | 0.052842115 | 0.3924 | 0.12631 | 22.40% | ||
| T3 | 0.268782721 | 0.048345749 | 0.42107 | 0.15907 | 17.99% | ||
| T4 | 0.296613676 | 0.052516168 | 0.43803 | 0.17819 | 17.71% | ||
| T5 | 0.352496299 | 0.060643137 | 0.51194 | 0.19983 | 17.20% | ||
| T6 | 0.437217574 | 0.065422835 | 0.60941 | 0.26519 | 14.96% | ||
| SALT | Digital Biomass (DB) | T1 | 1,201,841.525 | 645,468.8941 | 3,180,000 | 5418.77 | 53.71% |
| T2 | 1,188,189.466 | 644,786.7089 | 3,421,400 | 47,520 | 54.27% | ||
| T3 | 1,949,282.993 | 1,168,493.057 | 6,756,980 | 127,041 | 59.94% | ||
| T4 | 2,560,512.914 | 1,600,916.684 | 9,038,970 | 127,459 | 62.52% | ||
| T5 | 3,896,388.289 | 2,435,638.425 | 13,519,700 | 152,862 | 62.51% | ||
| T6 | 5,823,881.936 | 3260235.245 | 16,729,400 | 158,282 | 55.98% | ||
| Plant Height (PH) | T1 | 38.15027941 | 14.81555384 | 96.553 | 7.286 | 38.83% | |
| T2 | 68.75126716 | 37.36236572 | 202.904 | 9.007 | 54.34% | ||
| T3 | 86.5950049 | 47.94333381 | 243.713 | 9.273 | 55.37% | ||
| T4 | 114.2672721 | 63.3829814 | 342.474 | 10.002 | 55.47% | ||
| T5 | 172.0369314 | 84.31686079 | 426.225 | 9.993 | 49.01% | ||
| T6 | 264.4116691 | 114.350432 | 600.701 | 10.987 | 43.25% | ||
| Normalized Difference Vegetation Index (NDVI) | T1 | 0.156698186 | 0.018441621 | 0.20908 | 0.11504 | 11.77% | |
| T2 | 0.174882745 | 0.027428217 | 0.28381 | 0.12399 | 15.68% | ||
| T3 | 0.184999363 | 0.032914124 | 0.29628 | 0.11996 | 17.79% | ||
| T4 | 0.202460049 | 0.038459752 | 0.34213 | 0.12383 | 19.00% | ||
| T5 | 0.22793924 | 0.049238959 | 0.39175 | 0.12893 | 21.60% | ||
| T6 | 0.278152034 | 0.068728279 | 0.46674 | 0.14181 | 24.71% |
| Treat | Trait | Time | Chr | Ps | p_Value | Gene |
|---|---|---|---|---|---|---|
| SALT | Normalized Difference Vegetation Index (NDVI) | T1 | 4 | chr4.s_240389218 | 7.42 × 10−7 | Zm00001d053747 |
| T2 | 1 | chr1.s_300266616 | 4.82 × 10−7 | Zm00001d034702 | ||
| 8 | chr8.s_109466855 | 5.65 × 10−7 | Zm00001d010321 | |||
| T3 | 1 | chr1.s_300266616 | 1.25 × 10−6 | Zm00001d010321 | ||
| T5 | 2 | chr2.s_22584208 | 1.08 × 10−6 | Zm00001d002784 | ||
| 7 | chr7.s_167828937 | 3.79 × 10−7 | Zm00001d022005 | |||
| T6 | 3 | chr3.s_200476054 | 9.49 × 10−7 | Zm00001d043454 | ||
| Plant Height (PH) | T1 | 6 | chr6.s_163542090 | 9.53 × 10−7 | Zm00001d038733 | |
| 6 | chr6.s_163542669 | 6.43 × 10−7 | ||||
| 6 | chr6.s_164734587 | 1.96 × 10−8 | Zm00001d038801 | |||
| 9 | chr9.s_13122222 | 1.00 × 10−6 | Zm00001d045112 | |||
| T6 | 2 | chr2.s_51949192 | 5.09 × 10−7 | Zm00001d003648 | ||
| Digital Biomass (DB) | T1 | 1 | chr1.s_2026279 | 3.84 × 10−7 | Zm00001d027293 | |
| T2 | 6 | chr6.s_164734587 | 7.87 × 10−8 | Zm00001d038801 | ||
| T3 | 2 | chr2.s_13293975 | 4.01 × 10−7 | Zm00001d002462 | ||
| 2 | chr2.s_13293982 | 4.01 × 10−7 | ||||
| 2 | chr2.s_13294061 | 3.94 × 10−7 | ||||
| 8 | chr8.s_77012316 | 9.62 × 10−7 | Zm00001d009707 | |||
