Selection and Evaluation of Reference Genes for RT-qPCR Analysis in Amorphophallus Konjac Based on Transcriptome Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sample Collection
2.2. RNA Extraction and cDNA Synthesis
2.3. Screening of Candidate Reference Genes, Primer Design, and RT-qPCR Assay
2.4. Data Analysis of Gene Expression Stability
3. Results
3.1. Primer Specificity and Amplification Efficiency of PCR Reaction
3.2. Ct Value Distribution and Expression Profile of the Eight Reference Genes
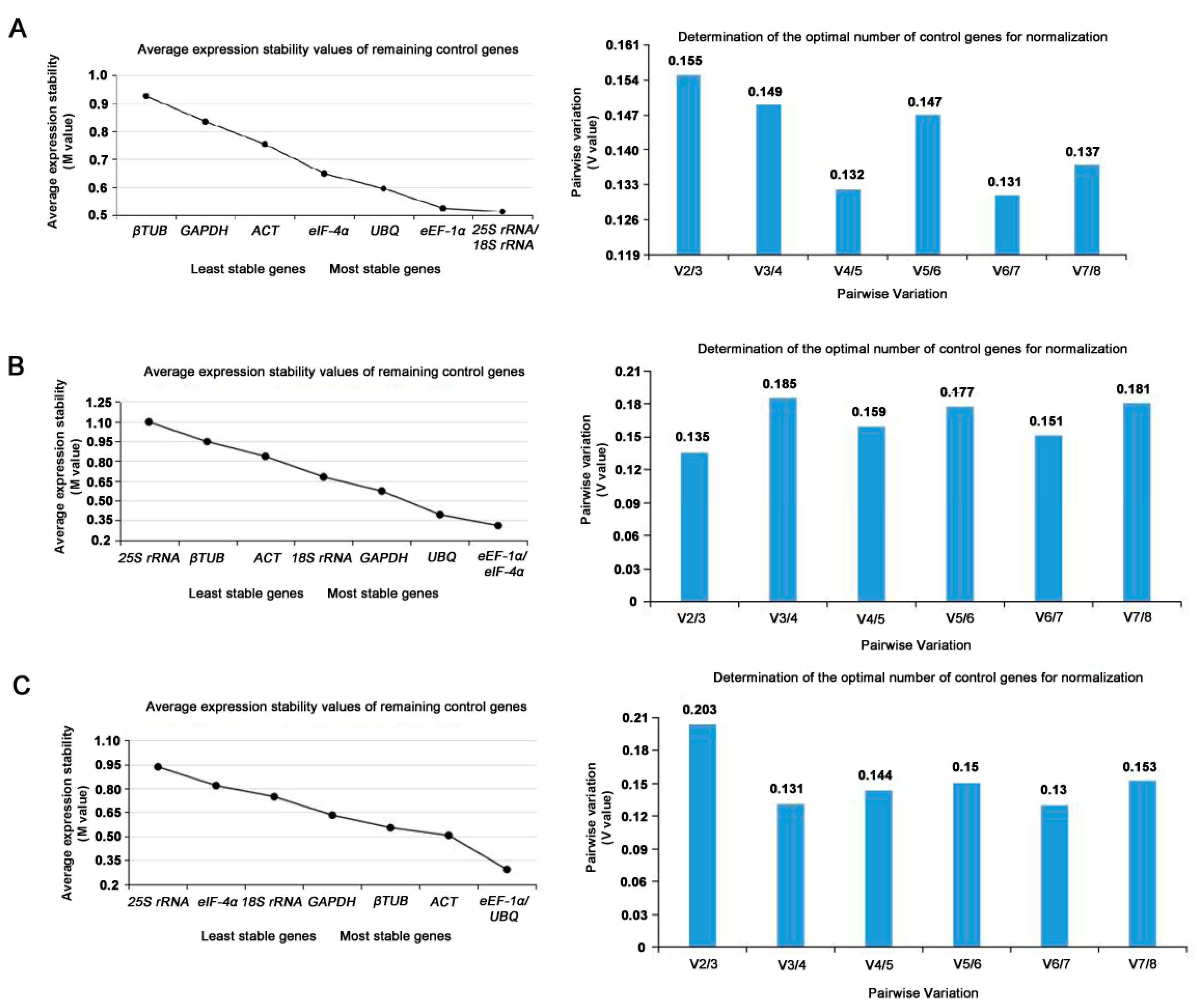
3.3. Stability Analysis of Reference Genes by GeNorm
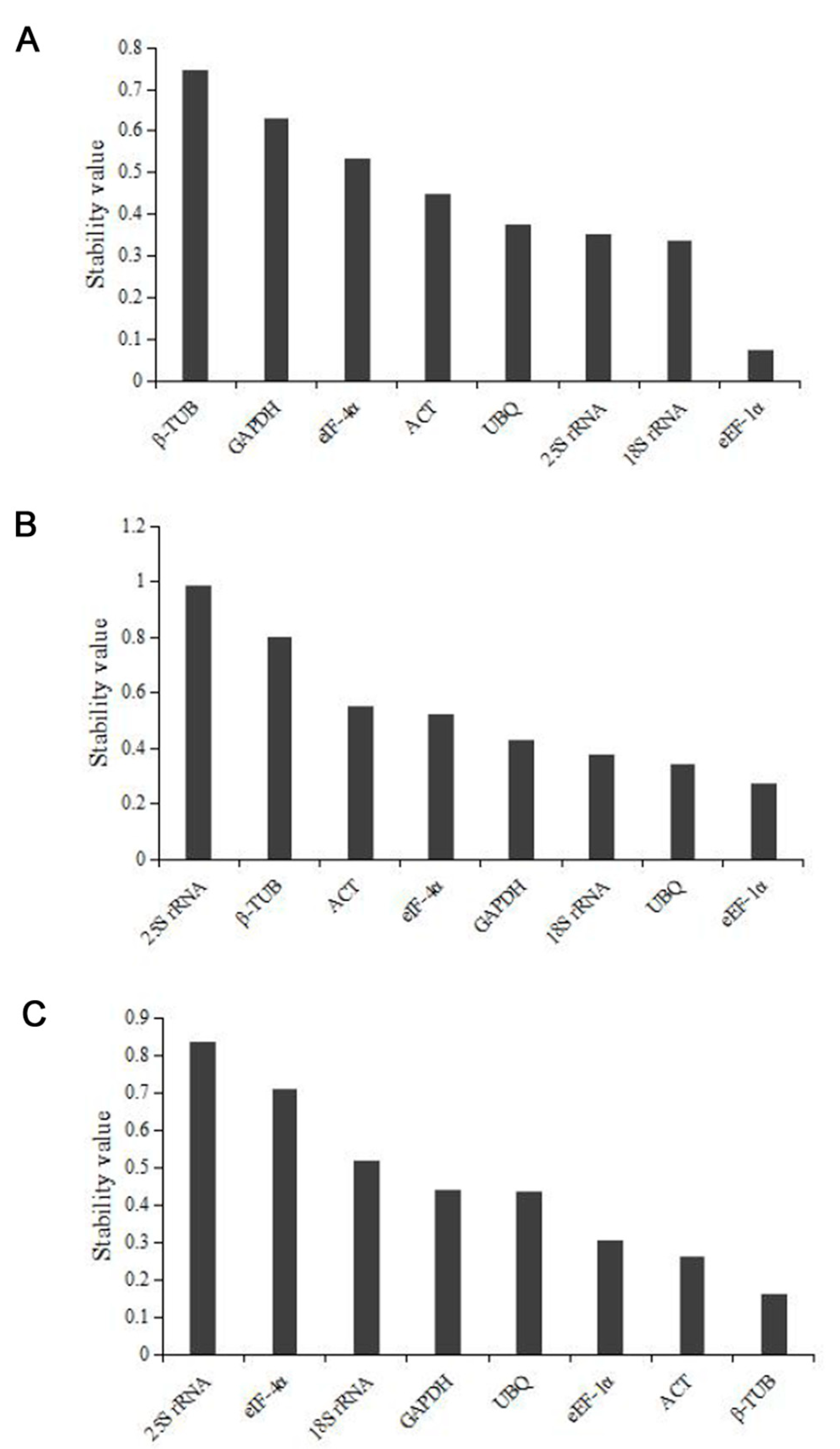
3.4. Stability Analysis of Reference Genes by Normfinder
3.5. Stability Analysis of Reference Genes by BestKeeper
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, M.; Baldwin, T.C.; Hocking, T.J.; Chan, K. Traditional Uses and Potential Health Benefits of Amorphophallus Konjac K. Koch Ex N.E.Br. J. Ethnopharmacol. 2010, 128, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Yang, C.; Yan, M.; Zheng, X.; Jin, S.; Wang, Y.; Hu, Z. De Novo Transcriptome and Small RNA Analyses of Two Amorphophallus Species. PLoS ONE 2014, 9, e95428. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yin, J.; Zhang, L.; Huang, X.; Nie, S. Studies on O-acetyl-glucomannans from Amorphophallus species: Comparison of physicochemical properties and primary structures. Food Hydrocoll. 2019, 89, 503–511. [Google Scholar] [CrossRef]
- Sood, N.; Baker, W.L.; Coleman, C.I. Effect of Glucomannan on Plasma Lipid and Glucose Concentrations, Body Weight, and Blood Pressure: Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2008, 88, 1167–1175. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Feng, C.; Chu, H.; Feng, C.; Wang, H.; Wu, L.; Yin, S.; Liu, C.; Chen, H.; et al. A Chromosome-Level Genome Assembly of Amorphophallus Konjac Provides Insights into Konjac Glucomannan Biosynthesis. Comput. Struct. Biotechnol. J. 2022, 20, 1002–1011. [Google Scholar] [CrossRef]
