Racial and Ethnic Disparities in Genomic Healthcare Utilization, Patient Activation, and Intrafamilial Communication of Risk among Females Tested for BRCA Variants: A Mixed Methods Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
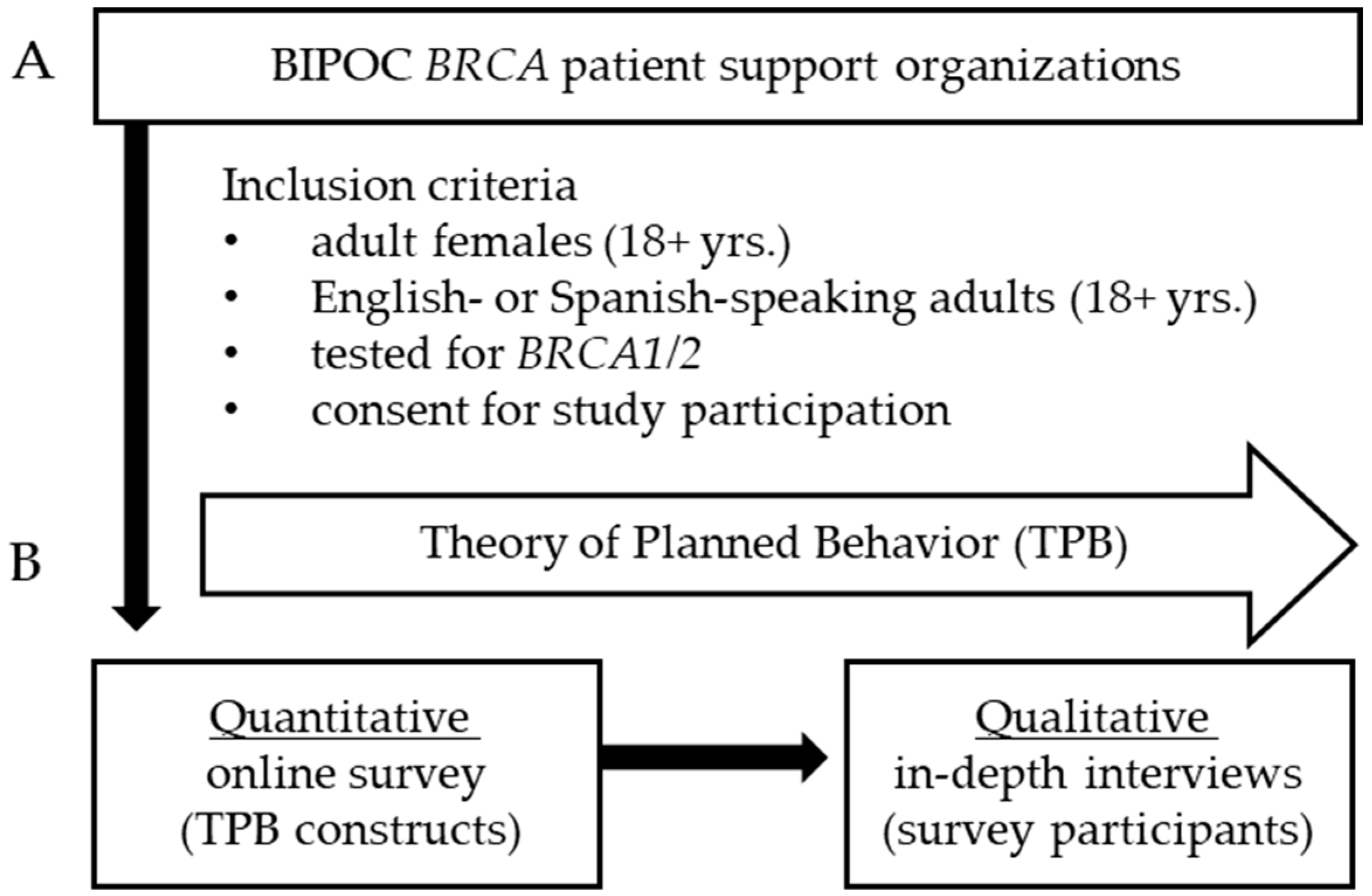
2.1. Participants and Procedures
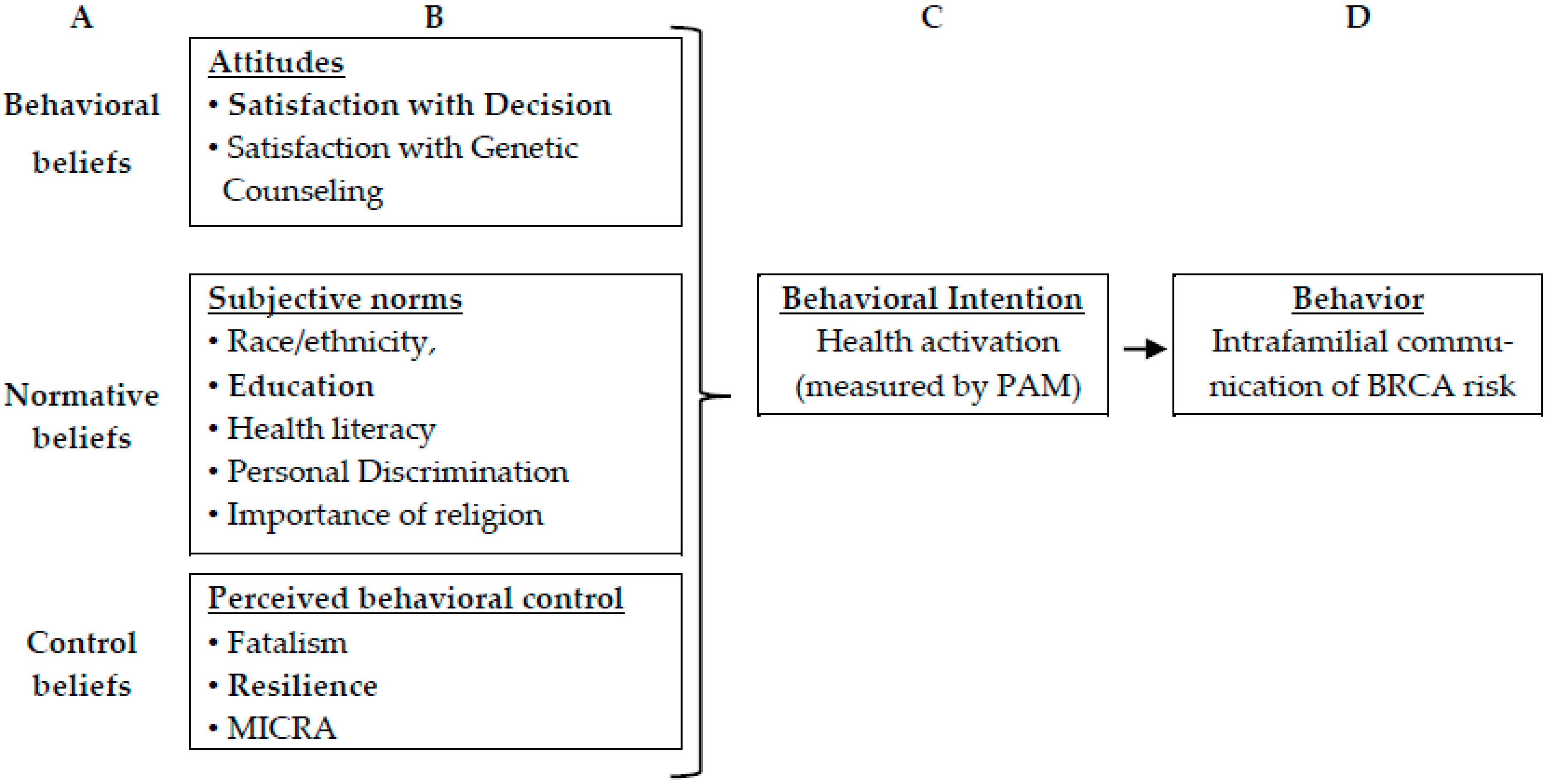
2.2. Theoretical Framework
2.3. Quantitative Survey
2.4. Qualitative Interviews
2.5. Analyses
3. Results
3.1. Participant Characteristics
3.2. Participant Medical Information and Genetic Testing
3.3. Participant Interactions with Healthcare, Coping Response, and Patient Activation
3.4. Predictors of Patient Activation (Intention) and Communicating Test Results (Behavior)
3.4.1. Qualitative Findings Related to the Theory of Planned Behavior “Behavioral Beliefs”
3.4.2. Qualitative Findings Related to the Theory of Planned Behavior “Normative Beliefs”
3.4.3. Qualitative Findings Related to the Theory of Planned Behavior “Behavioral Control Beliefs”
3.4.4. Qualitative Findings Related to the Theory of Planned Behavior “Behavioral Intention”
3.4.5. Qualitative Findings Related to the Theory of Planned Behavior “Behavior”
3.5. Expanding Survey Findings with Qualitative Interviews
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CancerDisparitiesProgressReport.org [Internet]. Philadelphia: American Association for Cancer Research; ©2022 [11/10/2022]. Available online: http://www.CancerDisparitiesProgressReport.org/ (accessed on 2 December 2022).
- American Cancer Society. Cancer Facts & Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf (accessed on 5 November 2022).
- Cragun, D.; Weidner, A.; Lewis, C.; Bonner, D.; Kim, J.; Vadaparampil, S.T.; Pal, T. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 2017, 123, 2497–2505. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- National Cancer Institute, Surveillance, Epidemiology, and End Result Program. Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/ (accessed on 5 November 2022).
