Antioxidant Behavioural Phenotype in the Immp2l Gene Knock-Out Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. SDS–PAGE and Western Analysis
2.3. Antioxidant Treatment
2.4. Behavioural Studies
2.4.1. Elevated Plus-Maze
2.4.2. Open Field Test
2.4.3. Dexamphetamine-Induced Hyperlocomotion
2.4.4. Fear Conditioning
2.4.5. Pre-Pulse Inhibition (PPI)
2.4.6. Grooming Behaviour
2.5. Assessment of MitoQ Brain and Kidney Concentration
2.6. Assessment of Mitochondrial Reactive Oxygen Species
2.6.1. Mitochondrial Isolation
2.6.2. Hydrogen Peroxide/Superoxide Production in Isolated Mitochondria
2.7. Electron Microscopy (EM) of the Adult Mouse Brain
2.7.1. Tissue Processing for Electron Microscopy
2.7.2. Field Emission Scanning Electron Microscopy
2.8. AlphaFold2-Multimer High-Accuracy Prediction of Complex Structures
2.9. Statistical Analysis
3. Results
3.1. Immp2lKD −/− KO Mouse Model
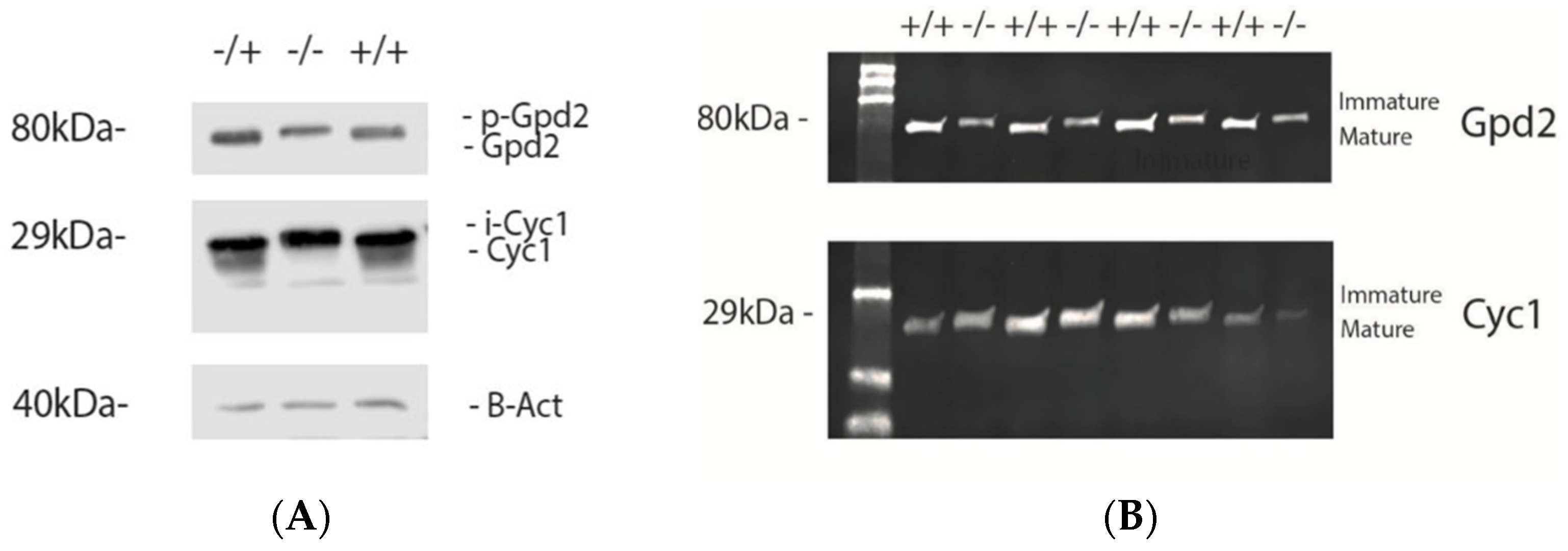
3.2. IMMP2L Activity Absent from Immp2lKD −/− KO Mice
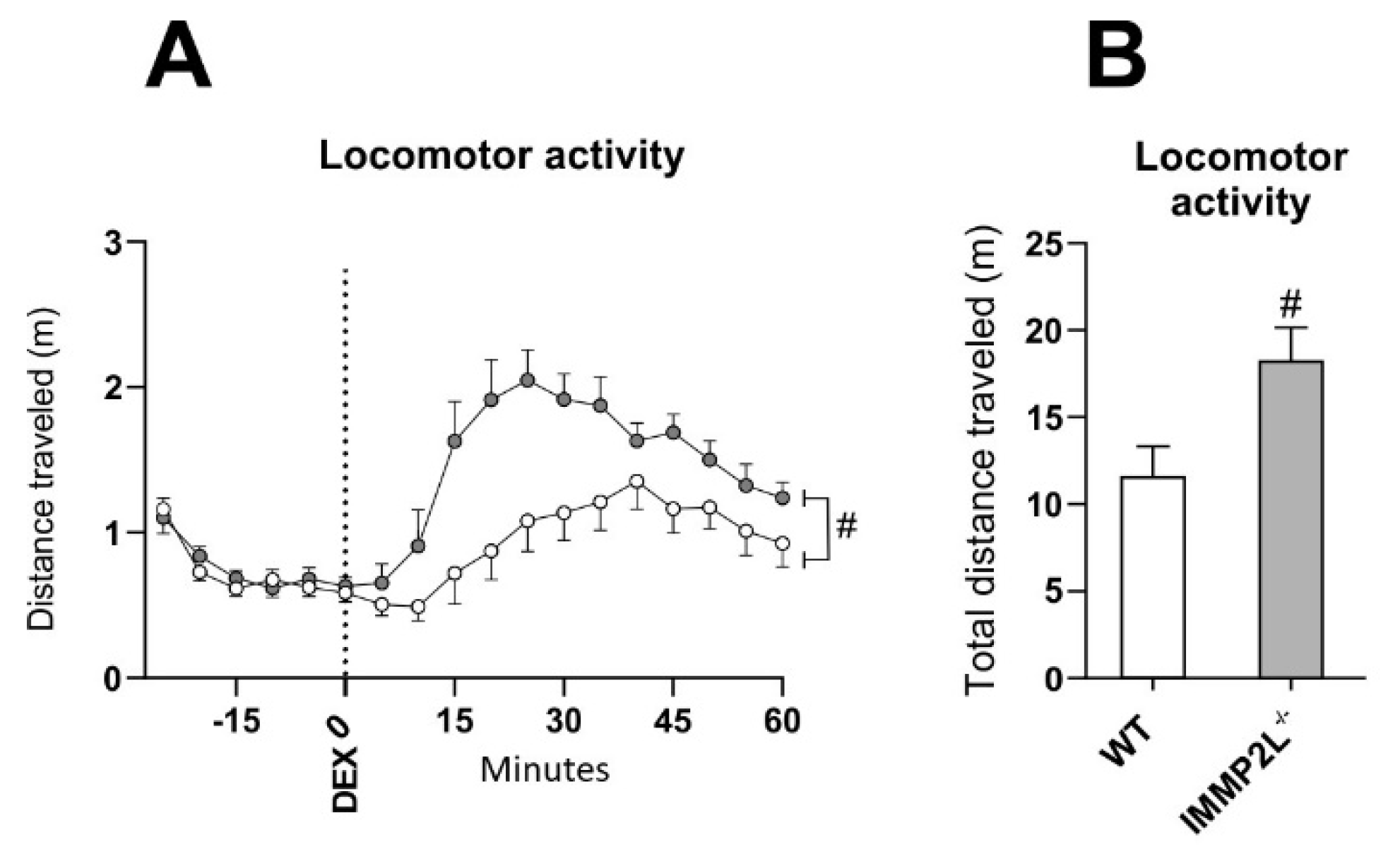
3.3. Immp2lKD −/− KO Enhances Dexamphetamine-Induced Hyperlocomotion
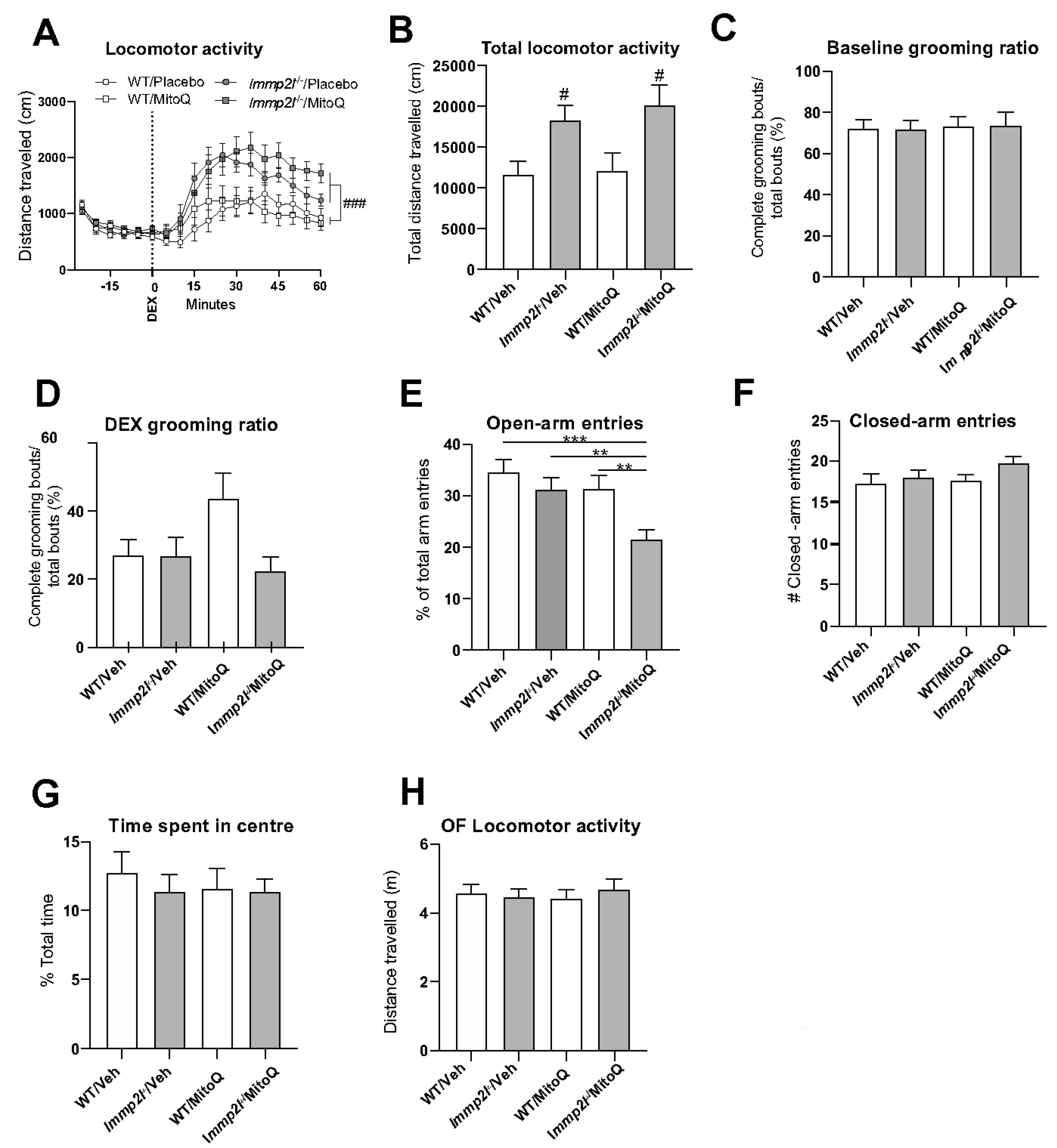
3.4. Antioxidant Treatment Using MitoQ Does Not Reverse the Behavioural Changes Associated with Immp2lKD −/− KO on Dopamine-Mediated Behaviour
3.4.1. MitoQ Is Anxiogenic in Immp2lKD −/− KO Mice in the EPM.
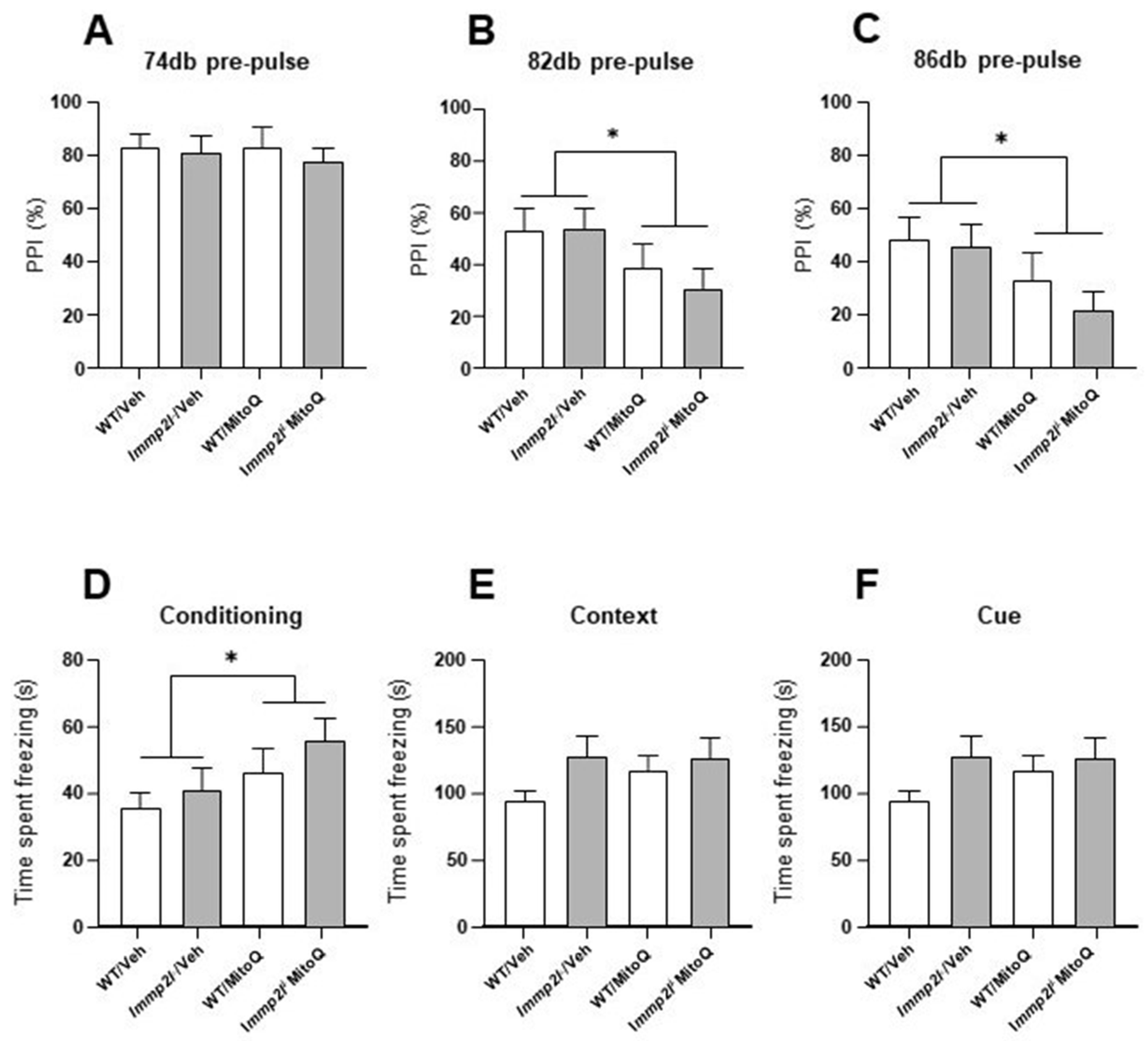
3.4.2. MitoQ Impairs Sensoriomotor Gating Which Is Not Impacted by Immp2l
3.4.3. Fear Conditioned Memory Not Affected by Immp2l KO or Treatment with MitoQ
3.5. Oral Treatment with MitoQ Reaches the Brain
3.6. Immp2lKD −/− KO Did Not Increase Oxidative Stress
3.7. Immp2lKD −/− KO Is Not Associated with Neurodegeneration in the Prefrontal Cortex
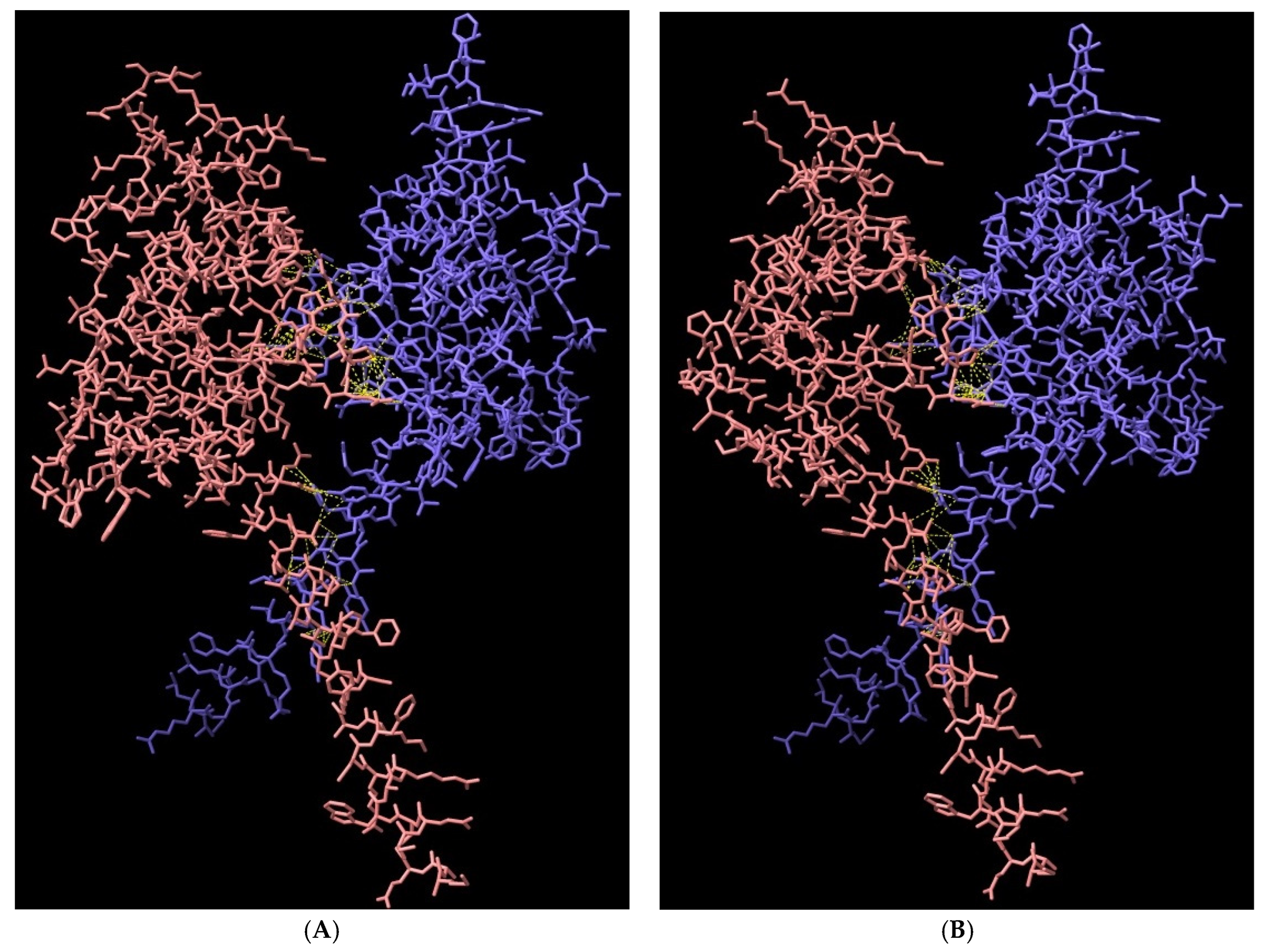
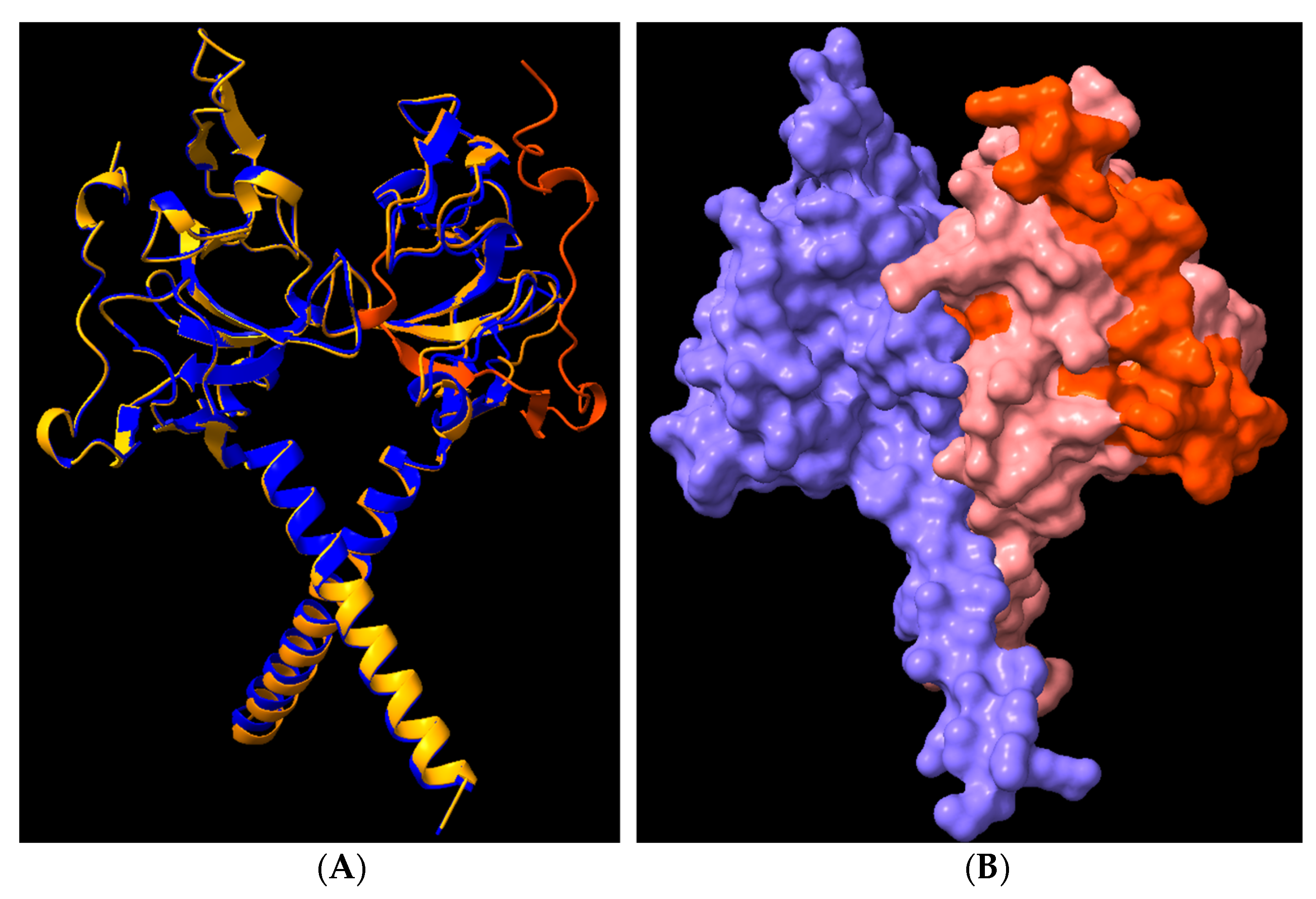
3.8. Structural Analysis of Heterodimerisation between Immp1l and ‘Truncated Immp2l’
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpita, B.; Muti, D.; Dell’Osso, L. Oxidative Stress, Maternal Diabetes, and Autism Spectrum Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 3717215. [Google Scholar] [CrossRef]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; SaravanaBabu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of Oxidative Stress and Antioxidants in Autism. Adv. Neurobiol. 2020, 24, 193–206. [Google Scholar] [PubMed]
- Lu, B.; Poirier, C.; Gaspar, T.; Gratzke, C.; Harrison, W.; Busija, D.; Matzuk, M.M.; Andersson, K.E.; Overbeek, P.A.; Bishop, C.E. A mutation in the inner mitochondrial membrane peptidase 2-like gene (Immp2l) affects mitochondrial function and impairs fertility in mice. Biol. Reprod. 2008, 78, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Hashimoto, T.; Kitaoka, T.; Kyotani, S. Impact of Oxidative Stress and Newer Antiepileptic Drugs on the Albumin and Cortisol Value in Severe Motor and Intellectual Disabilities with Epilepsy. J. Clin. Med. Res. 2018, 10, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015, 19, 915–924. [Google Scholar] [CrossRef]
- Mousavinejad, E.; Ghaffari, M.A.; Riahi, F.; Hajmohammadi, M.; Tiznobeyk, Z.; Mousavinejad, M. Coenzyme Q(10) supplementation reduces oxidative stress and decreases antioxidant enzyme activity in children with autism spectrum disorders. Psychiatry Res. 2018, 265, 62–69. [Google Scholar] [CrossRef]
- Kern, J.K.; Geier, D.A.; King, P.G.; Sykes, L.K.; Mehta, J.A.; Geier, M.R. Shared Brain Connectivity Issues, Symptoms, and Comorbidities in Autism Spectrum Disorder, Attention Deficit/Hyperactivity Disorder, and Tourette Syndrome. Brain Connect. 2015, 5, 321–335. [Google Scholar] [CrossRef]