- Nie, C.Y.; Gao, Q.Y. Deep Processing and Application of Konjac. Food Sci. Technol. 2022, 47, 153–158. [Google Scholar] [CrossRef]
- Das, D.; Mondal, S.; Roy, S.K.; Maiti, D.; Bhunia, B.; Maiti, T.K.; Islam, S.S. Isolation and Characterization of a Heteropolysaccharide from the Corm of Amorphophallus Campanulatus. Carbohydr. Res. 2009, 344, 2581–2585. [Google Scholar] [CrossRef]
- Fei, J.; Liao, Z.; Chai, Y.; Pang, Y.; Yao, J.; Sun, X.; Tang, K. Molecular Cloning and Characterization of a Novel Mannose-Binding Lectin Gene from Amorphophallus Konjac. Mol. Biol. Rep. 2003, 30, 177–183. [Google Scholar] [CrossRef]
- Grob, G.B.J.; Gravendeel, B.; Eurlings, M.C.M. Potential Phylogenetic Utility of the Nuclear FLORICAULA/LEAFY Second Intron: Comparison with Three Chloroplast DNA Regions in Amorphophallus (Araceae). Mol. Phylogenet. Evol. 2004, 30, 13–23. [Google Scholar] [CrossRef]
- Liu, E.; Yang, C.; Liu, J.; Jin, S.; Harijati, N.; Hu, Z.; Diao, Y.; Zhao, L. Comparative Analysis of Complete Chloroplast Genome Sequences of Four Major Amorphophallus Species. Sci. Rep. 2019, 9, 809. [Google Scholar] [CrossRef]
- Zheng, X.; Pan, C.; Diao, Y.; You, Y.; Yang, C.; Hu, Z. Development of Microsatellite Markers by Transcriptome Sequencing in Two Species of Amorphophallus (Araceae). BMC Genom. 2013, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Ginzinger, D.G. Gene Quantification Using Real-Time Quantitative PCR: An Emerging Technology Hits the Mainstream. Exp. Hematol. 2002, 30, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Güven, E. Gene Expression Analysis of MCF7 Cell Lines of Breast Cancer Treated with Herbal Extract of Cissampelos Pareira Revealed Association with Viral Diseases. Gene Rep. 2021, 23, 101169. