Predicting Patterns of Distant Metastasis in Breast Cancer Patients following Local Regional Therapy Using Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
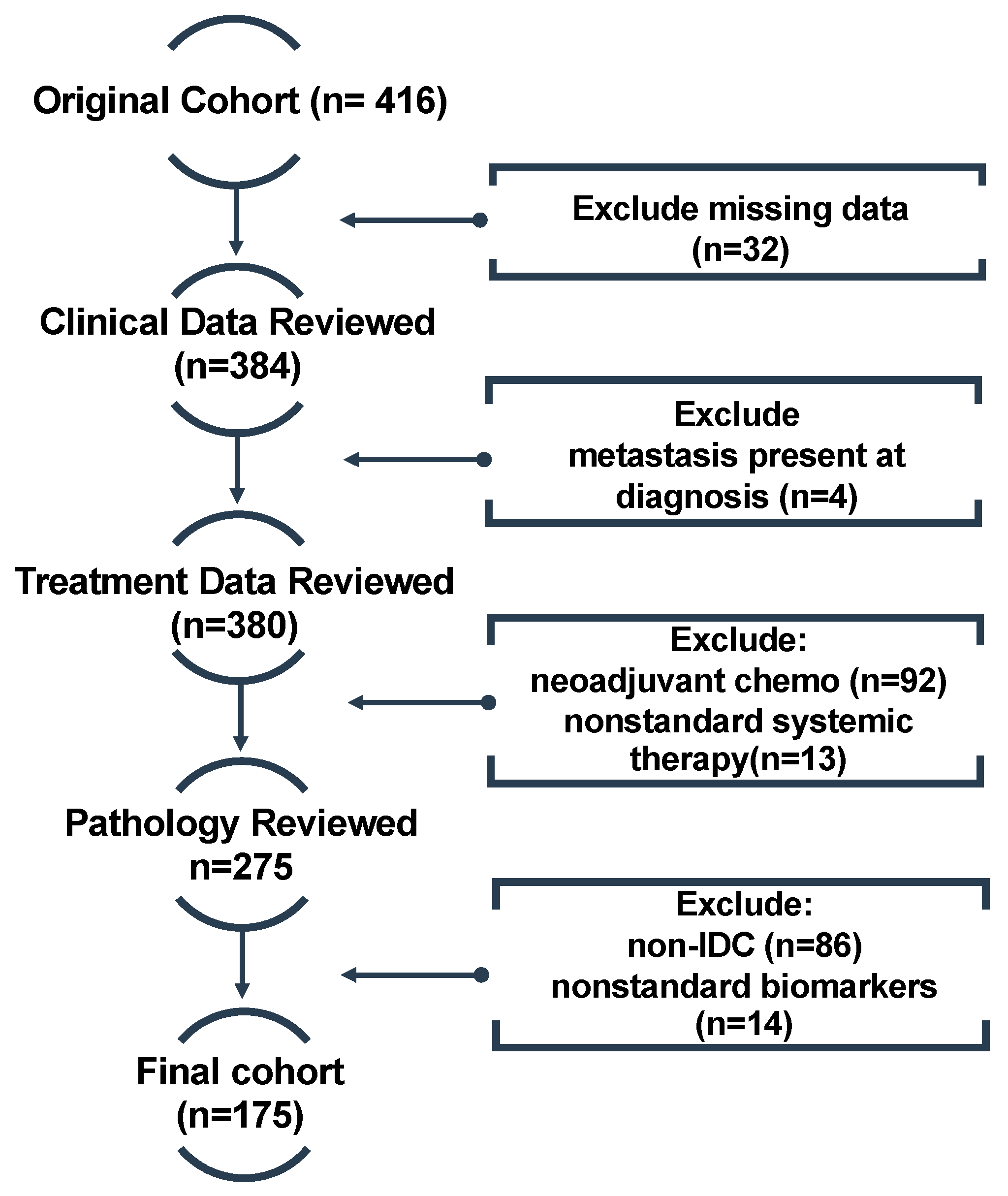
2.1. Cohort and Dataset
2.2. Clinicopathological Variables
2.3. Treatment Characteristics
2.3.1. Clinical Endpoints
2.3.2. Statistical Analyses
2.3.3. Machine Learning Classifiers
3. Results
3.1. Clinicopathological Characteristics
3.2. Outcome Measures
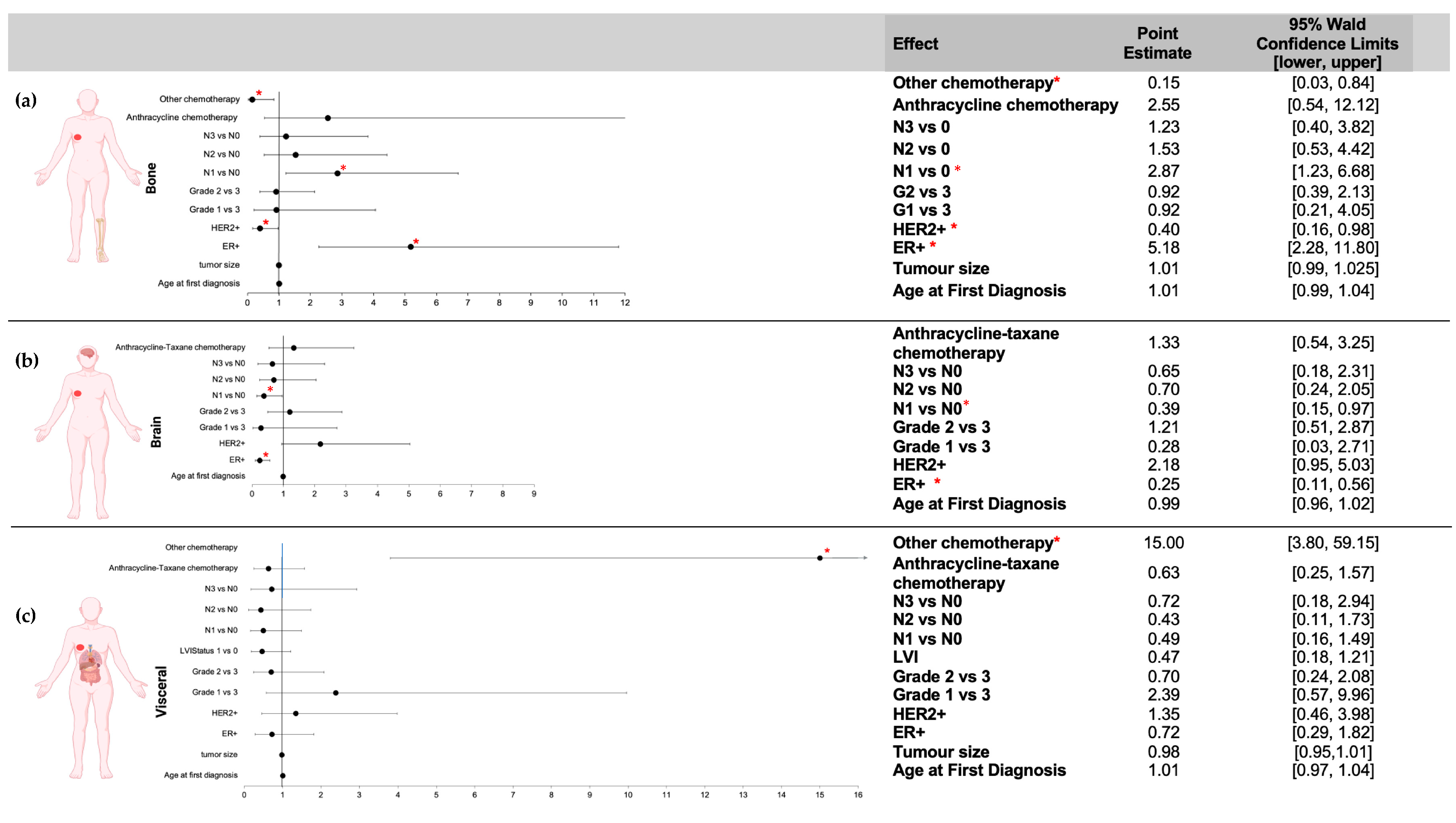
3.2.1. First Site of Distant Metastasis
3.2.2. Time to Distant Metastasis
3.3. Machine Learning Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Shaughnessy, J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncologist 2005, 10, 20–29. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.G.; Dawood, S.; Sopik, V.; Haaland, B.; Tan, P.S.; Bhoo-Pathy, N.; Warner, E.; Iqbal, J.; Narod, S.A.; Dent, R. Ten-year survival in women with primary stage IV breast cancer. Breast Cancer Res. Treat. 2016, 160, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhong, G.; Yu, K.; Lei, K.; Yang, Q. Individualized Prediction of Survival Benefit From Locoregional Surgical Treatment for Patients With Metastatic Breast Cancer. Front. Oncol. 2020, 10, 148. [Google Scholar] [CrossRef]
- Frank, S.; Carton, M.; Dubot, C.; Campone, M.; Pistilli, B.; Dalenc, F.; Mailliez, A.; Levy, C.; D’hondt, V.; Debled, M.; et al. Impact of age at diagnosis of metastatic breast cancer on overall survival in the real-life ESME metastatic breast cancer cohort. Breast 2020, 52, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Dowsett, M.; Sestak, I.; Regan, M.M.; Dodson, A.; Viale, G.; Thürlimann, B.; Colleoni, M.; Cuzick, J. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J. Clin. Oncol. 2018, 36, 1941–1948. [Google Scholar] [CrossRef]
