Identification and Expression of Integrins during Testicular Fusion in Spodoptera litura
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insects
2.2. Prediction and Bioinformatic Analysis of Integrin Subunits
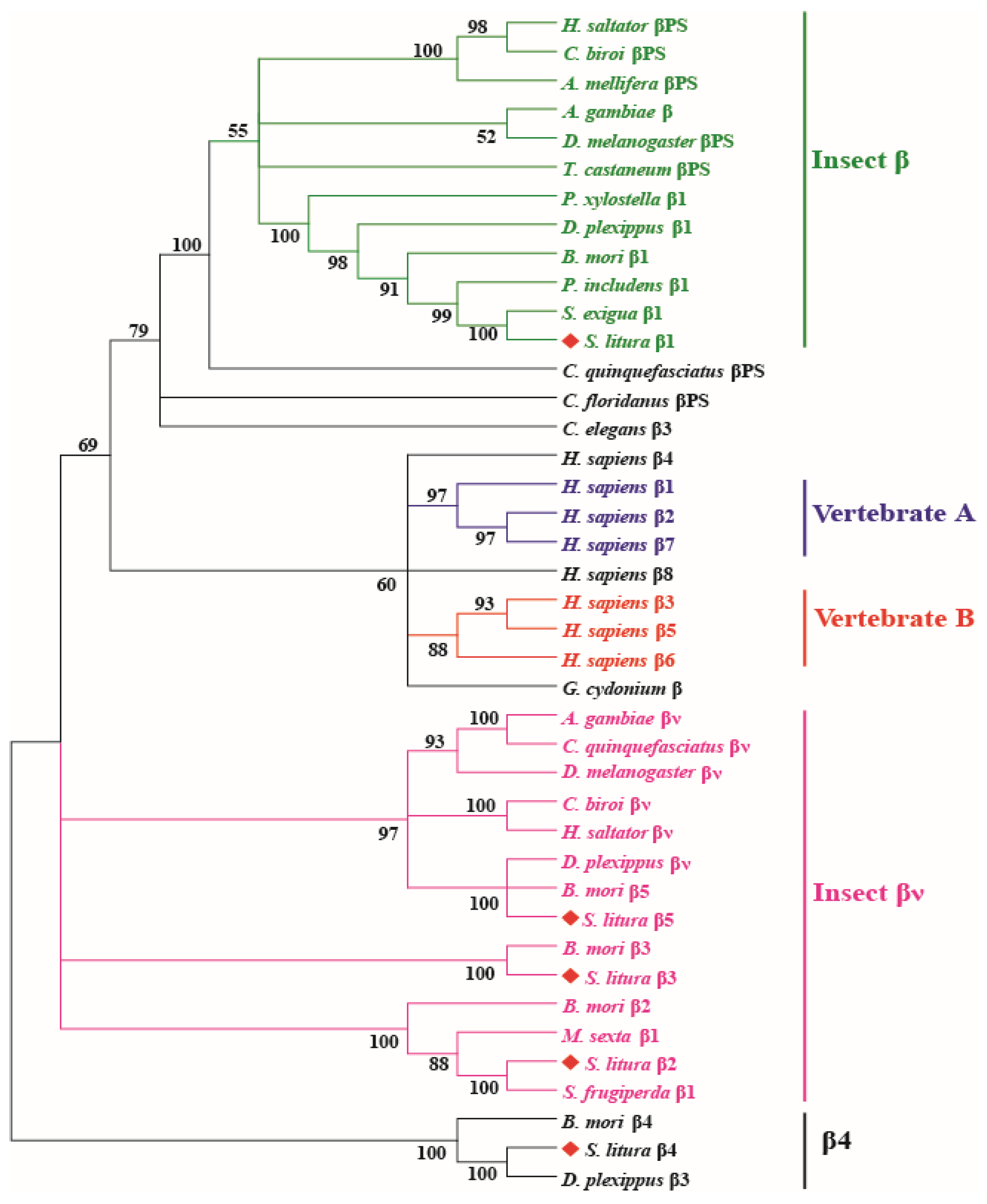
2.3. Phylogeny Analysis of Integrins
2.4. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
2.5. Separation of Testicular Peritoneal Sheath and Sperm Cells
2.6. Western Blotting Analysis
2.7. Immunohistochemistry Analysis
2.8. Statistical Analysis
3. Results
3.1. Identification of Integrin Members in S. litura
3.2. Phylogenetic Analysis of Integrin Members
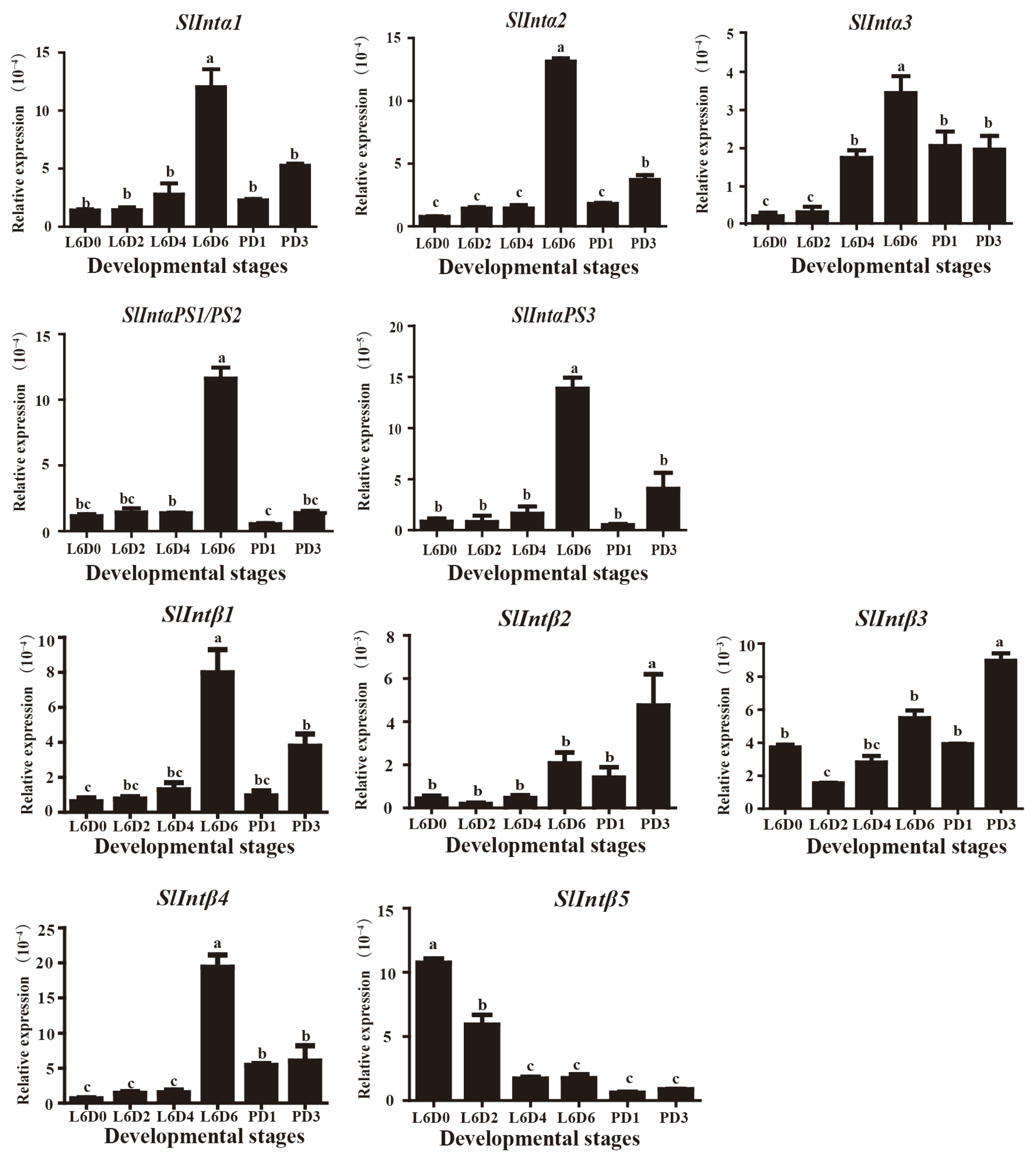
3.3. Expression Patterns of Integrins in the Testis of S. litura at Different Developmental Stages
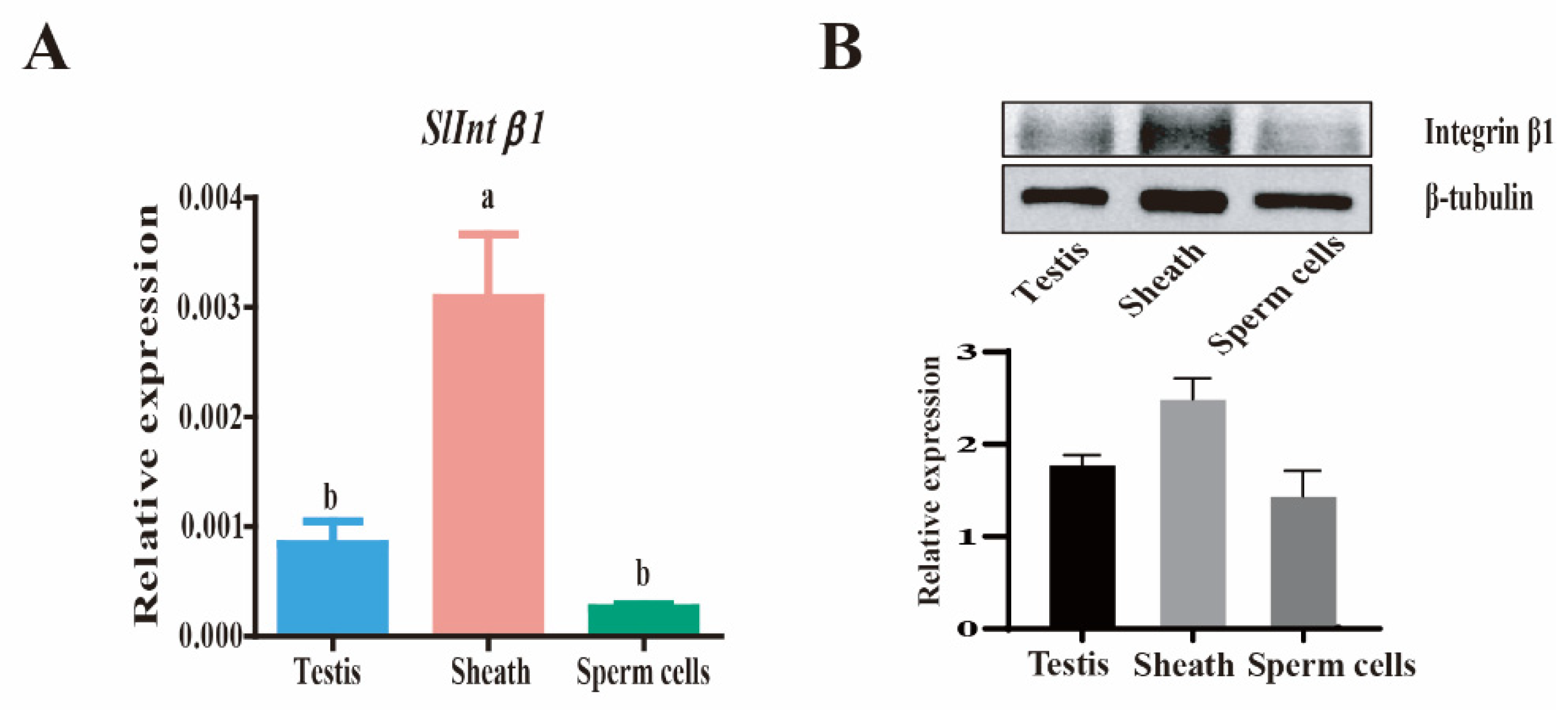
3.4. Expression and Localization of Integrin β1 at Different Parts in the Testis of S. litura
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A. Integrin activation. J. Cell Sci. 2004, 117, 657–666. [Google Scholar] [PubMed]
- Luo, B.H.; Springer, T.A. Integrin structures and conformational signaling. Curr. Opin. Cell Biol. 2006, 18, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef]
- Johnson, M.S.; Lu, N.; Denessiouk, K.; Heino, J.; Gullberg, D. Integrins during evolution: Evolutionary trees and model organisms. Biochim. Biophys. Acta 2009, 1788, 779–789. [Google Scholar]
- Bökel, C.; Brown, N.H. Integrins in development: Moving on, responding to, and sticking to the extracellular matrix. Dev. Cell 2002, 3, 311–321. [Google Scholar]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 1472. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar]
- Kanchanawong, P.; Calderwood, D.A. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161. [Google Scholar]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar]
- Gilcrease, M.Z. Integrin signaling in epithelial cells. Cancer Lett. 2007, 247, 1–25. [Google Scholar] [PubMed]
- Breuss, J.M.; Gallo, J.; De Lisser, H.M.; Klimanskaya, I.V.; Folkesson, H.G.; Pittet, J.F.; Nishimura, S.L.; Aldape, K.; Landers, D.V.; Carpenter, W.; et al. Expression of the β 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 1995, 108 Pt 6, 2241–2251. [Google Scholar] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar]