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Mauriat, M.; Gunin, S.; Pelloux, J.; Lefebvre, J.-F.; Louvet, R.; Rusterucci, C.; Moritz, T.; Guerineau, F.; Bellini, C.; et al. The Lack of a Systematic Validation of Reference Genes: A Serious Pitfall Undervalued in Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis in Plants. Plant Biotechnol. J. 2008, 6, 609–618. [Google Scholar] [CrossRef]
- Thellin, O.; Zorzi, W.; Lakaye, B.; De Borman, B.; Coumans, B.; Hennen, G.; Grisar, T.; Igout, A.; Heinen, E. Housekeeping Genes as Internal Standards: Use and Limits. J. Biotechnol. 1999, 75, 291–295. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Huo, X.P.; Zhang, L.P.; Zhao, X.R.; Liu, Q.S.; Wang, H.F. Effect of RNA quality on internal reference gene selection and qRT PCR result evaluation. Biotechnol. News 2018, 29, 415–418. [Google Scholar]
- Cheng, T.; Zhu, F.; Sheng, J.; Zhao, L.; Zhou, F.; Hu, Z.; Diao, Y.; Jin, S. Selection of Suitable Reference Genes for Quantitive Real-Time PCR Normalization in Miscanthus Lutarioriparia. Mol. Biol. Rep. 2019, 46, 4545–4553. [Google Scholar] [CrossRef]
- Huang, L.; Yan, H.; Jiang, X.; Zhang, X.; Zhang, Y.; Huang, X.; Zhang, Y.; Miao, J.; Xu, B.; Frazier, T.; et al. Evaluation of Candidate Reference Genes for Normalization of Quantitative RT-PCR in Switchgrass Under Various Abiotic Stress Conditions. Bioenerg. Res. 2014, 7, 1201–1211. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and Validation of Reference Genes for Quantitative RT-PCR Normalization in Wheat. BMC Mol. Biol 2009, 10, 11. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Tian, C.; Jiang, Q.; Li, X.