Abstract
Endometrial cancer (EC) is a prevalent malignancy in women, and those who are proficient in the DNA mismatch repair (pMMR) pathway may have a family history (FH) that meets the criteria for a hereditary neoplastic condition (HNS). This study aimed to estimate the risk of HNS in women with pMMR endometrial tumors by analyzing their FH. To achieve this, we collaborated with a primary study and collected FH information by telephone. The final sample comprised 42 women who responded to the Primary Screening Questionnaire. Their family pedigrees were drawn and categorized according to internationally standardized criteria for the risk of HNS. Results showed that 26 women (61%) were found to be at risk for HNS, with Bethesda criteria being met by 23%, Amsterdam criteria by 15%, and 4% met the attenuated familial adenomatous polyposis criteria. Our results emphasize the importance of FH and the need to encourage healthcare professionals to collect and document FH more frequently, even if it is self-reported. By identifying individuals with HNS, we can improve their outcomes and reduce the burden of cancer in families with a predisposition to cancer.
1. Introduction
Endometrial cancer (EC) is the most common gynecological malignancy associated with Lynch syndrome (LS) [1]. Hereditary factors account for 2–6% of all EC cases and result from germline mutations that affect the function of the four key genes in the mismatch repair (MMR) pathway, MLH1, MSH2, MSH6, PMS2, or deletions at the final exons of EPCAM [1,2]. Women with LS have a 28–60% chance of developing EC, depending on the affected gene [3]. Although colorectal and EC are the most common tumors associated with LS, it has also been linked to tumors in other organs [2]. Moreover, other diseases such as breast cancer have been associated with LS, but more research is needed to explore these associations [4]. Families with the same tumor spectrum as LS but no MMR germline mutations are categorized as “familial colorectal cancer type X (FCCTX),” which meets the Amsterdam criteria [2]. An advanced comprehension of the clinical and histological manifestations associated with hereditary non-polyposis colorectal cancer (HNPCC) allowed the formulation of the Bethesda Guidelines. These guidelines systematically outline specific criteria for the identification of colorectal cancer, wherein criteria were delineated for identifying colorectal tumors eligible for microsatellite instability (MSI) testing [2]. Germline mutations in other genes, such as TP53 or PTEN, cause hereditary EC and are associated with Li-Fraumeni and Cowden hereditary neoplastic syndromes [5,6]. Having a first-degree relative with EC increases a woman’s risk of developing the disease by two-fold [1,7].
It is worth noting that 20% of all cancer patients may meet the criteria for HNS based on their family history [8,9,10]. However, recording a family history is not typically prioritized [11]. EC is often initially considered sporadic because it is diagnosed after the age of 50 and does not exhibit morphological or molecular abnormalities in the DNA repair system [12,13,14,15]. Nonetheless, EC may have hereditary components that require investigation, even in patients with proficient mismatch repair (pMMR) tumors [12]. Therefore, identifying and assessing the personal and family history of cancer in women diagnosed with EC, initially classified as sporadic, is critical. The aim of this study is to assess the risk for hereditary neoplastic syndromes in women with EC who are proficient in MMR by analyzing their family history.
2. Materials and Methods
This is a cross-sectional study utilizing a sample obtained from a primary study that evaluated EC patients in terms of proficiency of the MMR in the Brazilian population. The primary study retrospectively analyzed 127 EC patients who underwent surgery at a university general hospital in Southeast Brazil between 2005 and 2017. Among these patients, 58 women were identified as pMMR, using immunohistochemistry, indicating a negative family history of cancer and no risk of hereditary neoplastic syndromes (HNS), thus comprising our initial sample [16].
The Scientific and Research Committee of the Ribeirão Preto Medical School of the University of Sao Paulo, Brazil (HC-FMRP-USP) approved the research protocol and consent form for this study (protocol number 1.578.206/2016). Participants in the primary study provided written consent to collect information on their family history of cancer, which is used in the present study [16].
The inclusion criteria were as follows: in addition to having pMMR endometrial tumors, participants had to be over 18 years old and have a working phone contact, which was the method used to collect data. Those with obsolete or non-existent phone numbers recorded in the hospital database, as well as those who could not be contacted after 15 attempts, were excluded from the study.
From April to July 2018, the primary researcher attempted to contact and collect the personal cancer history (PH) and family history (FH) of the 58 participants. FH of cancer in family members of at least three generations was evaluated. PH included current age and age at diagnosis, the occurrence of other primary cancers, and recurrence. Clinical data, such as histological subtype, tumor grade, and staging according to the International Federation of Gynecology and Obstetrics (FIGO), and numerical variables, such as age at cancer diagnosis, were obtained from the primary study’s database and hospital records, including electronic medical records and phone contacts of participants [16].