- Clarke, R.A.; Lee, S.; Eapen, V. Pathogenetic model for Tourette syndrome delineates overlap with related neurodevelopmental disorders including Autism. Transl. Psychiatry 2012, 2, e158. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. A full genome screen for autism with evidence for linkage to a region on chromosome 7q. International Molecular Genetic Study of Autism Consortium. Hum. Mol. Genet. 1998, 7, 571–578. [Google Scholar] [CrossRef]
- Barrett, S.; Beck, J.C.; Bernier, R.; Bisson, E.; Braun, T.A.; Casavant, T.L.; Childress, D.; Folstein, S.E.; Garcia, M.; Gardiner, M.B.; et al. An autosomal genomic screen for autism. Collaborative linkage study of autism. Am. J. Med. Genet. 1999, 88, 609–615. [Google Scholar]
- Shao, Y.; Wolpert, C.; Raiford, K.; Menold, M.; Donnelly, S.; Ravan, S.; Bass, M.; McClain, C.; von Wendt, L.; Vance, J.; et al. Genomic screen and follow-up analysis for autistic disorder. Am. J. Med. Genet. 2002, 114, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, G.D.; Dawson, G.; Sung, Y.J.; Estes, A.; Munson, J.; Rosenthal, E.; Rothstein, J.; Flodman, P.; Smith, M.; Coon, H.; et al. Evidence for multiple loci from a genome scan of autism kindreds. Mol. Psychiatry 2006, 11, 1049–1060, 979. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, E.; Pagnamenta, A.T.; Lamb, J.A.; Bacchelli, E.; Sykes, N.H.; Sousa, I.; Toma, C.; Barnby, G.; Butler, H.; Winchester, L.; et al. High-density SNP association study and copy number variation analysis of the AUTS1 and AUTS5 loci implicate the IMMP2L-DOCK4 gene region in autism susceptibility. Mol. Psychiatry 2010, 15, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Fombonne, E. Pervasive developmental disorders in preschool children: Confirmation of high prevalence. Am. J. Psychiatry 2005, 162, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Fombonne, E. Epidemiology of autistic disorder and other pervasive developmental disorders. J. Clin. Psychiatry 2005, 66 (Suppl. S10), 3–8. [Google Scholar]
- Baird, G.; Simonoff, E.; Pickles, A.; Chandler, S.; Loucas, T.; Meldrum, D.; Charman, T. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The Special Needs and Autism Project (SNAP). Lancet 2006, 368, 210–215. [Google Scholar] [CrossRef]
- Ashley-Koch, A.; Wolpert, C.M.; Menold, M.M.; Zaeem, L.; Basu, S.; Donnelly, S.L.; Ravan, S.A.; Powell, C.M.; Qumsiyeh, M.B.; Aylsworth, A.S.; et al. Genetic studies of autistic disorder and chromosome 7. Genomics 1999, 61, 227–236. [Google Scholar] [CrossRef]
- International Molecular Genetic Study of Autism Consortium (IMGSAC). A genomewide screen for autism: Strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am. J. Hum. Genet. 2001, 69, 570–581. [Google Scholar] [CrossRef]
- Auranen, M.; Vanhala, R.; Varilo, T.; Ayers, K.; Kempas, E.; Ylisaukko-Oja, T.; Sinsheimer, J.S.; Peltonen, L.; Järvelä, I. A genomewide screen for autism-spectrum disorders: Evidence for a major susceptibility locus on chromosome 3q25-27. Am. J. Hum. Genet. 2002, 71, 777–790. [Google Scholar] [CrossRef]
- Lamb, J.A.; Barnby, G.; Bonora, E.; Sykes, N.; Bacchelli, E.; Blasi, F.; Maestrini, E.; Broxholme, J.; Tzenova, J.; Weeks, D.; et al. Analysis of IMGSAC autism susceptibility loci: Evidence for sex limited and parent of origin specific effects. J. Med. Genet. 2005, 42, 132–137. [Google Scholar] [CrossRef]
- Buxbaum, J.D.; Silverman, J.M.; Smith, C.J.; Kilifarski, M.; Reichert, J.; Hollander, E.; Lawlor, B.A.; Fitzgerald, M.; Greenberg, D.A.; Davis, K.L. Evidence for a susceptibility gene for autism on chromosome 2 and for genetic heterogeneity. Am. J. Hum. Genet. 2001, 68, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Badner, J.A.; Gershon, E.S. Regional meta-analysis of published data supports linkage of autism with markers on chromosome 7. Mol. Psychiatry 2002, 7, 56–66. [Google Scholar] [CrossRef]