-H.; Zhuang, J. Selection of Suitable Reference Genes for QRT-PCR Normalization during Leaf Development and Hormonal Stimuli in Tea Plant (Camellia sinensis). Sci. Rep. 2016, 6, 19748. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Q.; Zhang, B. Evaluation and Selection of Reliable Reference Genes for Gene Expression under Abiotic Stress in Cotton (Gossypium hirsutum L.). Gene 2013, 530, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Niu, Y.; Wang, Q.; Liu, H.; Jin, Y.; Zhang, S. Cloning and Evaluation of Reference Genes for Quantitative Real-Time PCR Analysis in Amorphophallus. PeerJ 2017, 5, e3260. [Google Scholar] [CrossRef]
- Dheda, K.; Huggett, J.F.; Bustin, S.A.; Johnson, M.A.; Rook, G.; Zumla, A. Validation of Housekeeping Genes for Normalizing RNA Expression in Real-Time PCR. BioTechniques 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Hisbergues, M.; Christiansen, G.; Rouhiainen, L.; Sivonen, K.; Börner, T. PCR-Based Identification of Microcystin-Producing Genotypes of Different Cyanobacterial Genera. Arch. Microbiol. 2003, 180, 402–410. [Google Scholar] [CrossRef]
- Lee, P.D.; Sladek, R.; Greenwood, C.M.T.; Hudson, T.J. Control Genes and Variability: Absence of Ubiquitous Reference Transcripts in Diverse Mammalian Expression Studies. Genome Res. 2002, 12, 292–297. [Google Scholar] [CrossRef]
- Zmienko, A.; Samelak-Czajka, A.; Goralski, M.; Sobieszczuk-Nowicka, E.; Kozlowski, P.; Figlerowicz, M. Selection of Reference Genes for QPCR- and DdPCR-Based Analyses of Gene Expression in Senescing Barley Leaves. PLoS ONE 2015, 10, e0118226. [Google Scholar] [CrossRef] [PubMed]
- Nicot, N.; Hausman, J.-F.; Hoffmann, L.; Evers, D. Housekeeping Gene Selection for Real-Time RT-PCR Normalization in Potato during Biotic and Abiotic Stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
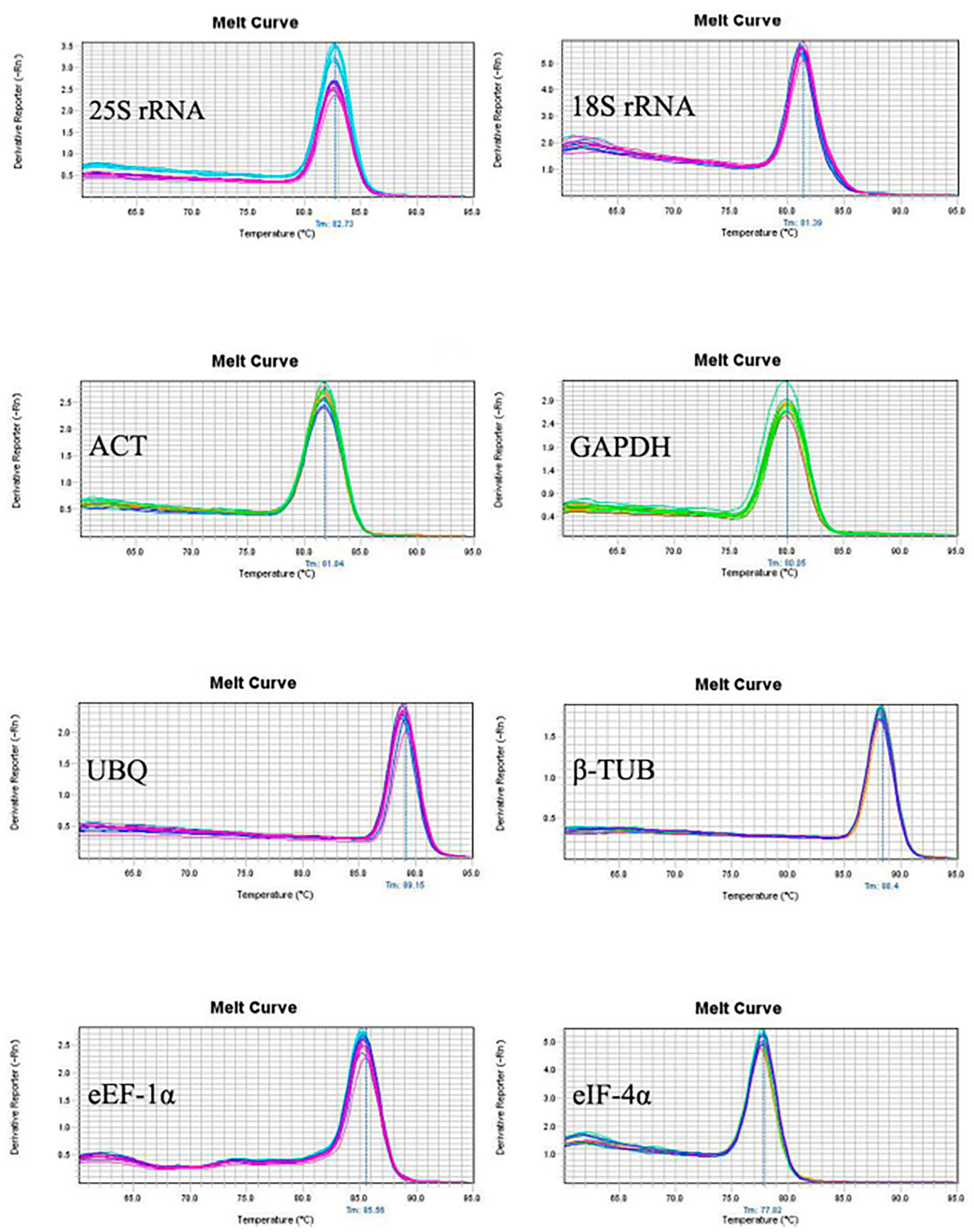