- Purushotham, A.; Shamil, E.; Cariati, M.; Agbaje, O.; Muhidin, A.; Gillett, C.; Mera, A.; Sivanadiyan, K.; Harries, M.; Sullivan, R.; et al. Age at diagnosis and distant metastasis in breast cancer—A surprising inverse relationship. Eur. J. Cancer 2014, 50, 1697–1705. [Google Scholar] [CrossRef]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci. Rep. 2019, 39, BSR20190288. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Baig, A.; Kasymjanova, G.; Kafi, K.; Holcroft, C.; Mekouar, H.; Carbonneau, A.; Bahoric, B.; Sultanem, K.; Muanza, T. Pattern of Local Recurrence and Distant Metastasis in Breast Cancer By Molecular Subtype. Cureus 2016, 8, e924. [Google Scholar] [CrossRef] [PubMed]
- Rueda, O.M.; Sammut, S.-J.; Seoane, J.A.; Chin, S.-F.; Caswell-Jin, J.L.; Callari, M.; Batra, R.; Pereira, B.; Bruna, A.; Ali, H.R.; et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature 2019, 567, 399–404. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, L.; Cai, J.; Wu, H.; Xu, Y.; Lin, Y.; Li, Z.; Chekhonin, V.P.; Peltzer, K.; Cao, M.; et al. Clinicomics-guided distant metastasis prediction in breast cancer via artificial intelligence. BMC Cancer 2023, 23, 239. [Google Scholar] [CrossRef] [PubMed]