A single, fully qualified interviewer, the main researcher, conducted all the interviews. She used the telephone approach, contacted patients diagnosed with EC, and explained the project’s goals. After reading the consent form and obtaining verbalized consent, the ‘Primary Screening Questionnaire’ (PSQ—Supplementary Material S1) was administered. It comprises a previously validated instrument with three ‘Yes’ or ‘No’ questions and one open-ended question [10]. The first question pertained to the personal history of cancer before the age of 50. The second question included sub-items about the presence of breast, bowel, and/or ovarian cancer in first- or second-degree relatives before the age of 50. The third question, open-ended, inquired about the presence of cancer in three or more first- or second-degree relatives before the age of 50.
To collect information on cancer cases that were not initially addressed in the PSQ, direct questioning of each family member was performed. This allowed for data collection from at least three generations, involving affected and healthy people, in both the maternal and paternal lineages [17,18] Using the PedigreeDraw software (Genial Genetic Solutions, discontinued on 1 May 2020) in its free edition, the information gathered during the interviews was exclusively from the proband. This data was then utilized to construct the pedigrees. These pedigrees were separately assessed by the main researcher and, subsequently, by two partner oncogeneticists. These professionals collaborated to develop plausible diagnostic hypotheses regarding HNS after collecting and documenting the self-reported family history. Families were investigated to determine whether they met the Amsterdam criteria and the revised Bethesda criteria [19]. They were then categorized according to the National Comprehensive Cancer Network (NCCN) criteria for the risk of HNS.
The data were analyzed using descriptive statistics, and Fisher’s exact test, running on R, was employed to check for a statistical association between FIGO tumor stage, suspected HNS, and age at diagnosis, with p-values of less than 0.05 being considered significant.
3. Results
Initially, 58 women were deemed eligible for this study, out of which 43 patients were successfully contacted by phone, but one of them declined to participate. Ultimately, a final sample of 42 women was obtained. The study design flowchart is depicted in Figure 1. The average age at the time of EC diagnosis was 58.4 years, with a range of 33 to 91 years. Other pertinent features are shown in Table 1.
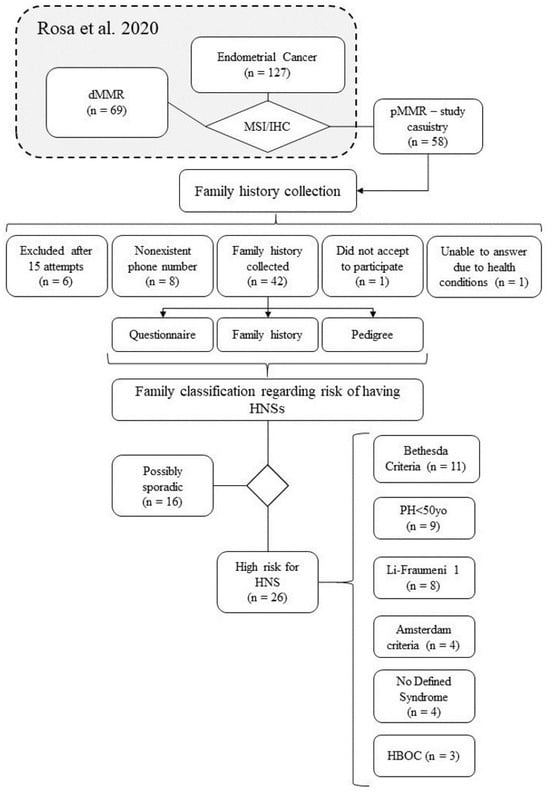
Figure 1.
Workflow depicting the primary study’s casuistry (n = 127), the current study’s (n = 58), and the composition of our final sample (n = 42). The diagram also showcases the distribution of samples meeting the criteria for each listed HNS, with the acknowledgment that a single sample may fall under multiple syndrome criteria [16].

Table 1.
Sample characterization according to age, tumor histology, FIGO staging and myometrial invasion.
Of the 42 women who participated in the study, personal and family cancer history was collected. Out of these, 26 (61.9%) individuals met the primary questionnaire and National Comprehensive Cancer Network (NCCN) criteria (Supplementary Material), which put them at risk for a HNS, as shown in Table 2. Three families (7.14%) allowed access to data from up to five generations through the use of pedigree, while 15 families (35.71%) allowed access to data from up to the fourth generation, and 23 families (54.76%) allowed access to data from up to the third generation. Only one family (2.38%) recorded data for two generations.