| Growing Stage | Spathe | Append Age | Stamen | Pistil | Inflorescence Axis | Inflorescence Stalk | Tuber | Root | Leaf | Petiole |
|---|---|---|---|---|---|---|---|---|---|---|
| Reproductive period | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Nutritional growing period | √ | √ | √ | √ | ||||||
| Germinating period | √ | |||||||||
| Leaf opening period | √ | |||||||||
| Leaf mature period | √ |
| Gene Name | Sequence Primer 5′-3′ | Size (bp) | PCR Efficiency (%) | R2 |
|---|---|---|---|---|
| 25S rRNA | F: CGCCCTCGACCTATTCTCAAA R: CTTACCAAAAATGGCCCACTT | 103 | 97.56 | 0.995 |
| 18S rRNA | F: AGACGAACAACTGCGAAAGC R: GGCGGAGTCCTAAGAGCAAC | 149 | 99.41 | 0.998 |
| ACT | F: TGAACGTGAAATTGTAAGGGAC R: CAGATGAGCTAGTCTTGGCAGT | 95 | 101.40 | 0.999 |
| GAPDH | F: AGAGGAGCGAGGCAGTTAGTG R: CCCATGTTTGTTGTTGGTGTA | 95 | 103.73 | 0.991 |
| UBQ | F: CCAGCAGCGCCTCATCTTTG R: CTTGGGCTTGGTGTAGGTCTTC | 151 | 96.06 | 0.998 |
| β-TUB | F: CGGATGATGCTGACCTTCTCG R: ATGCACTCGTCGGCGTTCTC | 116 | 90.19 | 0.997 |
| eEF-1α | F: CTGAAGAATGGCGATGCTGG R: ACCGTCTGCCTCATGTCCCT | 119 | 102.20 | 0.994 |
| eIF-4α | F: AGCATTTCATCCGCTTCGTC R: TTGTGGGTACTCCTGGTCGT | 100 | 103.71 | 0.997 |
| Different Developmental Stage of Konjac | Gene Name | GeNorm Stability (M) | Normfinder Stability (SV) | BestKeeper | Stability | |
|---|---|---|---|---|---|---|
| SD | CV% | r | ||||
| Reproductive phase | 25S rRNA | 0.513 | 0.354 | 0.56 | 3.83 | 0.89 |
| 18S rRNA | 0.513 | 0.336 | 0.70 | 4.38 | 0.91 | |
| ACT | 0.754 | 0.448 | 1.64 | 5.58 | 0.95 | |
| GAPDH | 0.835 | 0.630 | 1.64 | 6.27 | 0.93 | |
| UBQ | 0.595 | 0.375 | 0.90 | 3.4 | 0.91 | |
| β-TUB | 0.926 | 0.745 | 1.17 | 4.11 | 0.81 | |
| eEF-1α | 0.525 | 0.075 | 0.81 | 3.06 | 0.86 | |
| eIF-4α | 0.649 | 0.532 | 0.69 | 2.38 | 0.83 | |
| Nutritional phase | 25S rRNA | 1.097 | 0.987 | 1.27 | 6.51 | 0.99 |