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group); McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Wang, Y. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- Gui, P.; Bivona, T.G. Evolution of metastasis: New tools and insights. Trends Cancer 2022, 8, 98–109. [Google Scholar] [CrossRef]
- Nathanson, S.D.; Detmar, M.; Padera, T.P.; Yates, L.R.; Welch, D.R.; Beadnell, T.C.; Scheid, A.D.; Wrenn, E.D.; Cheung, K. Mechanisms of breast cancer metastasis. Clin. Exp. Metastasis 2022, 39, 117–137. [Google Scholar] [CrossRef]
- MacEachern, S.J.; Forkert, N.D. Machine learning for precision medicine. Genome 2021, 64, 416–425. [Google Scholar] [CrossRef]
- Carels, N.; Spinassé, L.B.; Tilli, T.M.; Tuszynski, J.A. Toward precision medicine of breast cancer. Theor. Biol. Med Model. 2016, 13, 7. [Google Scholar] [CrossRef]
- Mathew, A.; Rajagopal, P.S.; Villgran, V.; Sandhu, G.S.; Jankowitz, R.C.; Jacob, M.; Rosenzweig, M.; Oesterreich, S.; Brufsky, A. Distinct Pattern of Metastases in Patients with Invasive Lobular Carcinoma of the Breast. Geburtshilfe Und Frauenheilkd. 2017, 77, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Kalli, S.; Semine, A.; Cohen, S.; Naber, S.P.; Makim, S.S.; Bahl, M. American joint committee on cancer’s staging system for breast cancer, eighth edition: What the radiologist needs to know. Radiographics 2018, 38, 1921–1933. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Voduc, K.D.; Cheang, M.C.U.; Tyldesley, S.; Gelmon, K.; Nielsen, T.O.; Kennecke, H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010, 28, 1684–1691. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS® 9.4 Language Reference: Concepts, Sixth Edition; SAS Institute Inc.: Cary, NC, USA, 2016.