- Casey, J.P.; Magalhaes, T.; Conroy, J.M.; Regan, R.; Shah, N.; Anney, R.; Shields, D.C.; Abrahams, B.S.; Almeida, J.; Bacchelli, E.; et al. A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum. Genet. 2012, 131, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Anzaldúa, A.; Joober, R.; Rivière, J.-B.; Dion, Y.; Lespérance, P.; Chouinard, S.; Richer, F.; Rouleau, G.A.; Montreal Tourette Syndrome Study Group. Association between 7q31 markers and Tourette syndrome. Am. J. Med. Genet. A 2004, 127a, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, X.; Li, Y.; Ni, Y.; Xue, L. Identification of methylation markers for diagnosis of autism spectrum disorder. Metab. Brain Dis. 2022, 37, 219–228. [Google Scholar] [CrossRef]
- Trikalinos, T.A.; Karvouni, A.; Zintzaras, E.; Ylisaukko-oja, T.; Peltonen, L.; Järvelä, I.; Ioannidis, J.P.A. A heterogeneity-based genome search meta-analysis for autism-spectrum disorders. Mol. Psychiatry 2006, 11, 29–36. [Google Scholar] [CrossRef][Green Version]
- Pagliaroli, L.; Vereczkei, A.; Padmanabhuni, S.S.; Tarnok, Z.; Farkas, L.; Nagy, P.; Rizzo, R.; Wolanczyk, T.; Szymanska, U.; Kapisyzi, M.; et al. Association of Genetic Variation in the 3’UTR of LHX6, IMMP2L, and AADAC With Tourette Syndrome. Front. Neurol. 2020, 11, 803. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Lei, B.; Xu, X.; Lu, B. Mitochondria-targeted antioxidant SkQ1 improves spermatogenesis in Immp2l mutant mice. Andrologia 2018, 50, e12848. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Lu, B. The Immp2l mutation causes age-dependent degeneration of cerebellar granule neurons prevented by antioxidant treatment. Aging Cell 2016, 15, 167–176. [Google Scholar] [CrossRef][Green Version]
- Tauskela, J.S. MitoQ—A mitochondria-targeted antioxidant. IDrugs 2007, 10, 399–412. [Google Scholar]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochemistry 2008, 73, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Barahona, A.; Alonso-Barroso, E.; Pérez, B.; Murphy, M.P.; Richard, E.; Desviat, L.R. Treatment with antioxidants ameliorates oxidative damage in a mouse model of propionic acidemia. Mol. Genet. Metab. 2017, 122, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chan, Y.L.; Sukjamnong, S.; Anwer, A.G.; Vindin, H.; Padula, M.; Zakarya, R.; George, J.; Oliver, B.G.; Saad, S.; et al. A Mitochondrial Specific Antioxidant Reverses Metabolic Dysfunction and Fatty Liver Induced by Maternal Cigarette Smoke in Mice. Nutrients 2019, 11, 1669. [Google Scholar] [CrossRef]
- Sukjamnong, S.; Chan, Y.L.; Zakarya, R.; Nguyen, L.T.; Anwer, A.G.; Zaky, A.A.; Santiyanont, R.; Oliver, B.G.; Goldys, E.; Pollock, C.A.; et al. MitoQ supplementation prevent long-term impact of maternal smoking on renal development, oxidative stress and mitochondrial density in male mice offspring. Sci. Rep. 2018, 8, 6631. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Eapen, V.; Singer, H.S.; Martino, D.; Scharf, J.M.; Paschou, P.; Roessner, V.; Woods, D.W.; Hariz, M.; Mathews, C.A.; et al. Gilles de la Tourette syndrome. Nat. Rev. Dis. Primers 2017, 3, 16097. [Google Scholar] [CrossRef]
- Gabriels, R.L.; Cuccaro, M.L.; Hill, D.E.; Ivers, B.J.; Goldson, E. Repetitive behaviors in autism: Relationships with associated clinical features. Res. Dev. Disabil. 2005, 26, 169–181. [Google Scholar] [CrossRef]
- Leblond, C.S.; Cliquet, F.; Carton, C.; Huguet, G.; Mathieu, A.; Kergrohen, T.; Buratti, J.; Lemière, N.; Cuisset, L.; Bienvenu, T.; et al. Both rare and common genetic variants contribute to autism in the Faroe Islands. NPJ Genom. Med. 2019, 4, 1. [Google Scholar] [CrossRef]
- Baldan, F.; Gnan, C.; Franzoni, A.; Ferino, L.; Allegri, L.; Passon, N.; Damante, G. Genomic Deletion Involving the IMMP2L Gene in Two Cases of Autism Spectrum Disorder. Cytogenet. Genome Res. 2018, 154, 196–200. [Google Scholar] [CrossRef]