- Krishn, S.R.; Garcia, V.; Naranjo, N.M.; Quaglia, F.; Shields, C.D.; Harris, M.A.; Kossenkov, A.V.; Liu, Q.; Corey, E.; Altieri, D.C.; et al. Small extracellular vesicle-mediated ITGB6 siRNA delivery downregulates the αVβ6 integrin and inhibits adhesion and migration of recipient prostate cancer cells. Cancer Biol. Ther. 2022, 23, 173–185. [Google Scholar]
- Sun, Q.; Shang, Y.; Sun, F.; Dong, X.; Niu, J.; Li, F. Interleukin-6 Promotes Epithelial-Mesenchymal Transition and Cell Invasion through Integrin β6 Upregulation in Colorectal Cancer. Oxidative Med. Cell Longev. 2020, 2020, 8032187. [Google Scholar] [CrossRef]
- Ishii, M.; Tay, T.W.; Matsui, T.; Kidokoro, T.; Mizukami, T.; Kanai, Y.; Hayashi, Y.; Kurohmaru, M. Expression pattern of alphavbeta3 and alphavbeta5 integrin mRNA in mouse fetal gonads. J. Reprod. Dev. 2006, 52, 461–468. [Google Scholar] [CrossRef]
- Itami, S.; Tamotsu, S.; Sakai, A.; Yasuda, K. The roles of THY1 and integrin beta3 in cell adhesion during theca cell layer formation and the effect of follicle-stimulating hormone on THY1 and integrin beta3 localization in mouse ovarian follicles. Biol. Reprod. 2011, 84, 986–995. [Google Scholar] [PubMed]
- Burns, K.H.; Owens, G.E.; Fernandez, J.M.; Nilson, J.H.; Matzuk, M.M. Characterization of integrin expression in the mouse ovary. Biol. Reprod. 2002, 67, 743–751. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Zhan, J.; Chen, Y.; Fang, W.; Zhang, L.; Zhang, H. Smurf1 inhibits integrin activation by controlling Kindlin-2 ubiquitination and degradation. J. Cell Biol. 2017, 216, 1455–1471. [Google Scholar] [CrossRef]
- Antalíková, J.; Sečová, P.; Michalková, K.; Horovská, Ľ.; Páleníková, V.; Jankovičová, J. Expression of αV integrin and its potential partners in bull reproductive tissues, germ cells and spermatozoa. Int. J. Biol. Macromol. 2022, 209, 542–551. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 2002, 82, 825–874. [Google Scholar] [CrossRef]
- Frolikova, M.; Valaskova, E.; Cerny, J.; Lumeau, A.; Sebkova, N.; Palenikova, V.; Sanches-Hernandez, N.; Pohlova, A.; Manaskova-Postlerova, P.; Dvorakova-Hortova, K. Addressing the Compartmentalization of Specific Integrin Heterodimers in Mouse Sperm. Int. J. Mol. Sci. 2019, 20, 1004. [Google Scholar] [CrossRef]