- Putri, D.A.; Kristiyanti, D.A.; Indrayuni, E.; Nurhadi, A.; Hadinata, D.R. Comparison of Naive Bayes Algorithm and Support Vector Machine using PSO Feature Selection for Sentiment Analysis on E-Wallet Review. J. Phy. Conf. Ser. 2020, 1641, 012085. [Google Scholar] [CrossRef]
- Uddin, S.; Khan, A.; Hossain, M.E.; Moni, M.A. Comparing different supervised machine learning algorithms for disease prediction. BMC Med Inform. Decis. Mak. 2019, 19, 281. [Google Scholar] [CrossRef]
- Ramraj, S.; Uzir, N.; Sunil, R.; Banerjee, S. Experimenting XGBoost Algorithm for Prediction and Classification of Different Datasets. Int. J. Control. Theory Appl. 2016, 9, 651–662. [Google Scholar]
- Natekin, A.; Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobotics 2013, 7, 21. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A scalable tree boosting system. In Proceedings of the KDD’16: 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Zeng, X.; Martinez, T.R. Distribution-balanced stratified cross-validation for accuracy estimation. J. Exp. Theor. Artif. Intell. 2000, 12, 681140. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Cohen, M.; Classe, J.; Reyal, F.; Mazouni, C.; Chopin, N.; Martinez, A.; Daraï, E.; Coutant, C.; Colombo, P.; et al. Lymphovascular invasion has a significant prognostic impact in patients with early breast cancer, results from a large, national, multicenter, retrospective cohort study. . ESMO Open 2021, 6, 100316. [Google Scholar] [CrossRef] [PubMed]
- Tabor, S.; Szostakowska-rodzos, M.; Fabisiewicz, A.; Grzybowska, E.A. How to predict metastasis in luminal breast cancer? Current solutions and future prospects. Int. J. Mol. Sci. 2020, 21, 8415. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, V.K. Gradient Boosting Machine BT—Pro Machine Learning Algorithms: A Hands-On Approach to Implementing Algorithms in Python and R. In Pro Machine Learning Algorithms; Apress: Berkeley, CA, USA, 2018. [Google Scholar] [CrossRef]
- Colzani, E.; Johansson, A.L.V.; Liljegren, A.; Foukakis, T.; Clements, M.; Adolfsson, J.; Hall, P.; Czene, K. Time-dependent risk of developing distant metastasis in breast cancer patients according to treatment, age and tumour characteristics. Br. J. Cancer 2014, 110, 1378–1384. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Zhang, J.; Kong, L.; Zhu, H.; Yu, J. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: A SEER based study. Oncotarget 2017, 8, 26368–26379. [Google Scholar] [CrossRef]
- Arciero, C.A.; Guo, Y.; Jiang, R.; Behera, M.; O’regan, R.; Peng, L.; Li, X. ER+/HER2+ Breast Cancer Has Different Metastatic Patterns and Better Survival Than ER−/HER2+ Breast Cancer. Clin. Breast Cancer 2019, 19, 236–245. [Google Scholar] [CrossRef]
- Shouket, T.; Mahmood, S.; Hassan, M.T.; Iftikhar, A. Overall and disease-free survival prediction of postoperative breast cancer patients using machine learning techniques. In Proceedings of the 22nd International Multitopic Conference, INMIC 2019, Islamabad, Pakistan, 29–30 November 2019. [Google Scholar] [CrossRef]