- Williams, C.D.; Bullard, A.J.; O’Leary, M.; Thomas, R.; Redding, T.S., 4th; Goldstein, K. Racial/Ethnic Disparities in BRCA Counseling and Testing: A Narrative Review. J. Racial. Ethn. Health Disparities 2019, 6, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Committee on Gynecologic Practice. ACOG Committee Opinion No. 727: Cascade Testing: Testing Women for Known Hereditary Genetic Mutations Associated With Cancer. Obstet. Gynecol. 2018, 131, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, K.D.; Obeid, E.; Daly, M.B.; Hall, M.J. Cascade Genetic Testing for Hereditary Cancer Risk: An Underutilized Tool for Cancer Prevention. JCO Precis Oncol. 2021, 5, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Yenter, D.; Vagher, J.; Clayton, M.F.; Rindler, M.; Shukovich, M.; Kaphingst, K.A. “Being proactive, not reactive”: Exploring perceptions of genetic testing among White, Latinx, and Pacific Islander Populations. J. Community Genet. 2021, 12, 617–630. [Google Scholar] [CrossRef]
- NCCN Guidelines Version 1.2023, Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503 (accessed on 1 December 2022).
- Seven, M.; Shah, L.L.; Daack-Hirsch, S.; Yazici, H. Experiences of BRCA1/2 Gene Mutation-Positive Women With Cancer in Communicating Genetic Risk to Their Relatives. Cancer Nurs. 2021, 44, E142–E150. [Google Scholar] [CrossRef]
- Makhnoon, S.; Arun, B.; Bedrosian, I. Helping Patients Understand and Cope with BRCA Mutations. Curr. Oncol. Rep. 2022, 24, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, J.H.; Mahoney, E.R.; Stockard, J.; Tusler, M. Development and testing of a short form of the patient activation measure. Health Serv. Res. 2005, 40 Pt 1, 1918–1930. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Hesse-Biber, S.; Shea, H.; Zeng, Z.; Yi, S. Coping response and family communication of cancer risk in men harboring a BRCA mutation: A mixed methods study. Psychooncology 2022, 31, 486–495. [Google Scholar] [CrossRef]
- Underhill, M.L.; Jones, T.; Habin, K. Disparities in Cancer Genetic Risk Assessment and Testing. Oncol. Nurs. Forum. 2016, 43, 519–523. [Google Scholar] [CrossRef]
- Fehniger, J.; Lin, F.; Beattie, M.S.; Joseph, G.; Kaplan, C. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J. Genet. Couns. 2013, 22, 603–612. [Google Scholar] [CrossRef]
- Cheung, E.L.; Olson, A.D.; Yu, T.M.; Han, P.Z.; Beattie, M.S. Communication of BRCA results and family testing in 1103 high-risk women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2211–2219. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: Guidelines for reporting observational studies. J Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behavior. Org. Behav. Hum. Dec. Process 1991, 50, 179–211. [Google Scholar] [CrossRef]
- O’Cathain, A.; Croot, L.; Duncan, E.; Rousseau, N.; Sworn, K.; Turner, K.M.; Yardley, L.; Hoddinott, P. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019, 9, e029954. [Google Scholar] [CrossRef]
- Chew, L.D.; Griffin, J.M.; Partin, M.R.; Noorbaloochi, S.; Grill, J.P.; Snyder, A.; Bradley, K.A.; Nugent, S.M.; Baines, A.D.; Vanryn, M. Validation of screening questions for limited health literacy in a large VA outpatient population. J. Gen. Intern. Med. 2008, 23, 561–566. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, T.A.; Peshkin, B.N.; Mars, B.D.; Tercyak, K.P. Patient satisfaction with cancer genetic counseling: A psychometric analysis of the Genetic Counseling Satisfaction Scale. J. Genet. Couns. 2004, 13, 293–304. [Google Scholar] [CrossRef]
- Holmes-Rovner, M.; Kroll, J.; Schmitt, N.; Rovner, D.R.; Breer, M.L.; Rothert, M.L.; Padonu, G.; Talarczyk, G. Patient satisfaction with health care decisions: The satisfaction with decision scale. Med. Decis. Making. 1996, 16, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Brehaut, J.C.; O’Connor, A.M.; Wood, T.J.; Hack, T.F.; Siminoff, L.; Gordon, E.; Feldman-Stewart, D. Validation of a decision regret scale. Med. Decis. Making. 2003, 23, 281–292. [Google Scholar] [CrossRef]
- Hausmann LR, Kressin NR, Hanusa BH, Ibrahim SA. Perceived racial discrimination in health care and its association with patients’ healthcare experiences: Does the measure matter? Ethn. Dis. 2010, 20, 40–47. [Google Scholar]