- Giebel, J.; Löster, K.; Rune, G.M. Localization of integrin β 1, α 1, α 5 and α 9 subunits in the rat testis. Int. J. Androl. 1997, 20, 3–9. [Google Scholar] [CrossRef]
- Pines, M.; Das, R.; Ellis, S.J.; Morin, A.; Czerniecki, S.; Yuan, L.; Klose, M.; Coombs, D.; Tanentzapf, G. Mechanical force regulates integrin turnover in Drosophila in vivo. Nat. Cell Biol. 2012, 14, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Ortega, L.; Valencia-Expósito, A.; Kabanova, A.; González-Reyes, A.; Martin-Bermudo, M.D. Integrins control epithelial stem cell proliferation in the Drosophila ovary by modulating the Notch pathway. Front. Cell Dev. Biol. 2023, 11, 1114458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tan, J.; Xu, M.; Su, J.; Hu, R.; Chen, Y.; Xuan, F.; Yang, R.; Cui, H. A novel granulocyte-specific α integrin is essential for cellular immunity in the silkworm Bombyx mori. J. Insect Physiol. 2014, 71, 61–67. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Hu, X.; Yan, X.; Chen, S.; Li, C.; Pan, G.; Chang, H.; Tian, W.; Abbas, M.N.; et al. Integrin β2 and β3: Two plasmatocyte markers deepen our understanding of the development of plasmatocytes in the silkworm Bombyx mori. Insect Sci. 2022, 29, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Park, Y.; Kim, Y. A Transformed Bacterium Expressing Double-Stranded RNA Specific to Integrin β1 Enhances Bt Toxin Efficacy against a Polyphagous Insect Pest, Spodoptera exigua. PLoS ONE 2015, 10, e0132631. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhuo, X.R.; Tang, L.; Liu, X.S.; Wang, Y.F.; Wang, G.X.; Yu, X.Q.; Wang, J.L. C-type lectin interacting with β-integrin enhances hemocytic encapsulation in the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 2017, 86, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, H.; Yu, X.; Liu, J.; Wang, P.; Chen, J.; Xu, Q.; Zhang, W. Integrin β1 subunit from Ostrinia furnacalis hemocytes: Molecular characterization, expression, and effects on the spreading of plasmatocytes. J. Insect Physiol. 2010, 56, 1846–1856. [Google Scholar] [PubMed]