- Pagnamenta, A.T.; Bacchelli, E.; de Jonge, M.V.; Mirza, G.; Scerri, T.S.; Minopoli, F.; Chiocchetti, A.; Ludwig, K.U.; Hoffmann, P.; Paracchini, S.; et al. Characterization of a family with rare deletions in CNTNAP5 and DOCK4 suggests novel risk loci for autism and dyslexia. Biol. Psychiatry 2010, 68, 320–328. [Google Scholar] [CrossRef]
- Bertelsen, B.; Melchior, L.; Jensen, L.R.; Growth, C.; Glenthøj, B.; Rizzo, R.; Debes, N.M.; Skov, L.; Brøndum-Nielsen, K.; Paschou, P.; et al. Intragenic deletions affecting two alternative transcripts of the IMMP2L gene in patients with Tourette syndrome. Eur. J. Hum. Genet. 2014, 22, 1283–1289. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zarrei, M.; Tong, W.; Dong, R.; Wang, Y.; Zhang, H.; Yang, X.; MacDonald, J.R.; Uddin, M.; et al. Association of IMMP2L deletions with autism spectrum disorder: A trio family study and meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Gimelli, S.; Capra, V.; Di Rocco, M.; Leoni, M.; Mirabelli-Badenier, M.; Schiaffino, M.C.; Fiorio, P.; Cuoco, C.; Gimelli, G.; Tassano, E. Interstitial 7q31.1 copy number variations disrupting IMMP2L gene are associated with a wide spectrum of neurodevelopmental disorders. Mol. Cytogenet. 2014, 7, 54. [Google Scholar] [CrossRef]
- Jang, W.; Kim, Y.; Han, E.; Park, J.; Chae, H.; Kwon, A.; Choi, H.; Kim, J.; Son, J.O.; Lee, S.J.; et al. Chromosomal Microarray Analysis as a First-Tier Clinical Diagnostic Test in Patients with Developmental Delay/Intellectual Disability, Autism Spectrum Disorders, and Multiple Congenital Anomalies: A Prospective Multicenter Study in Korea. Ann. Lab. Med. 2019, 39, 299–310. [Google Scholar] [CrossRef]
- Elia, J.; Gai, X.; Xie, H.M.; Perin, J.C.; Geiger, E.; Glessner, J.T.; D′arcy, M.; Deberardinis, R.; Frackelton, E.; Kim, C.; et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol. Psychiatry 2010, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Viñas-Jornet, M.; Esteba-Castillo, S.; Baena, N.; Ribas-Vidal, N.; Ruiz, A.; Torrents-Rodas, D.; Gabau, E.; Vilella, E.; Martorell, L.; Armengol, L.; et al. High Incidence of Copy Number Variants in Adults with Intellectual Disability and Co-morbid Psychiatric Disorders. Behav. Genet. 2018, 48, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Qaiser, F.; Yin, Y.; Mervis, C.B.; Morris, C.A.; Klein-Tasman, B.P.; Tam, E.; Osborne, L.R.; Yuen, R.K. Rare and low frequency genomic variants impacting neuronal functions modify the Dup7q11.23 phenotype. Orphanet J. Rare Dis. 2021, 16, 6. [Google Scholar] [CrossRef]
- Clarke, R.A.; Furlong, T.M.; Eapen, V. Tourette Syndrome Risk Genes Regulate Mitochondrial Dynamics, Structure, and Function. Front. Psychiatry 2020, 11, 556803. [Google Scholar] [CrossRef]
- Boghosian-Sell, L.; Comings, D.E.; Overhauser, J. Tourette syndrome in a pedigree with a 7;18 translocation: Identification of a YAC spanning the translocation breakpoint at 18q22.3. Am. J. Hum. Genet. 1996, 59, 999–1005. [Google Scholar] [PubMed]
- Petek, E.; Windpassinger, C.; Vincent, J.B.; Cheung, J.; Boright, A.P.; Scherer, S.W.; Kroisel, P.M.; Wagner, K. Disruption of a novel gene (IMMP2L) by a breakpoint in 7q31 associated with Tourette syndrome. Am. J. Hum. Genet. 2001, 68, 848–858. [Google Scholar] [CrossRef]
- Patel, C.; Cooper-Charles, L.; McMullan, D.J.; Walker, J.M.; Davison, V.; Morton, J. Translocation breakpoint at 7q31 associated with tics: Further evidence for IMMP2L as a candidate gene for Tourette syndrome. Eur. J. Hum. Genet. 2011, 19, 634–639. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.M.; Eapen, V.; Clarke, R.A. CTNNA3 discordant regulation of nested LRRTM3, implications for autism spectrum disorder and Tourette syndrome. Meta Gene 2017, 11, 43–48. [Google Scholar] [CrossRef]