- Cella, D.; Hughes, C.; Peterman, A.; Chang, C.H.; Peshkin, B.N.; Schwartz, M.D.; Wenzel, L.; Lemke, A.; Marcus, A.C.; Lerman, C. A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002, 21, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Condit, C.M.; Wright, L. The psychometric property and validation of a fatalism scale. Psychol. Health 2009, 24, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.W.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The brief resilience scale: Assessing the ability to bounce back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Fetters, M.D.; Curry, L.A.; Creswell, J.W. Achieving integration in mixed methods designs—Principles and practices. Health Serv. Res. 2013, 48 Pt 2, 2134–2156. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, version 28.0; IBM Corp.: Armonk, NY, USA, 2021. [Google Scholar]
- Wolf, E.J.; Harrington, K.M.; Clark, S.L.; Miller, M.W. Sample Size Requirements for Structural Equation Models: An Evaluation of Power, Bias, and Solution Propriety. Educ. Psychol. Meas. 2013, 76, 913–934. [Google Scholar] [CrossRef]
- Crabtree, B.F.; Miller, W.L. Using codes and code manuals: A template organizing style of interpretation. In Doing Qualitative Research, 2nd ed.; Crabtree, B.F., Miller, W.L., Eds.; Sage: Thousand Oaks, CA, USA, 1999; pp. 163–177. [Google Scholar]
- Crabtree, B.F.; Miller, W.L. (Eds.) Doing Qualitative Research; Sage: Newbury Park, CA, USA, 1992; pp. 256–270. [Google Scholar]
- King, N. Using templates in the thematic analysis of text. In Essential Guide to Qualitative Methods in Organizational Research; Symon, G., Cassell, C., Eds.; Sage: London, UK, 2004. [Google Scholar]
- Reid, S.; Cadiz, S.; Pal, T. Disparities in Genetic Testing and Care among Black women with Hereditary Breast Cancer. Curr. Breast Cancer Rep. 2020, 12, 125–131. [Google Scholar] [CrossRef]
- Frey, M.K.; Finch, A.; Kulkarni, A.; Akbari, M.R.; Chapman-Davis, E. Genetic Testing for All: Overcoming Disparities in Ovarian Cancer Genetic Testing. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 1–12. [Google Scholar] [CrossRef]
- PDQ Cancer Genetics Editorial Board. Cancer Genetics Risk Assessment and Counseling (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries [Internet]; National Cancer Institute (US): Bethesda, MD, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65817/ (accessed on 13 September 2022).
- Hesse-Biber, S.; Seven, M.; Jiang, J.; Schaik, S.V.; Dwyer, A.A. Impact of BRCA Status on Reproductive Decision-Making and Self-Concept: A Mixed-Methods Study Informing the Development of Tailored Interventions. Cancers 2022, 14, 1494. [Google Scholar] [CrossRef]
- Radley, D.C.; Baumgartner, J.C.; Collins, S.R.; Zephyrin, L.C.; Schneider, E.C. Achieving Racial and Ethnic Equity in U.S. Health Care A Scorecard of State Performance. Available online: https://www.commonwealthfund.org/publications/scorecard/2021/nov/achieving-racial-ethnic-equity-us-health-care-state-performance (accessed on 4 June 2023).
- Conley, C.C.; Ketcher, D.; Reblin, M.; Kasting, M.L.; Cragun, D.; Kim, J.; Ashing, K.T.; Knott, C.L.; Hughes-Halbert, C.; Pal, T.; et al. The big reveal: Family disclosure patterns of BRCA genetic test results among young Black women with invasive breast cancer. J. Genet. Couns. 2020, 29, 410–422. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Au, M.G.; Smith, N.; Plummer, L.; Lippincott, M.F.; Balasubramanian, R.; Seminara, S.B. Evaluating co-created patient-facing materials to increase understanding of genetic test results. J. Genet. Couns. 2021, 30, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzani, C.; Aceti, M.; Schweighoffer, R.; Kaiser-Grolimund, A.; Bürki, N.; Chappuis, P.O.; Graffeo, R.; Monnerat, C.; Pagani, O.; Rabaglio, M.; et al. The Communication Chain of Genetic Risk: Analyses of Narrative Data Exploring Proband-Provider and Proband-Family Communication in Hereditary Breast and Ovarian Cancer. J. Pers. Med. 2022, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Seven, M.; Shah, L.L.; Yazici, H.; Daack-Hirsch, S. From Probands to Relatives: Communication of Genetic Risk for Hereditary Breast-Ovarian Cancer and Its Influence on Subsequent Testing. Cancer Nurs. 2022, 45, E91–E98. [Google Scholar] [CrossRef]
- Lieberman, S.; Lahad, A.; Tomer, A.; Koka, S.; BenUziyahu, M.; Raz, A.; Levy-Lahad, E. Familial communication and cascade testing among relatives of BRCA population screening participants. Genet. Med. 2018, 20, 1446–1454. [Google Scholar] [CrossRef]