- Gotwals, P.J.; Paine-Saunders, S.E.; Stark, K.A.; Hynes, R.O. Drosophila integrins and their ligands. Curr. Opin. Cell Biol. 1994, 6, 734–739. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, M.; Su, J.; Yu, S.; Sun, Z.; Li, Y.; Zhang, W.; Hou, J.; Shang, L.; Cui, H. Characterization and identification of the integrin family in silkworm, Bombyx mori. Gene 2014, 549, 149–155. [Google Scholar] [CrossRef]
- Mitter, C.; Davis, D.R.; Cummings, M.P. Phylogeny and Evolution of Lepidoptera. Annu. Rev. Entomol. 2017, 62, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Mancini, K.; Lino-Neto, J.; Dolder, H. Morphology of the male reproductive system and sperm ultrastructure of Leucoptera coffeella (Lepidoptera: Lyonetiidae). Acta Zoologica. 2006, 87, 131–139. [Google Scholar] [CrossRef]
- Bilha, J.K.; Conte, H. Testicles Fusion of Diatraea saccharalis F. (Lepidoptera; Crambidae) During Post-embryonic Development. J. Entomol. Res. Soc. 2012, 16, 1–7. [Google Scholar]
- Lachance, L.E.; Olstad, G. Spermiogenesis of Eupyrene Sperm in Prepupae, Pupae, and Adults of Heliothis virescens (Lepidoptera: Noctuidae): An Ultrastructural Study. Ann. Entomol. Soc. Am. 1988, 81, 292–300. [Google Scholar] [CrossRef]
- Mari, I.P.; Gigliolli AA, S.; Nanya, S.; Portela-Castro AL, B. Histological and electron microscopy observations on the testis and spermatogenesis of the butterfly Dione juno (Cramer, 1779) and Agraulis vanillae (Linnaeus, 1758) (Lepidoptera: Nymphalidae). Micron 2018, 109, 11–21. [Google Scholar] [CrossRef]
- Chen, Y.; Ou, J.; Liu, Y.; Wu, Q.; Wen, L.; Zheng, S.; Li, S.; Feng, Q.; Liu, L. Transcriptomic analysis of the testicular fusion in Spodoptera litura. BMC Genom. 2020, 21, 171. [Google Scholar] [CrossRef]
- He, X.; Ma, Q.; Jian, B.; Liu, Y.; Wu, Q.; Chen, M.; Feng, Q.; Zhao, P.; Liu, L. Microsurgical Obstruction of Testes Fusion in Spodoptera litura. J. Vis. Exp. 2021, 173, e62524. [Google Scholar]
- Qi, C. A simple artificial diet for mass rearing of some noctuid species. Entomol. Knowl. 2000, 6, 325–327. [Google Scholar]
- Cheng, T.; Wu, J.; Wu, Y.; Chilukuri, R.V.; Huang, L.; Yamamoto, K.; Feng, L.; Li, W.; Chen, Z.; Guo, H.; et al. Genomic adaptation to polyphagy and insecticides in a major East Asian noctuid pest. Nat. Ecol. Evol. 2017, 1, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L. Evolution of the integrin α and β protein families. J. Mol. Evol. 2001, 52, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, M.; Gao, X.; Kang, T.; Zhan, S.; Wan, H.; Li, J. Identification and validation of reference genes for gene expression analysis using quantitative PCR in Spodoptera litura (Lepidoptera: Noctuidae). PLoS ONE 2013, 8, e68059. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A. Principles and Methods of Immunohistochemistry. Methods Mol. Biol. 2017, 1641, 115–128. [Google Scholar]
- Shi, S.R.; Cote, R.J.; Taylor, C.R. Antigen retrieval techniques: Current perspectives. J. Histochem. Cytochem. 2001, 49, 931–937. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef] [PubMed]
- Huhtala, M.; Heino, J.; Casciari, D.; de Luise, A.; Johnson, M.S. Integrin evolution: Insights from ascidian and teleost fish genomes. Matrix Biol. 2005, 24, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P. Sperm-egg interaction. Annu. Rev. Physiol. 2012, 74, 477–502. [Google Scholar] [CrossRef]
- Vjugina, U.; Zhu, X.; Oh, E.; Bracero, N.J.; Evans, J.P. Reduction of mouse egg surface integrin alpha9 subunit (ITGA9) reduces the egg’s ability to support sperm-egg binding and fusion. Biol. Reprod. 2009, 80, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.M. Conserved signaling mechanisms in Drosophila heart development. Dev. Dyn. 2017, 246, 641–656. [Google Scholar] [CrossRef]
- Bate, M. The embryonic development of larval muscles in Drosophila. Development 1990, 110, 791–804. [Google Scholar] [CrossRef]
- Dominguez-Gimenez, P.; Brown, N.H.; Martin-Bermudo, M.D. Integrin-ECM interactions regulate the changes in cell shape driving the morphogenesis of the Drosophila wing epithelium. J. Cell Sci. 2007, 120, 1061–1071. [Google Scholar] [CrossRef]
- Perkins, A.D.; Ellis, S.J.; Asghari, P.; Shamsian, A.; Moore, E.D.; Tanentzapf, G. Integrin-mediated adhesion maintains sarcomeric integrity. Dev. Biol. 2010, 338, 15–27. [Google Scholar] [CrossRef]
- Vanderploeg, J.; Vazquez Paz, L.L.; MacMullin, A.; Jacobs, J.R. Integrins are required for cardioblast polarisation in Drosophila. BMC Dev. Biol. 2012, 12, 8. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Fratto, V.M.; Lemosy, E.K. Integrin α chains exhibit distinct temporal and spatial localization patterns in epithelial cells of the Drosophila ovary. Dev. Dyn. 2008, 237, 3927–3939. [Google Scholar] [CrossRef]