- Kreilaus, F.; Chesworth, R.; Eapen, V.; Clarke, R.; Karl, T. First behavioural assessment of a novel Immp2l knockdown mouse model with relevance for Gilles de la Tourette syndrome and Autism spectrum disorder. Behav. Brain Res. 2019, 374, 112057. [Google Scholar] [CrossRef]
- Leung, B.K.; Merlin, S.; Walker, A.K.; Lawther, A.J.; Paxinos, G.; Eapen, V.; Clarke, R.; Balleine, B.W.; Furlong, T.M. Immp2l knockdown in male mice increases stimulus-driven instrumental behaviour but does not alter goal-directed learning or neuron density in cortico-striatal circuits in a model of Tourette syndrome and autism spectrum disorder. Behav. Brain Res. 2023, 452, 114610. [Google Scholar] [CrossRef]
- George, S.K.; Jiao, Y.; Bishop, C.E.; Lu, B. Oxidative stress is involved in age-dependent spermatogenic damage of Immp2l mutant mice. Free Radic. Biol. Med. 2012, 52, 2223–2233. [Google Scholar] [CrossRef][Green Version]
- Ma, Y.; Mehta, S.L.; Lu, B.; Li, P.A. Deficiency in the inner mitochondrial membrane peptidase 2-like (Immp21) gene increases ischemic brain damage and impairs mitochondrial function. Neurobiol. Dis. 2011, 44, 270–276. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.; Chen, Z.; Ma, N.; Sun, S.; Zhang, J.; Ni, X.; Zhang, J.; Li, P.A. Suppression of Inner Mitochondrial Membrane Peptidase 2-Like (IMMP2L) Gene Exacerbates Hypoxia-Induced Neural Death Under High Glucose Condition. Neurochem. Res. 2017, 42, 1504–1514. [Google Scholar] [CrossRef]
- He, Q.; Gu, L.; Lin, Q.; Ma, Y.; Liu, C.; Pei, X.; Li, P.A.; Yang, Y. The Immp2l Mutation Causes Ovarian Aging Through ROS-Wnt/β-Catenin-Estrogen Pathway: Preventive Effect of Melatonin. Endocrinology 2020, 161, bqaa119. [Google Scholar] [CrossRef]
- George, S.K.; Jiao, Y.; Bishop, C.E.; Lu, B. Mitochondrial peptidase IMMP2L mutation causes early onset of age-associated disorders and impairs adult stem cell self-renewal. Aging Cell 2011, 10, 584–594. [Google Scholar] [CrossRef]
- Soler, R.; Füllhase, C.; Lu, B.; Bishop, C.E.; Andersson, K.E. Bladder dysfunction in a new mutant mouse model with increased superoxide—Lack of nitric oxide? J. Urol. 2010, 183, 780–785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, C.; Zhao, Q.; Lu, B. The role of nitric oxide signaling in food intake; insights from the inner mitochondrial membrane peptidase 2 mutant mice. Redox. Biol. 2013, 1, 498–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guimarães-Souza, N.K.; Yamaleyeva, L.M.; Lu, B.; Ramos, A.C.M.D.S.; Bishop, C.E.; Andersson, K.E. Superoxide overproduction and kidney fibrosis: A new animal model. Einstein 2015, 13, 79–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, Y.; Liang, R.M.; Ma, N.; Mi, X.J.; Cheng, Z.Y.; Zhang, Z.J.; Lu, B.S.; Li, P.A. Immp2l Mutation Induces Mitochondrial Membrane Depolarization and Complex III Activity Suppression after Middle Cerebral Artery Occlusion in Mice. Curr. Med. Sci. 2023, 43, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Mi, X.; Zhang, Z.; Ma, Y. IMMP2L gene mutation activates mitochondrial apoptotic pathway to aggravate cerebral ischemic injury in mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2021, 37, 616–622. [Google Scholar]
- Liu, C.; Gu, J.; Ma, W.; Zhang, Q.; Song, M.; Ha, L.; Xu, X.; Jiao, H.; Huo, Z. Lycium barbarum polysaccharide protects against ethanol-induced spermiotoxicity and testicular degeneration in Immp2l(+/−) mice. Andrologia 2020, 52, e13554. [Google Scholar] [CrossRef]
- Liu, C.L.; Zhang, Q.; Zhang, S.-H.; Mu, C.-L.; Yao, P.; Jiao, H.-Y.; Xu, X.; Huo, Z.-H. Lycium barbarum polysaccharide reduces testicular spermatogenic injury in Immp2l−/−mice through GPX4 and AIF pathways. Zhonghua Nan Ke Xue 2021, 27, 387–393. [Google Scholar]
- Bharadwaj, M.S.; Zhou, Y.; Molina, A.J.; Criswell, T.; Lu, B. Examination of bioenergetic function in the inner mitochondrial membrane peptidase 2-like (Immp2l) mutant mice. Redox. Biol. 2014, 2, 1008–1015. [Google Scholar] [CrossRef]