- Schaa, K.L.; Roter, D.L.; Biesecker, B.B.; Cooper, L.A.; Erby, L.H. Genetic counselors’ implicit racial attitudes and their relationship to communication. Health Psychol. 2015, 34, 111–119. [Google Scholar] [CrossRef]
- Grafft, N.; Dwyer, A.A.; Pineros-Leano, M. Latinx individuals’ knowledge of, preferences for, and experiences with prenatal genetic testing: A scoping review. Reprod. Health 2022, 19, 134. [Google Scholar] [CrossRef]
- Niitsu, K.; Rice, M.J.; Houfek, J.F.; Stoltenberg, S.F.; Kupzyk, K.A.; Barron, C.R. A Systematic Review of Genetic Influence on Psychological Resilience. Biol. Res. Nurs. 2019, 21, 61–71. [Google Scholar] [CrossRef]
- Seiler, A.; Jenewein, J. Resilience in Cancer Patients. Front Psychiatry 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Andrasik, M.P.; Broder, G.B.; Wallace, S.E.; Chaturvedi, R.; Michael, N.L.; Bock, S.; Beyrer, C.; Oseso, L.; Aina, J.; Lucas, J.; et al. Increasing Black, Indigenous and People of Color participation in clinical trials through community engagement and recruitment goal establishment. PLoS ONE 2021, 16, e0258858. [Google Scholar] [CrossRef] [PubMed]
- Scharff, D.P.; Mathews, K.J.; Jackson, P.; Hoffsuemmer, J.; Martin, E.; Edwards, D. More than Tuskegee: Understanding mistrust about research participation. J. Health Care Poor Underserved 2010, 21, 879–897. [Google Scholar] [CrossRef]

| Characteristics | Survey Participants | Interview Participants (n = 18) | ||
|---|---|---|---|---|
| NHW (n = 197) | BIPOC (n = 45) | All (n = 242) | ||
| n (%) | n (%) | n (%) | ||
| Age (years) | ||||
| min.–max. | 20–76 | 20–70 | 20–76 | 25–68 |
| mean ± S.D. | 44.1 ± 13.0 | 35.1 ± 12.2 * | 42.4 ± 13.3 | 41.8 ± 13.5 |
| Gender (current) | ||||
| female (cis) | 193 (98%) | 28 (96.6%) | 221 (97.8%) | 18 (100%) |
| male | 1 (0.5) | - | 1 (0.4%) | - |
| transgender male | - | 1 (3.4%) | 1 (0.4%) | - |
| nonbinary/queer | 3 (1.5%) | - | 3 (1.3%) | - |
| Marital status | ||||
| married/in a relationship | 155 (78.7%) | 20 (69%) | 175 (77.4%) | 13 (72.2%) |
| single | 24 (12.2%) | 7 (24.1%) | 31 (13.7%) | 3 (16.7%) |
| separated/widowed | 18 (9.1%) | 2 (6.9%) | 20 (8.8%) | 2 (11.1%) |
| Education | ||||
| high school | 6 (3%) | 3 (10.3%) * | 9 (4%) | - |
| some college/Associate’s degree | 34 (17.3%) | 3 (10.3%) * | 37 (16.4%) | 2 (11.1%) |
| college or advanced degree | 157 (79.7%) | 23 (79.3%) | 180 (79.6%) | 16 (88.9%) |
| Health literacy (scale range: 0–5; low is better) | 1.24 ± 0.52 | 1.59 ± 0.81 * | −3.145/0.002 | 1.31 ± 0.63 |
| Household income (annual) | ||||
| <$75,000/year. | 106 (56.4%) | 12 (41.4%) | 118 (54.4%) | 7 (38.9%) |
| $75,000–125,000/year | 52 (27.7%) | 13 (44.8%) | 65 (30%) | 6 (33.3%) |
| >$125,000/year | 30 (16%) | 4 (13.8%) | 34 (15.7%) | 5 (27.8%) |
| Children | ||||
| number of children (range: 1–4) | 1.53 ± 0.67 | 1.98 ± 0.80 * | 1.89 ± 0.79 | 2.33 ± 1.11 |
| biological child(ren) | 112 (57.4%) | 21 (72.4%) | 133 (59.4%) | 5 (27.8%) |
| adopted child(ren) | 14 (7.2%) | 1 (3.4%) | 15 (6.7%) | 2 (11.1%) |
| both biological and adopted children | 6 (3.1%) | - | 6 (2.7%) | 2 (11.1%) |
| no children | 63 (32.3%) | 7 (24.1%) | 70 (31.3%) | 9 (50%) |
| Importance of religion (0–10) | 6.04 ± 2.38 | 4.58 ± 3.37 * | 4.88 ± 3.24 | 5.52 ± 3.90 |
| Survey Participants | |||
|---|---|---|---|
| Characteristics | NHW (n = 197) | BIPOC (n = 45) | All (n = 242) |
| n (%) | n (%) | n (%) | |
| Personal history of cancer | 80 (40.6%) | 18 (62.1%) * | 98 (43.4%) |
| Breast/ovarian cancer in 1st female relatives | 87 (44.2%) | 6 (20.7%) * | 93 (41.2%) |
| Breast cancer in 1st male relatives | 21 (10.9%) | 12 (41.4%) * | 33 (14.9%) |
| Age at BRCA genetic testing (years) | |||
| min.–max. | 18–73 | 18–68 | 18–73 |
| mean ± S.D. | 38.8 ± 12.53 | 31.0 ± 12.26* | 37.5 ± 12.80 |
| Pathogenic BRCA variant | |||
| yes | 179 (90.9%) | 25 (86.2%) | 204 (90.3%) |
| no | 15 (7.6%) | 4 (13.8%) | 19 (8.4%) |
| uncertain | 3 (1.5%) | - | 3 (1.3%) |
| Inheritance of BRCA variant | |||
| maternal | 93 (52%) | 14 (56%) | 107 (52.5%) |
| paternal | 59 (33%) | 8 (32%) | 67 (32.8%) |
| both | 2 (1.1%) | 2 (8%) | 4 (2%) |
| unknown | 25 (14%) | 1 (4%) | 26 (12.7%) |
| Medical decisions after BRCA testing | |||
| increased surveillance | 42 (23.5%) | 11 (29.7%) | 54 (21.9%) |