- Kalafi, E.Y.; Nor, N.A.M.; Taib, N.A.; Ganggayah, M.D.; Town, C.; Dhillon, S.K. Machine learning and deep learning approaches in breast cancer survival prediction using clinical data. Folia Biol. 2019, 65, 212–220. [Google Scholar]
- Fu, B.; Liu, P.; Lin, J.; Deng, L.; Hu, K.; Zheng, H. Predicting Invasive Disease-Free Survival for Early Stage Breast Cancer Patients Using Follow-Up Clinical Data. IEEE Trans. Biomed. Eng. 2019, 66, 2053–2064. [Google Scholar] [CrossRef]
- Sun, D.; Li, A.; Tang, B.; Wang, M. Integrating genomic data and pathological images to effectively predict breast cancer clinical outcome. Comput. Methods Programs Biomed. 2018, 161, 45–53. [Google Scholar] [CrossRef]
- Song, B.I. A machine learning-based radiomics model for the prediction of axillary lymph-node metastasis in breast cancer. Breast Cancer 2021, 28, 664–671. [Google Scholar] [CrossRef]
- Tapak, L.; Shirmohammadi-Khorram, N.; Amini, P.; Alafchi, B.; Hamidi, O.; Poorolajal, J. Prediction of survival and metastasis in breast cancer patients using machine learning classifiers. Clin. Epidemiology Glob. Health 2018, 7, 293–299. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2015, 66, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Azarin, S.M.; Yi, J.; Gower, R.M.; Aguado, B.A.; Sullivan, M.E.; Goodman, A.G.; Jiang, E.J.; Rao, S.S.; Ren, Y.; Tucker, S.L.; et al. In vivo capture and label-free detection of early metastatic cells. Nat. Commun. 2015, 6, 8094. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Chen, Z.; Zhu, T.; Wu, J.; Su, F.; Deng, H. Development and validation of a novel nomogram for predicting distant metastasis-free survival among breast cancer patients. Ann. Transl. Med. 2019, 7, 537. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lee, S.-W.; Choi, N.; Kwon, J.; Eom, K.-Y.; Kang, E.; Kim, E.-K.; Kim, J.H.; Kim, Y.J.; Kim, S.H.; et al. A novel prognostic nomogram for predicting risks of distant failure in patients with invasive breast cancer following postoperative adjuvant radiotherapy. Cancer Res. Treat. 2018, 50, 1140–1148. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Xiang, Y.; Guo, K.; Jin, H.; Ruan, S.; Guan, Z. Nomograms for Predicting Specific Distant Metastatic Sites and Overall Survival of Breast Invasive Ductal Carcinoma Patients after Surgery: A Large Population-Based Study. Front. Surg. 2022, 9, 779220. [Google Scholar] [CrossRef]
- Ali, B.; Mubarik, F.; Zahid, N.; Sattar, A.K. Clinicopathologic Features Predictive of Distant Metastasis in Patients Diagnosed with Invasive Breast Cancer. JCO Glob. Oncol. 2020, 6, 1346–1351. [Google Scholar] [CrossRef]
- Ye, L.-J.; Suo, H.-D.; Liang, C.-Y.; Zhang, L.; Jin, Z.-N.; Yu, C.-Z.; Chen, B. Nomogram for predicting the risk of bone metastasis in breast cancer: A SEER population-based study. Transl. Cancer Res. 2020, 9, 6710–6719. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef]