| T4 | 8 | chr8.s_77012316 | 5.65 × 10−7 | Zm00001d009707 | ||
| 8 | chr8.s_77013610 | 1.25 × 10−6 | Zm00001d009708 | |||
| 8 | chr8.s_77013657 | 1.09 × 10−6 | ||||
| 8 | chr8.s_85585942 | 6.96 × 10−7 | Zm00001d009860 | |||
| T5 | 2 | chr2.s_236506507 | 8.84 × 10−7 | Zm00001d007630 | ||
| T6 | 2 | chr2.s_236506507 | 2.83 × 10−7 | Zm00001d007630 | ||
| 10 | chr10.s_16712100 | 1.17 × 10−6 | Zm00001d023717 | |||
| 10 | chr10.s_16712849 | 7.51 × 10−7 | ||||
| 10 | chr10.s_16713353 | 1.04 × 10−6 | ||||
| 10 | chr10.s_16713406 | 5.92 × 10−7 | ||||
| CK | Digital Biomass (DB) | T1 | 1 | chr1.s_2233766 | 2.00 × 10−7 | Zm00001d027300 |
| 1 | chr1.s_7312522 | 1.77 × 10−7 | Zm00001d027518 | |||
| 1 | chr1.s_198080808 | 1.26 × 10−6 | Zm00001d031665 | |||
| 2 | chr2.s_41052633 | 6.16 × 10−7 | Zm00001d003349 | |||
| 2 | chr2.s_41097824 | 7.31 × 10−7 | ||||
| T2 | 3 | chr3.s_5143271 | 7.69 × 10−7 | Zm00001d039469 | ||
| 10 | chr10.s_10655807 | 5.96 × 10−7 | Zm00001d023580 | |||
| 10 | chr10.s_144727619 | 2.76 × 10−7 | ENSRNA049474907 | |||
| T5 | 1 | chr1.s_32354806 | 7.35 × 10−7 | Zm00001d028369 | ||
| T6 | 5 | chr5.s_1279003 | 1.08 × 10−6 | Zm00001d012861 | ||
| Plant Height (PH) | T2 | 4 | chr4.s_3408937 | 8.78 × 10−7 | Zm00001d048693 | |
| T4 | 10 | chr10.s_145969872 | 7.56 × 10−8 | Zm00001d026445 | ||
| T6 | 6 | chr6.s_119549311 | 1.02 × 10−6 | Zm00001d037290 | ||
| Normalized Difference Vegetation Index (NDVI) | T1 | 4 | chr4.s_4154287 | 4.95 × 10−7 | Zm00001d048718 | |
| 5 | chr5.s_7988332 | 4.41 × 10−9 | Zm00001d013294 | |||
| T2 | 5 | chr5.s_7988332 | 7.48 × 10−8 | Zm00001d013294 | ||
| 8 | chr8.s_112914569 | 1.26 × 10−7 | Zm00001d010401 | |||
| T3 | 3 | chr3.s_170135758 | 5.41 × 10−7 | Zm00001d042508 | ||
| 3 | chr3.s_170135828 | 5.41 × 10−7 | ||||
| 3 | chr3.s_170273718 | 7.15 × 10−7 | Zm00001d042512 | |||
| 3 | chr3.s_170275456 | 3.49 × 10−7 | ||||
| 3 | chr3.s_170275775 | 9.95 × 10−8 | ||||
| 3 | chr3.s_170275799 | 4.58 × 10−7 | ||||
| 3 | chr3.s_170374752 | 8.71 × 10−7 | Zm00001d042519 | |||
| 3 | chr3.s_170374757 | 8.71 × 10−7 | ||||
| 3 | chr3.s_170374936 | 3.47 × 10−7 | ||||
| 3 | chr3.s_170374939 | 3.47 × 10−7 | ||||
| 3 | chr3.s_170385027 | 3.19 × 10−7 | ||||
| 3 | chr3.s_170387082 | 6.15 × 10−7 | ||||
| 3 | chr3.s_170390974 | 2.05 × 10−7 | Zm00001d042520 | |||
| 3 | chr3.s_173423411 | 2.90 × 10−8 | Zm00001d042608 | |||
| 3 | chr3.s_173423420 | 5.13 × 10−9 | ||||
| 3 | chr3.s_175638756 | 9.37 × 10−7 | Zm00001d042656 | |||
| 7 | chr7.s_133912573 | 1.15 × 10−6 | Zm00001d020821 | |||
| T4 | 3 | chr3.s_170275775 | 5.45 × 10−7 | Zm00001d042512 | ||
| 3 | chr3.s_209949573 | 1.21 × 10−6 | Zm00001d043778 | |||
| 7 | chr7.s_132496442 | 8.24 × 10−7 | Zm00001d020782 | |||
| T5 | 2 | chr2.s_1543350 | 7.93 × 10−7 | Zm00001d001827 | ||
| 4 | chr4.s_169992759 | 1.08 × 10−6 | Zm00001d051800 | |||