| risk-reducing medication | 6 (3.4%) | 16 (43.2%) * | 22 (9.1%) |
| risk-reducing surgery | 111 (62.0%) | 9 (24.3%) * | 120 (49.6%) |
| combination of surveillance/risk-reducing surgery | 7 (3.9%) | 1 (2.7%) | 12 (5%) |
| none | 2 (1.1%) | - | 2 (0.8%) |
| Other † | 11 (6.1) | - | 7 (2.9%) |
| Primary reason for genetic testing | |||
| personal desire for information | 74 (37.6%) | 11 (37.9%) | 85 (37.6%) |
| provider suggestion/encouragement | 52 (26.4%) | 12 (41.4%) | 64 (28.5%) |
| family suggestion/encouragement | 50 (25.4%) | 4 (13.8%) | 54 (23.9%) |
| other (suggestions from others) | 21 (10.7%) | 2 (6.9%) | 23 (10.2%) |
| Communicated BRCA risk with: | |||
| healthcare provider(s) | 161 (81.7%) | 13 (44.8%) * | 174 (77%) |
| family members/blood relatives | 190 (96.4%) | 23 (79.3%) * | 213 (94.2%) |
| friends | 152 (77.2%) | 12 (42.9%) * | 164 (72.9%) |
| NHW Participants | BIPOC Participants | z Value/p Value | All Participants | |
|---|---|---|---|---|
| Genetic Counseling Satisfaction Scale (scale range: 0–100) | 81.5 ±17.8 | 71.8 ± 22.8 * | −2.658/0.008 | 79.5 ± 19.3 |
| Satisfaction with Testing Decision (scale range: 1–5) | 4.4 ± 0.6 | 3.80±.95 * | −4.116/<0.001 | 4.3 ± 0.7 |
| Decision Regret Scale (scale range: 0–100) | 51.2 ±5.6 | 49.68±10.90 | −.390/0.697 | 50.9 ± 6.9 |
| Personal Discrimination Single-Item (scale range: 0–1) | 0.04 ± 0.18 | 0.73 ± 0.44 * | −11.34/<0.001 | 0.17 ± 0.37 |
| Multi-item (scale range: 0–7) | 4.0 ± 0.9 | 5.4 ± 1.7 * | −2.193/0.028 | 5.2 ± 1.6 |
| General Racism in Healthcare System (scale range: 0–4) | 2.0 ± 1.7 | 1.9 ± 1.3 | −0.073/0.942 | 1.9 ± 1.4 |
| Fatalism scale (scale range: 0–5) | 2.2 ± 0.6 | 2.7 ± 0.6 * | −2.319/0.020 | 2.2 ± 0.6 |
| Predetermination | 2.2 ± 0.7 | 2.9 ± 0.6 * | −3.138/0.002 | 2.3 ± 0.7 |
| Luck | 2.3 ± 0.9 | 2.9 ± 1.1 * | −2.993/0.003 | 2.40 ± 0.99 |
| Pessimism | 2.2 ± 0.8 | 3.0 ± 1.0 * | −5.021/<0.001 | 2.35 ± 0.86 |
| MICRA(scale range: 0–5) | 2.0 ± 0.8 | 2.3 ± 0.8 * | −2.084/0.037 | 2.06 ± 0.78 |
| Distress | 2.1 ± 1.2 | 2.4 ± 1.3 | −1.630/0.103 | 2.16 ± 1.20 |
| Uncertainty | 1.7 ± 0.8 | 2.0 ± 0.9 * | −2.204/0.027 | 1.73 ± 0.85 |
| Positive experience | 2.2 ± 1.0 | 2.0 ± 1.2 | −1.369/0.171 | 2.13 ± 1.07 |
| The Brief Resilience Scale (scale range: 0–6) | 3.0 ± 0.3 | 3.2 ± 0.7 * | −2.767/0.006 | 3.01 ± 0.39 |
| The Patient Activation Measure (scale range: 0–100) | 3.5 ± 0.5 | 3.2 ± 0.6 * | −3.039/0.002 | 3.43 ± 0.53 |
| Variables | Coefficient | S.D. | z | p Value |
|---|---|---|---|---|
| Direct effects of variables on patient activation score | ||||
| race/ethnicity (BIPOC vs. NHW) | −0.0189 | 0.0868 | −0.22 | 0.827 |
| education (B.S. vs. non-B.S. degree) | 0.0362 | 0.0707 | 0.51 | 0.608 |
| health Literacy | −0.1077 | 0.0642 | −1.67 | 0.094 |
| importance of religion | 0.0023 | 0.0093 | 0.25 | 0.802 |
| Personal Discrimination Scale | −0.0049 | 0.1038 | −0.05 | 0.962 |
| Genetic Counseling Satisfaction Scale | 0.0027 | 0.0018 | 1.44 | 0.149 |
| Satisfaction with Decision Scale | 0.3239 | 0.0559 | 5.79 | <0.001 |
| Fatalism Scale | −0.2481 | 0.0771 | −3.21 | 0.001 |
| Brief Resilience Scale | 0.2495 | 0.0779 | 3.20 | 0.001 |
| MICRA (uncertainty) | −0.0084 | 0.0372 | −0.23 | 0.821 |
| Indirect effect of variables (with mediating effect of patient activation score) on communicating results | ||||
| Patient Activation Measure | 0.0347 | 0.0581 | 0.60 | 0.550 |
| race/ethnicity | −0.0772 | 0.0754 | −1.02 | 0.306 |
| education | −10.102 | 0.0484 | −2.11 | 0.035 |
| health literacy | −0.0092 | 0.0337 | −0.27 | 0.785 |
| importance of religion | 0.0034 | 0.0040 | 0.86 | 0.388 |
| Personal Discrimination Scale | −0.1057 | 0.0868 | −1.22 | 0.223 |
| Genetic Counseling Satisfaction Scale | 0.0009 | 0.0012 | 0.73 | 0.463 |
| Satisfaction with Decision Scale | 0.0768 | 0.0344 | 2.23 | 0.026 |
| Fatalism Scale | 0.0878 | 0.0583 | 1.51 | 0.132 |
| Brief Resilience Scale | −0.1459 | 0.0608 | −2.40 | 0.017 |
| MICRA (uncertainty) | 0.0021 | 0.0247 | 0.09 | 0.930 |
| Significant Quantitative Findings | Representative Qualitative Interview Quotes (Providing Context and Insight into Quantitative Findings) |
|---|---|