- Van’T Veer, L.J.; Dai, H.; Van De Vijver, M.J.; He, Y.D.; Hart, A.A.M.; Mao, M.; Peterse, H.L.; Van Der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536. [Google Scholar] [CrossRef]
- Filipits, M.; Rudas, M.; Jakesz, R.; Dubsky, P.; Fitzal, F.; Singer, C.F.; Dietze, O.; Greil, R.; Jelen, A.; Sevelda, P.; et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin. Cancer Res. 2011, 17, 6012–6020. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, X.; Huang, Y.; Wang, M.; Cen, C.; Tang, S.; Dique, M.R.; Cai, L.; Luis, M.A.; Smollar, J.; et al. Detection Methods and Clinical Applications of Circulating Tumor Cells in Breast Cancer. Front. Oncol. 2021, 11, 652253. [Google Scholar] [CrossRef] [PubMed]
- Fabisiewicz, A.; Szostakowska-Rodzos, M.; Zaczek, A.J.; Grzybowska, E.A. Circulating tumor cells in early and advanced breast cancer; biology and prognostic value. Int. J. Mol. Sci. 2020, 21, 1671. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M. Tumor markers of breast cancer: New prospectives. J. Oncol. Sci. 2017, 3, 5–11. [Google Scholar] [CrossRef]
- Abdeltawab, A.A.; Ali, S.A.; Mostafa, H.G.; Hassan, M.A. Predictive Factors Increasing the Risk of Radiation Toxicity in Patients with Early Breast Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 145–149. [Google Scholar] [CrossRef]
- De Placido, S.; De Angelis, C.; Giuliano, M.; Pizzi, C.; Ruocco, R.; Perrone, V.; Bruzzese, D.; Tommasielli, G.; De Laurentiis, M.; Cammarota, S.; et al. Imaging tests in staging and surveillance of non-metastatic breast cancer: Changes in routine clinical practice and cost implications. Br. J. Cancer 2017, 116, 821–827. [Google Scholar] [CrossRef]
| Clinicopathological Characteristics | Study Cohort (n = 175) |
|---|---|
| Pre-surgical Characteristics | |
| Age | |
| Mean Age ± SD (years) | 55.6 ± 13.4 |
| 20–49 years | 66 (38%) |
| ≥50 years | 109 (62%) |
| Laterality | |
| Left | 93 (53%) |
| Right | 82 (47%) |
| Surgical Pathology Characteristics | |
| Type of surgery | |
| Lumpectomy | 122 (69%) |
| Mastectomy | 53 (30%) |
| T Stage | |
| Mean size ± SD (mm) | 31.52 ± 18.80 |
| N Stage | |
| N0 | 52 (30%) |
| N1 | 64 (37%) |
| N2 | 36 (21%) |
| N3 | 23 (13%) |
| Nottingham Grade | |
| G1 | 12 (7%) |
| G2 | 56 (32%) |
| G3 | 106 (61%) |
| NA | 1 (1%) |
| Receptor Status | |
| ER+ | 112 (64%) |
| PR+ | 109 (62%) |
| HER2+ | 34 (20%) |
| Subtype | |
| Luminal A | 99 (57%) |
| Luminal B | 20 (11%) |
| HER2-Enriched | 14 (8%) |
| TNBC | 49 (28%) |
| LVI Status | |
| LVI- | 69 (39%) |
| LVI+ | 92 (53%) |
| NA | 14 (8%) |
| Adjuvant Treatments | |
| Chemotherapy (n = 135 (77%)) | |
| Anthracycline backbone alone | 9 (5%) |
| Anthracycline–taxane backbone | 101 (58%) |
| Other | 10 (6%) |
| Unknown | 15 (9%) |
| Endocrine Therapy (n = 103 (59%)) | |
| Aromatase Inhibitors | 43 (25%) |
| Tamoxifen | 47 (27%) |
| Unknown | 13 (7%) |
| Trastuzumab | 23 (13%) |
| Outcome Variables | Study Cohort (n = 175) |