- Bui, T.; Rennhack, J.; Mok, S.; Ling, C.; Perez, M.; Roccamo, J.; Andrechek, E.R.; Moraes, C.; Muller, W.J. Functional Redundancy between β1 and β3 Integrin in Activating the IR/Akt/mTORC1 Signaling Axis to Promote ErbB2-Driven Breast Cancer. Cell Rep. 2019, 29, 589–602.e6. [Google Scholar] [CrossRef]
- Bell, L.V.; Else, K.J. Mechanisms of leucocyte recruitment to the inflamed large intestine: Redundancy in integrin and addressin usage. Parasite Immunol. 2008, 30, 163–170. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.W.; Lee, J.H.; Park, J.Y.; Roshandell, M.; Brennan, C.A.; Choe, K.M. Requirement for and polarized localization of integrin proteins during Drosophila wound closure. Mol. Biol. Cell 2018, 29, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, X.L.; Liu, T.H.; Chen, P.; Jiang, X.; Dong, Z.Q.; Pan, M.H.; Lu, C. A Matrix Metalloproteinase Mediates Tracheal Development in Bombyx mori. Int. J. Mol. Sci. 2021, 22, 5618. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.M.; Breuer, L.N.; Zhuang, S.; Anderson, S.A.; Nardi, J.B.; Kanost, M.R. A hemocyte-specific integrin required for hemocytic encapsulation in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2005, 35, 369–380. [Google Scholar] [CrossRef]
- Van De Bor, V.; Loreau, V.; Malbouyres, M.; Cerezo, D.; Placenti, A.; Ruggiero, F.; Noselli, S. A dynamic and mosaic basement membrane controls cell intercalation in Drosophila ovaries. Development 2021, 148, dev195511. [Google Scholar] [CrossRef]
- Castillo-Briceño, P.; Cabas, I.; Arizcun, M.; Meseguer, J.; Mulero, V.; García-Ayala, A. Identification of a β1 integrin isoform with restricted tissue expression in a teleost fish. Reprod. Fertil. Dev. 2011, 23, 654–664. [Google Scholar] [CrossRef]
- Pai, Y.J.; Abdullah, N.L.; Mohd-Zin, S.W.; Mohammed, R.S.; Rolo, A.; Greene, N.D.; Abdul-Aziz, N.M.; Copp, A.J. Epithelial fusion during neural tube morphogenesis. Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Ray, H.J.; Niswander, L. Mechanisms of tissue fusion during development. Development 2012, 139, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Critchley, D.R.; Holt, M.R.; Barry, S.T.; Priddle, H.; Hemmings, L.; Norman, J. Integrin-mediated cell adhesion: The cytoskeletal connection. Biochem. Soc. Symp. 1999, 65, 79–99. [Google Scholar] [PubMed]