- Sun, F. Commentary on “The Immp2l Mutation Causes Ovarian Aging Through ROS-Wnt/beta-Catenin-Estrogen Pathway: Preventive Effect of Melatonin”. Endocrinology 2020, 161, bqaa197. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Y.; Chen, H.; Yao, J.; Lv, L.; Li, Y.; Deng, C.; Zhang, M.; Sun, X.; Liu, G. Guilingji Protects Against Spermatogenesis Dysfunction from Oxidative Stress via Regulation of MAPK and Apoptotic Signaling Pathways in Immp2l Mutant Mice. Front. Pharmacol. 2021, 12, 771161. [Google Scholar] [CrossRef]
- Escalier, D. Knockout mice in the service of reproduction. Gynecol. Obstet. Fertil. 2008, 36, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Fang, H.; Green, N. Substrate specificity of inner membrane peptidase in yeast mitochondria. Mol. Genet. Genom. 2006, 275, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Riddick, N.V.; Nikolova, V.D.; Teng, B.L.; Agster, K.L.; Nonneman, R.J.; Young, N.B.; Baker, L.K.; Nadler, J.J.; Bodfish, J.W. Repetitive behavior profile and supersensitivity to amphetamine in the C58/J mouse model of autism. Behav. Brain Res. 2014, 259, 200–214. [Google Scholar] [CrossRef]
- Berridge, K.C.; Aldridge, J.W.; Houchard, K.R.; Zhuang, X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: A model of obsessive compulsive disorder and Tourette’s. BMC Biol. 2005, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Aldridge, J.W.; LaPorte, J.L.; Murphy, D.L.; Tuohimaa, P. Analyzing grooming microstructure in neurobehavioral experiments. Nat. Protoc. 2007, 2, 2538–2544. [Google Scholar] [CrossRef]
- Bentley, N.L.; Fiveash, C.E.; Osborne, B.; Quek, L.E.; Ogura, M.; Inagaki, N.; Cooney, G.J.; Polly, P.; Montgomery, M.K.; Turner, N. Protein hypoacylation induced by Sirt5 overexpression has minimal metabolic effect in mice. Biochem. Biophys. Res. Commun. 2018, 503, 1349–1355. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Osborne, B.; Brandon, A.E.; O′Reilly, L.; Fiveash, C.E.; Brown, S.H.; Wilkins, B.P.; Samsudeen, A.; Yu, J.; Devanapalli, B.; et al. Regulation of mitochondrial metabolism in murine skeletal muscle by the medium-chain fatty acid receptor Gpr84. FASEB J. 2019, 33, 12264–12276. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Osborne, B.; Brown, S.H.J.; Small, L.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. J. Lipid Res. 2013, 54, 3322–3333. [Google Scholar] [CrossRef]
- Cohen Hyams, T.; Mam, K.; Killingsworth, M.C. Scanning electron microscopy as a new tool for diagnostic pathology and cell biology. Micron 2020, 130, 102797. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Elfmann, C.; Stulke, J. PAE viewer: A webserver for the interactive visualization of the predicted aligned error for multimer structure predictions and crosslinks. Nucleic Acids Res. 2023, 51, W404–W410. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Fox, T.D.; Walter, P. A mitochondrial protease with two catalytic subunits of nonoverlapping specificities. Science 1993, 262, 1997–2004. [Google Scholar] [CrossRef]
- Escobar, A.P.; Martínez-Pinto, J.; Silva-Olivares, F.; Sotomayor-Zárate, R.; Moya, P.R. Altered Grooming Syntax and Amphetamine-Induced Dopamine Release in EAAT3 Overexpressing Mice. Front. Cell. Neurosci. 2021, 15, 661478. [Google Scholar] [CrossRef] [PubMed]
- Ng-Cordell, E.; Wardell, V.; Stewardson, C.; Kerns, C.M. Anxiety and Trauma-Related Disorders in Children on the Autism Spectrum. Curr. Psychiatry Rep. 2022, 24, 171–180. [Google Scholar] [CrossRef]
- Perry, W.; Minassian, A.; Lopez, B.; Maron, L.; Lincoln, A. Sensorimotor Gating Deficits in Adults with Autism. Biol. Psychiatry 2007, 61, 482–486. [Google Scholar] [CrossRef]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef]