| 7 | chr7.s_132496442 | 1.05 × 10−6 | Zm00001d020782 | |||
| T6 | 5 | chr5.s_216550034 | 3.82 × 10−7 | Zm00001d018204 | ||
| 5 | chr5.s_216550127 | 6.56 × 10−7 |
| Gene | Description | |
|---|---|---|
| Zm00001d023717 | RICESLEEPR1 | Zinc finger BED domain-containing protein RICESLEEPER 1 |
| Zm00001d002462 | PEX6 | Peroxisome biogenesis protein 6 |
| Zm00001d018204 | DUF1644 | Putative DUF1644 and RING zinc finger domain protein |
| Zm00001d009708 | CDPK1 | Calcium-dependent protein kinase 1 |
| Zm00001d007630 | RPS2 | Disease resistance protein RPS2 |
| Zm00001d003352 | Per1 | Per1-like family protein |
| Zm00001d009707 | Probable protein ABIL4 | |
| Zm00001d042608 | PUB4 | U-box domain-containing protein 4 |
| Zm00001d010321 | PPDK2 | Pyruvate, phosphate dikinase 2-like |
| Zm00001d027293 | SRP Receptor | Signal recognition particle receptor homolog 1 |
| Zm00001d037290 | Mitochondrion protein | |
| Zm00001d012861 | PPF1 | Inner membrane protein PPF-1, chloroplastic |
| Zm00001d039468 | Grx_A2—glutaredoxin subgroup III | |
| Zm00001d023580 | NADP | Aldehyde dehydrogenase, dimeric NADP-preferring |
| Zm00001d045112 | Putative two-component response regulator family protein | |
| Zm00001d042656 | BGAL7 | β-galactosidase 7 |
| Zm00001d020821 | pentatricopeptide repeat-containing protein At1g76280 | |
| Zm00001d027518 | SRP43 | Probable signal recognition particle 43 kDa protein, chloroplastic-like |
| Zm00001d043778 | HCF152 | Pentatricopeptide repeat-containing protein At3g09650, chloroplastic |
| Zm00001d001827 | HBS1 | HBS1-like protein |
| Zm00001d027300 | PAIR1 | Protein PAIR1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Lv, L.; Zhao, Y.; Wang, J.; Lu, X.; Zhang, M.; Wang, R.; Zhang, Y.; Guo, X. Using High-Throughput Phenotyping Analysis to Decipher the Phenotypic Components and Genetic Architecture of Maize Seedling Salt Tolerance. Genes 2023, 14, 1771. https://doi.org/10.3390/genes14091771
Guo S, Lv L, Zhao Y, Wang J, Lu X, Zhang M, Wang R, Zhang Y, Guo X. Using High-Throughput Phenotyping Analysis to Decipher the Phenotypic Components and Genetic Architecture of Maize Seedling Salt Tolerance. Genes. 2023; 14(9):1771. https://doi.org/10.3390/genes14091771
Chicago/Turabian StyleGuo, Shangjing, Lujia Lv, Yanxin Zhao, Jinglu Wang, Xianju Lu, Minggang Zhang, Ronghuan Wang, Ying Zhang, and Xinyu Guo. 2023. "Using High-Throughput Phenotyping Analysis to Decipher the Phenotypic Components and Genetic Architecture of Maize Seedling Salt Tolerance" Genes 14, no. 9: 1771. https://doi.org/10.3390/genes14091771
APA StyleGuo, S., Lv, L., Zhao, Y., Wang, J., Lu, X., Zhang, M., Wang, R., Zhang, Y., & Guo, X. (2023). Using High-Throughput Phenotyping Analysis to Decipher the Phenotypic Components and Genetic Architecture of Maize Seedling Salt Tolerance. Genes, 14(9), 1771. https://doi.org/10.3390/genes14091771