| Behavioral beliefs Satisfaction with Decision (SWD): BIPOC: 3.8 ± 1.0 NHW: 4.4 ± 0.6 (p < 0.001) | Low Satisfaction P022: 48 y.o. female, Black/African American, SWD: 4.3/5, PAM: 3.3/5 communicated risk to 1st degree family members. “Because in spite of what I believed, even up to the moment I was diagnosed with the first lump, I still… I still… that day, I was like, [...] why am I going to get this stupid mammogram because it comes back negative in areas? The waste of my time, and it’s painful, and it’s uncomfortable, and I have a meeting to get to… I don’t want to be late for my meeting, you know? So even up until that point in time, I still didn’t believe that I could get breast cancer because of what I believe about… you know… my mother and my grandmothers not having it [cancer], not even thinking about my father’s side of the family.” High Satisfaction P019, 38 y.o. female, NHW, SWD: 5.0/5, PAM: 3.8/5, communicated risk to family members. “Sometimes it flares up in, like, weird situations. But um… but for the most part, I feel good. What I feel good about is the decisions that I made. I don’t regret them. And I feel like they were the right decisions. And I’m never going to live without any anxiety. So I think I did the best that I could, for, like, you know, being able to live the best that I can”. |
| Normative beliefs Education level (non-B.S. degree) BIPOC: 20.6% NHW: 20.3% (p < 0.05 ) | Associate’s degree P003: 28 y.o. female, mixed race health literacy score: 2/5, communicated risk to family members. “At the time, I was a little bit naïve. Before that appointment, I believed I was going to be the same as I was then [...] Maybe just media, maybe just naïvete… this, idea that like… well, he’s a plastic surgeon, and he says it’s—you know… he does all of this stuff. Like, it’ll look the same—[after risk-reducing surgery]” Graduate degree P005: 65 y.o. female, NHW health literacy score: 1/5, communicated risk to family members. “I think that [education/knowledge] takes off a huge amount of pressure, and stress, and worry. I was able to do what I did, because I… basically, I knew how to access information. I know how to pick up the phone, I wasn’t afraid to pick up the phone [...] I mean, you know, it was a lot easier for me, because I’m very, I’m very privileged”. |
| Behavioral control Total Fatalism Scale BIPOC: 2.68 ± 0.58 NHW: 2.20 ± 0.57 (p = 0.02) | Low fatalism P007: 57 y.o. female, NHW, Fatalism Score: 1.63/5, PAM: 4.33/5 “I have the information [genetic test results] and you know… my degree is in science, so I come from that background and the importance… just the absolute importance [of the result]. And then, if you do… if you do get diagnosed, this is what you can do! You know, just don’t stick your head in the sand.” High fatalism P003: 28 y.o. female, mixed race, Fatalism score: 3.04/5, PAM: 3.25/5 “You know, and it’s…it’s been so hard because I… I acknowledge the fact that it’s vital to ensure that this doesn’t happen. But it… it’s such a… I just wish I could have kids the normal way. Yeah, because I feel like my whole life has just not… it’s never been easy. And, yeah, I think because of that, some genetic things, I… I don’t… I have always been sick”. |
| Brief Resilience Scale BIPOC: 3.18 ± 0.65 NHW: 2.98 ± 0.30 (p = 0.006) higher resilience = less likely to communicate BRCA risk | Low resilience P020: 25 y.o. female, NHW, Resilience: 2.67/5, PAM: 3.25/5 Communicated risk to family members. “When I walked in, the first person that came in to see me was a young female surgeon. And she just instantly… I have to laugh ‘cause they went in and I burst into tears. And I said, Look, I don’t know why I’m crying because I’m totally fine with it. But the minute I get into hospital, I just […] I just think like, I just clam up and it’s not… my head is perfectly fine. But for some reason, my face is just expelling tears.” High resilience P011: 35 y.o. female, Black or African American, Resilience: 3.00/5, PAM: 4.00/5 Has a lower level of communicating risk to family. “And it does, it takes a lot out of you, it is exhausting that I have to go to this level to get even the same service when you know, I feel like it to be equitable. But it’s exhausting. It’s tiring. It is disappointing. But at the same time, you can’t, you can’t leave it unchecked, like you have to address it. Because if you don’t, then you’re, you’re setting the expectation that it’s okay. And it’s going to be done to every other woman of color or Black woman that comes into the door behind me. And I can promise you she never did that again to another person who looks like me or another person of color”. |