- Gakh, O.; Cavadini, P.; Isaya, G. Mitochondrial processing peptidases. Biochim. Biophys. Acta 2002, 1592, 63–77. [Google Scholar] [CrossRef]
- Schneider, A.; Oppliger, W.; Jeno, P. Purified inner membrane protease I of yeast mitochondria is a heterodimer. J. Biol. Chem. 1994, 269, 8635–8638. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Strahm, Y.; Hawkins, C.J.; Gentle, I.E.; Puryer, M.A.; Verhagen, A.; Callus, B.; Vaux, D.; Lithgow, T. Mature DIABLO/Smac is produced by the IMP protease complex on the mitochondrial inner membrane. Mol. Biol. Cell 2005, 16, 2926–2933. [Google Scholar] [CrossRef]
- Shalom, D.B. The medial prefrontal cortex and integration in autism. Neuroscientist 2009, 15, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Loh, M.; Deco, G.; Winterer, G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat. Rev. Neurosci. 2008, 9, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.L.; Breier, M.R.; Yang, A.; Swerdlow, N.R. Disparate effects of pramipexole on locomotor activity and sensorimotor gating in Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2011, 99, 634–638. [Google Scholar] [CrossRef]
- Chen, A.P.F.; Chen, L.; Kim, T.A.; Xiong, Q. Integrating the Roles of Midbrain Dopamine Circuits in Behavior and Neuropsychiatric Disease. Biomedicines 2021, 9, 647. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Geyer, M.A.; Braff, D.L. Neural circuit regulation of prepulse inhibition of startle in the rat: Current knowledge and future challenges. Psychopharmacology 2001, 156, 194–215. [Google Scholar] [CrossRef]
- Staton, D.M.; Solomon, P.R. Microinjections of d-amphetamine into the nucleus accumbens and caudate-putamen differentially affect stereotypy and locomotion in the rat. Physiol. Psychol. 1984, 12, 159–162. [Google Scholar] [CrossRef][Green Version]
- Shafiq, S.; Pringsheim, T. Using antipsychotics for behavioral problems in children. Expert Opin. Pharmacother. 2018, 19, 1475–1488. [Google Scholar] [CrossRef]
- Zegers-Delgado, J.; Blanlot, C.; Calderon, F.; Yarur, H.E.; Novoa, J.; Vega-Quiroga, I.; Bastias, C.P.; Gysling, K. Reactive oxygen species modulate locomotor activity and dopamine extracellular levels induced by amphetamine in rats. Behav. Brain Res. 2022, 427, 113857. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.-L.; Zou, M.-Y.; Wang, H.; Zhou, X.; Sun, C.-H.; Xia, W.; Wu, L.-J.; Fujisawa, T.X.; Tomoda, A. Family-based association study of ZNF533, DOCK4 and IMMP2L gene polymorphisms linked to autism in a northeastern Chinese Han population. J. Zhejiang Univ. Sci. B 2014, 15, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, V.A.; Schönewolf-Greulich, B.; Juel Rasmussen, L.; Desler, C.; Tümer, Z. Mitochondrial Function in Gilles de la Tourette Syndrome Patients with and Without Intragenic IMMP2L Deletions. Front. Neurol. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]




| Association | Locus | Reference |
|---|---|---|
| GWAS | 7q31 | IMGSAC 1998 [9] |
| GWAS | 7q31 | Ashley-Kock et al., 1999 [17] |
| GWAS | 7q31 | Barrett et al., 1999 [10] |
| GWAS | 7q31 | MGSAC 2001 [18] |
| GWAS | 7q31 | Shao et al., 2002 [11] |
| GWAS | 7q31 | Auranen et al., 2002 [19] |
| GWAS | 7q31 | Lamb et al., 2005 [20] |
| GWAS | 7q31 | Schellenberg et al., 2006 [12] |
| Meta-analysis | 7q31 (4 ASD GWAS) | Buxbaum et al., 2001 [21] |
| Meta-analysis | 7q31 (9 ASD GWAS) | Badner et al., 2002 [22] |
| Multiplex | 7q31 | Trikalinos et al., 2006 [26] |
| High Density Analysis | IMMP2L SNP rs12537269 | Maestrini et al., (IMGSAC) [13] |
| Haplotype Sharing | IMMP2L | Casey et al., [23] |
| e-TDT SNP Analysis | IMMP2L in GTS SNP D7S1516 | Diaz-Anzaldua et al., 2004 [24] |
| e-TDT SNP Analysis | IMMP2L in GTS SNP rs7795011 | Pagliaroli et al., 2020 [27] |
| Methylation analysis | IMMP2L | Zhang et al., 2022 [25] |