|---|---|
| Sites of Distant Metastasis | |
| Bone Metastasis | 99 (57%) |
| Brain Metastasis | 55 (31%) |
| Visceral Metastasis | 21 (12%) |
| Time to Distant Metastasis | |
| ≤1 year | 22 (13%) |
| >1–≤2 years | 40 (23%) |
| >2–≤3 years | 33 (19%) |
| >3–≤4 years | 21 (12%) |
| >4–≤5 years | 22 (13%) |
| >5 years | 37 (21%) |
| Contrast Estimate Results | |||
|---|---|---|---|
| Label | Incidence Rate Ratio | 95% CI | p-Value |
| Age | 0.99 | [0.99, 0.99] | <0.0001 |
| Tumor size | 0.99 | [0.99, 0.99] | <0.0001 |
| ER+ | 1.98 | [1.96, 2.01] | <0.0001 |
| HER2+ | 1.14 | [1.12, 1.15] | <0.0001 |
| Grade 1 vs. 3 | 1.15 | [1.13, 1.17] | <0.0001 |
| Grade 2 vs. 3 | 1.01 | [1.00, 1.02] | 0.0378 |
| LVI | 1.05 | [1.04, 1.06] | <0.0001 |
| N1 vs. 0 | 0.69 | [0.68, 0.69] | <0.0001 |
| N2 vs. 0 | 0.86 | [0.84, 0.87] | <0.0001 |
| N3 vs. 0 | 0.71 | [0.70, 0.73] | <0.0001 |
| Anthracycline–taxane-based | 1.57 | [1.54, 1.60] | <0.0001 |
| Anthracycline-based | 1.15 | [1.14, 1.17] | <0.0001 |
| Other chemo | 0.83 | [0.81, 0.84] | <0.0001 |
| Visceral | Brain | Skeletal | |
|---|---|---|---|
| Selected features |
|
|
|
| Tr Acc | 0.72 | 0.75 | 0.73 |
| Tr Sens | 0.64 | 0.73 | 0.75 |
| Tr Spec | 0.73 | 0.78 | 0.72 |
| Te Acc | 0.70 | 0.75 | 0.70 |
| Te Sens | 0.60 | 0.71 | 0.72 |
| Te Spec | 0.72 | 0.77 | 0.68 |
| Te AUC | 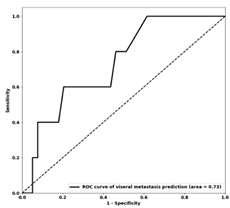 0.73 | 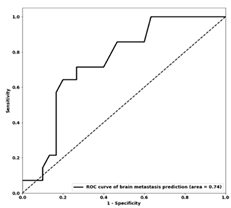 0.74 | 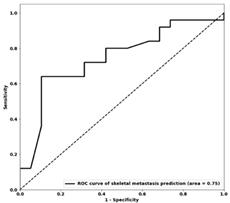 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiner, A.; Kiss, A.; Saednia, K.; Jerzak, K.J.; Gandhi, S.; Lu, F.-I.; Emmenegger, U.; Fleshner, L.; Lagree, A.; Alera, M.A.; et al. Predicting Patterns of Distant Metastasis in Breast Cancer Patients following Local Regional Therapy Using Machine Learning. Genes 2023, 14, 1768. https://doi.org/10.3390/genes14091768
Shiner A, Kiss A, Saednia K, Jerzak KJ, Gandhi S, Lu F-I, Emmenegger U, Fleshner L, Lagree A, Alera MA, et al. Predicting Patterns of Distant Metastasis in Breast Cancer Patients following Local Regional Therapy Using Machine Learning. Genes. 2023; 14(9):1768. https://doi.org/10.3390/genes14091768
Chicago/Turabian StyleShiner, Audrey, Alex Kiss, Khadijeh Saednia, Katarzyna J. Jerzak, Sonal Gandhi, Fang-I Lu, Urban Emmenegger, Lauren Fleshner, Andrew Lagree, Marie Angeli Alera, and et al. 2023. "Predicting Patterns of Distant Metastasis in Breast Cancer Patients following Local Regional Therapy Using Machine Learning" Genes 14, no. 9: 1768. https://doi.org/10.3390/genes14091768
APA StyleShiner, A., Kiss, A., Saednia, K., Jerzak, K. J., Gandhi, S., Lu, F.-I., Emmenegger, U., Fleshner, L., Lagree, A., Alera, M. A., Bielecki, M., Law, E., Law, B., Kam, D., Klein, J., Pinard, C. J., Shenfield, A., Sadeghi-Naini, A., & Tran, W. T. (2023). Predicting Patterns of Distant Metastasis in Breast Cancer Patients following Local Regional Therapy Using Machine Learning. Genes, 14(9), 1768. https://doi.org/10.3390/genes14091768