- Mercurio, A.M.; Rabinovitz, I.; Shaw, L.M. The α6β4 integrin and epithelial cell migration. Curr. Opin. Cell Biol. 2001, 13, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Raza, Q.S.; Vanderploeg, J.L.; Jacobs, J.R. Matrix Metalloproteinases are required for membrane motility and lumenogenesis during Drosophila heart development. PLoS ONE 2017, 12, e0171905. [Google Scholar] [CrossRef] [PubMed]


| Subunit | Gene | Scaffold | Position | Chr. | Accession | Exon | Size (AA) | MW/KDa |
|---|---|---|---|---|---|---|---|---|
| α | α1 | scaffold157 | 623,547–706,210 | 6 | XM_022982049 | 17 | 1118 | 124.21 |
| α2 | scaffold285 | 13,930–127,259 | 14 | XM_022963824 | 24 | 1567 | 174.05 | |
| α3 | scaffold417 | 212,791–229,830 | 7 | XM_022959037 | 21 | 960 | 106.67 | |
| αPS1/PS2 | Scaffold384 | 257,012–280,655 | 14 | XM_022964330 | 25 | 937 | 104.12 | |
| αPS3 | Scaffold384 | 217,418–233,535 | 14 | XM_022964516 | 23 | 876 | 97.35 | |
| β | β1 | scaffold315 | 223,689–230,408 | 14 | XM_022963710 | 16 | 838 | 93.13 |
| β2 | scaffold449(+) | 44,554–48,972 | 15 | XM_022964980 | 9 | 782 | 86.91 | |
| β3 | scaffold449(−) | 237,386–243,026 | 15 | XM_022965413 | 8 | 725 | 80.59 | |
| β4 | scaffold449(−) | 247,062–275,746 | 15 | XM_022965036 | 6 | 432 | 48.06 | |
| β5 | scaffold226 | 563,195–582,187 | 17 | XM_022967414 | 13 | 807 | 89.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, Y.; Jian, B.; Feng, Q.; Liu, L. Identification and Expression of Integrins during Testicular Fusion in Spodoptera litura. Genes 2023, 14, 1452. https://doi.org/10.3390/genes14071452
Chen Y, Chen Y, Jian B, Feng Q, Liu L. Identification and Expression of Integrins during Testicular Fusion in Spodoptera litura. Genes. 2023; 14(7):1452. https://doi.org/10.3390/genes14071452
Chicago/Turabian StyleChen, Yaqing, Yu Chen, Baozhu Jian, Qili Feng, and Lin Liu. 2023. "Identification and Expression of Integrins during Testicular Fusion in Spodoptera litura" Genes 14, no. 7: 1452. https://doi.org/10.3390/genes14071452
APA StyleChen, Y., Chen, Y., Jian, B., Feng, Q., & Liu, L. (2023). Identification and Expression of Integrins during Testicular Fusion in Spodoptera litura. Genes, 14(7), 1452. https://doi.org/10.3390/genes14071452