| Study | Deletion | Gender | Phenotypes |
|---|---|---|---|
| Maestrini [13] | Exonic | Male 13-0577-003 | ASD |
| Exonic | Male 15-0086-003 | ASD | |
| Maestrini and Pagnamenta [13,39] | Exonic | Male 15-0084 | ASD |
| Maestrini [13] | IMMP2L Linkage | Paternal | ASD |
| Bertelsen et al., 2014 [40] | Exonic | Male | ASD, GTS, ADHD, Asperger |
| Zhang et al., 2017 [41] | Exonic | Male | ASD, Speech Delay, Echo, MR |
| Exonic | Male | ASD, Speech Delay, Echo, MR | |
| Exonic | Male | ASD, Speech Delay, Echo MR | |
| Gimelli et al., 2014 [42] | Exonic | Male | Autistic, Speech Delay, ADHD |
| Baldan 2018 [38] | Intronic | Male | ASD, Speech Delay |
| Exonic | Male | ASD, Speech Delay | |
| Leblond et al., 2019 [37] | Exonic | - | ASD |
| Qaiser et al., 2021 [46] | Exonic | - | ASD |
| Vinas-Jornet 2018 [45] | Exonic | - | ASD |
| ASD Overlap Disorders | |||
| Jang et al., 2019 [43] | 3 × Exonic | - | Speech Delay, MR, Dev Delay |
| Gimelli et al., 2014 [42] | Exonic | Male | Speech Delay |
| Exonic | Male | Speech Delay | |
| Exonic | Female | Unaffected | |
| Bertelsen et al., 2014 [40] | 6 × Exonic | Males x 6 | GTS, ADHD/OCD |
| Elia et al., 2010 [44] | Exonic | Male | ADHD |
| Study | Type Association | Phenotypes | Gender | Family Phenotypes |
|---|---|---|---|---|
| Cytogenetic | Near Breakpoint | GTS, Temper | Male | Tics and OCD [48] |
| Cytogenetic | Disrupted | GTS | Male | [49] |
| CNV/LOH | IMMP2L Deleted | GTS, Speech delay | Male | [50] |
| + FOXP2 Deleted | + Verbal Dyspraxia | |||
| CNV/LOH | Exonic Deletion | GTS, ADHD | Male | Dyslexia, Temper [40] |
| CNV/LOH | Exonic Deletion | GTS, ADHD | Male | Tics and OCB [40] |
| CNV/LOH | Exonic Deletion | GTS, ADHD, Asperger | Male | Unaffected [40] |
| CNV/LOH | Exonic Deletion | GTS, OCD | Male | OCB, Stuttering [40] |
| CNV/LOH | Exonic Deletion | GTS | Male | Unaffected [40] |
| CNV/LOH | Exonic Deletion | GTS, ADHD, OCD | Male | Stubbornness [40] |
| CNV/LOH | Exonic Deletion | GTS, ADHD | Male | Unaffected [40] |
| eTDT SNP | Biased Transmission | GTS, ADHD, and OCD | - | Biased Transmission [24] |
| Pups | Homo × Homo | Female Homo × Male WT | Het × Het |
|---|---|---|---|
| Live Born (av/litter) | 4.7 | 7.3 | 6.7 |
| Weaned | 4.0 | 6.8 | 6.2 |
| Males | 56% | 50% | 56% |
| Females | 44% | 50% | 44% |
| Sex | Genotype | Percentage |
|---|---|---|
| Female | Homozygous | 9% |
| Female | Heterozygous | 24% |
| Female | Wild-type | 11% |
| Male | Homozygous | 15% |
| Male | Heterozygous | 28% |
| Male | Wild-type | 13% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawther, A.J.; Zieba, J.; Fang, Z.; Furlong, T.M.; Conn, I.; Govindaraju, H.; Choong, L.L.Y.; Turner, N.; Siddiqui, K.S.; Bridge, W.; et al. Antioxidant Behavioural Phenotype in the Immp2l Gene Knock-Out Mouse. Genes 2023, 14, 1717. https://doi.org/10.3390/genes14091717
Lawther AJ, Zieba J, Fang Z, Furlong TM, Conn I, Govindaraju H, Choong LLY, Turner N, Siddiqui KS, Bridge W, et al. Antioxidant Behavioural Phenotype in the Immp2l Gene Knock-Out Mouse. Genes. 2023; 14(9):1717. https://doi.org/10.3390/genes14091717
Chicago/Turabian StyleLawther, Adam J., Jerzy Zieba, Zhiming Fang, Teri M. Furlong, Illya Conn, Hemna Govindaraju, Laura L. Y. Choong, Nigel Turner, Khawar Sohail Siddiqui, Wallace Bridge, and et al. 2023. "Antioxidant Behavioural Phenotype in the Immp2l Gene Knock-Out Mouse" Genes 14, no. 9: 1717. https://doi.org/10.3390/genes14091717
APA StyleLawther, A. J., Zieba, J., Fang, Z., Furlong, T. M., Conn, I., Govindaraju, H., Choong, L. L. Y., Turner, N., Siddiqui, K. S., Bridge, W., Merlin, S., Hyams, T. C., Killingsworth, M., Eapen, V., Clarke, R. A., & Walker, A. K. (2023). Antioxidant Behavioural Phenotype in the Immp2l Gene Knock-Out Mouse. Genes, 14(9), 1717. https://doi.org/10.3390/genes14091717