| 18S rRNA | 0.679 | 0.375 | 1.08 | 5.37 | 0.98 | |
| ACT | 0.836 | 0.552 | 0.61 | 2.01 | 0.50 | |
| GAPDH | 0.572 | 0.430 | 0.90 | 3.38 | 0.82 | |
| UBQ | 0.393 | 0.344 | 0.42 | 1.41 | 0.50 | |
| β-TUB | 0.947 | 0.802 | 0.75 | 2.34 | 0.28 | |
| eEF-1α | 0.310 | 0.274 | 0.24 | 0.86 | 0.88 | |
| eIF-4α | 0.310 | 0.521 | 0.31 | 1.11 | −0.55 | |
| Three developmental stages of leaves | 25S rRNA | 0.935 | 0.834 | 1.11 | 5.62 | 0.82 |
| 18S rRNA | 0.748 | 0.517 | 0.46 | 2.35 | 0.85 | |
| ACT | 0.505 | 0.260 | 1.05 | 3.38 | 0.98 | |
| GAPDH | 0.632 | 0.440 | 0.83 | 2.97 | 1.00 | |
| UBQ | 0.291 | 0.434 | 0.55 | 1.82 | 0.60 | |
| β-TUB | 0.553 | 0.161 | 1.17 | 3.58 | 0.96 | |
| eEF-1α | 0.291 | 0.305 | 0.74 | 2.56 | 0.85 | |
| eIF-4α | 0.818 | 0.711 | 0.44 | 1.57 | −0.11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, C.; Harijati, N.; Diao, Y.; Liu, E.; Hu, Z. Selection and Evaluation of Reference Genes for RT-qPCR Analysis in Amorphophallus Konjac Based on Transcriptome Data. Genes 2023, 14, 1513. https://doi.org/10.3390/genes14081513
Liu Y, Zhang C, Harijati N, Diao Y, Liu E, Hu Z. Selection and Evaluation of Reference Genes for RT-qPCR Analysis in Amorphophallus Konjac Based on Transcriptome Data. Genes. 2023; 14(8):1513. https://doi.org/10.3390/genes14081513
Chicago/Turabian StyleLiu, Yanli, Chengcheng Zhang, Nunung Harijati, Ying Diao, Erxi Liu, and Zhongli Hu. 2023. "Selection and Evaluation of Reference Genes for RT-qPCR Analysis in Amorphophallus Konjac Based on Transcriptome Data" Genes 14, no. 8: 1513. https://doi.org/10.3390/genes14081513
APA StyleLiu, Y., Zhang, C., Harijati, N., Diao, Y., Liu, E., & Hu, Z. (2023). Selection and Evaluation of Reference Genes for RT-qPCR Analysis in Amorphophallus Konjac Based on Transcriptome Data. Genes, 14(8), 1513. https://doi.org/10.3390/genes14081513