| Behavioral Intention Patient Activation Measure (PAM) BIPOC: 3.16 ± 0.60 NHW: 3.49 ± 0.50 (p = 0.002) | Low PAM P009: 30 y.o. female, Black/African American, PAM:3.42/5 “Sometimes we [Black people] don’t get the best of care, or having people not really listen… having to really push, not having just all the information just given to us right away [...] I went back to the oncologist, and I was telling him what happened. It wasn’t common, so he didn’t hear this with anybody else. So it was kind of like… well, it could just have been the first time and that could also be symptoms of chemo… and it just wasn’t [chemotherapy effects]. He wasn’t… I felt like he didn’t believe me.” High PAM P005: 65 y.o. female, NHW, PAM: 4.00/5 “I need referrals immediately. But in fact… what I did was… I just, I can’t stand not knowing. So I pay. And I’m very, very disapproving [of] private medicine. But, you know… in this instance, I just thought, sorry. I’m going for it. [...] I’m going to use my money. I saw somebody private, privately. And, I just… I just threw everything I had at it [...] And in fact, I was in hospital within about four weeks”. |
| Behavior Intrafamilial communication of BRCA risk n = 213 (94.2%) BIPOC: n = 23 (79.3%) NHW: n = 190 (96.4%) | Did not communicate BRCA risk to family P011, Female, Black or African American, 35, 76–100k Communicated with first-degree family members only “Oh, I definitely have the support-strong family support my dad supported me, but I only told him limited information because he was fighting his own journey, and I didn’t want him concerned about me especially since I knew that this disease- that this diagnosis didn’t mean a disease you know when I was diagnosed but then I also didn’t want to kind of worry him either and so I kind of just told him but I told him it’s you know, I’m the reassuring I don’t have cancer just means I’m high risk. And then I really chose to not tell other family members and friends only because, you know, I just I was felt, again -grieving and processing this all for myself, and so I didn’t really share with them and almost 10 years later, when I had my double mastectomy, and then at that time, over 10 years, I was fully educated and equipped to be able to share this news.” Communicated BRCA risk to family P004: 65 y.o. female, Mexican-American Communicated with entire family “I think I’m going to continue to be pretty proactive in terms of like, my own family. Making sure my siblings who haven’t been tested yet get tested. Making sure my own child or children are aware of their possible genetic mutations, you know? And, making sure that they make decisions in the future in terms of being proactive… if they want to. Hopefully, they want to, right? I think those are my… my main sort of my main outlook” |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hesse-Biber, S.; Seven, M.; Shea, H.; Heaney, M.; Dwyer, A.A. Racial and Ethnic Disparities in Genomic Healthcare Utilization, Patient Activation, and Intrafamilial Communication of Risk among Females Tested for BRCA Variants: A Mixed Methods Study. Genes 2023, 14, 1450. https://doi.org/10.3390/genes14071450
Hesse-Biber S, Seven M, Shea H, Heaney M, Dwyer AA. Racial and Ethnic Disparities in Genomic Healthcare Utilization, Patient Activation, and Intrafamilial Communication of Risk among Females Tested for BRCA Variants: A Mixed Methods Study. Genes. 2023; 14(7):1450. https://doi.org/10.3390/genes14071450
Chicago/Turabian StyleHesse-Biber, Sharlene, Memnun Seven, Hannah Shea, Madeline Heaney, and Andrew A. Dwyer. 2023. "Racial and Ethnic Disparities in Genomic Healthcare Utilization, Patient Activation, and Intrafamilial Communication of Risk among Females Tested for BRCA Variants: A Mixed Methods Study" Genes 14, no. 7: 1450. https://doi.org/10.3390/genes14071450
APA StyleHesse-Biber, S., Seven, M., Shea, H., Heaney, M., & Dwyer, A. A. (2023). Racial and Ethnic Disparities in Genomic Healthcare Utilization, Patient Activation, and Intrafamilial Communication of Risk among Females Tested for BRCA Variants: A Mixed Methods Study. Genes, 14(7), 1450. https://doi.org/10.3390/genes14071450