Table 2.
Sample characterization according to NCCN criteria. Only samples with high risk are shown.
In the studied population, twenty probands (47.6%) had at least one first-degree relative with cancer, 28 (66.7%) had second-degree relatives with cancer, and 16 (38.1%) had third-degree relatives with cancer. Seven probands (16.7%) had at least one afflicted first-degree relative with cancer under the age of 50, six (14.3%) had second-degree relatives with cancer under the age of 50, and seven (16.7%) had third-degree relatives with cancer under the age of 50.
On average, three tumors were found in each family (SD = 2.01; minimum 0, maximum 8). Five cases (4.54% of all family members with neoplasms in the present study) of colorectal cancer (CRC), nine (8.18%) of breast cancer, and one case (0.91%) of ovarian cancer were observed to occur before the age of 50. Breast cancer (15.60%), prostate cancer (14.68%), and CRC (13.76%) were the most frequent types of cancer in the studied population, as presented in Table 3.

Table 3.
Distribution of malignant neoplasms in studied families based on their cancer history.
Pedigrees were examined to investigate whether any clinical criteria were present in the families. The average age of probands who met possibly sporadic cancer criteria was 62.7 years, whereas those suspected of having any HNS was 55.5 years.
This study found that the average age of individuals in pedigrees meeting the Bethesda criteria was 58.7 years (n = 6), while those who met the Amsterdam criteria had an average age of 63 years. Women who met the criteria for hereditary breast and ovarian cancer (HBOC) syndrome had a mean age of 48 years (n = 3), while those who met the criteria for Li-Fraumeni type 1 had a mean age of 61.3 years (n = 6). Only one patient with attenuated familial adenomatous polyposis (aFAP) was identified, and this patient was 43 years old.
Of the total patients, the majority (n = 28; 58.3%) had IA FIGO staging EC. The study further observed that women with an HNS had a higher degree of FIGO staging than sporadic cases (Fisher’s exact test, p < 0.05). However, there was no significant association found between FIGO staging and women diagnosed before the age of 50.
4. Discussion
In addition to personal cancer history, family history information is one of the most effective tools for identifying individuals at an increased risk of developing cancer [20,21,22]. This analysis enables health professionals to refer people to genetic counseling programs, perform more intensive screenings, follow up at a younger age, and offer genetic testing for HNS susceptibility genes [23,24,25].
Results of this study revealed that despite having pMMR tumors, 61.9% of the women investigated had at least one criterion for HNS based on FH and PH. The classification of such tumors would reduce the possibility of association with Lynch syndrome.
According to our data, 15% of the women in our study met the Amsterdam II criteria, which is indicative of familial colorectal cancer type X (FCCTX) [26,27]. FCCTX is a clinically heterogeneous group that encompasses several hereditary disorders and cancer aggregations [28,29,30]. Previous research has identified clinical and pathological differences between FCCTX and LS [31,32]. For example, FCCTX is associated with an older mean age at diagnosis of 50 to 65 years, compared to 40 years for LS [31,32]. This age difference was also observed in our study, where women with FCCTX had an average age of 63 years.
FCCTX includes several hereditary disorders and cancer aggregations with various candidate target genes, including BRCA2 [33], SEMA4A [30], KRAS [27], BRAF [34], APC [33], and BRIP1 [35]. In one of the pedigrees studied, several members who met the FCCTX criteria had a history of intestinal polyps. The BMPR1A gene has been previously associated with FCCTX [33], as well as with juvenile polyposis in 20% of cases and hereditary mixed polyposis syndrome in 50% of cases [36,37]. However, BRCA2 seems to have a more significant role in FCCTX cases [38]. Garre [39] reported the first evidence of a link between germline BRCA2 mutations and FCCTX in individuals who met the criteria. For instance, one of the probands in this study who met the FCCTX criteria had two first-degree relatives with prostate cancer, one second-degree cousin with prostate cancer, one third-degree relative with colorectal cancer, and four third-degree relatives with colorectal cancer. Although the existing data do not provide direct evidence of a link between BRCA1 and BRCA2 gene mutations and diagnoses of colorectal and EC, these findings support the notion that these mutations predispose to a broader spectrum of cancers than previously believed [38].
Two of the probands exhibited metachronous tumors, one had metachronic colorectal cancer and the other had thyroid cancer (Figure 2). Second primary malignancies may be linked to extensive medical surveillance after the initial diagnosis; treatments produced by exposure to X-rays and antineoplastic medicines; environmental factors, and high-risk genetic variants associated with HNS [40].
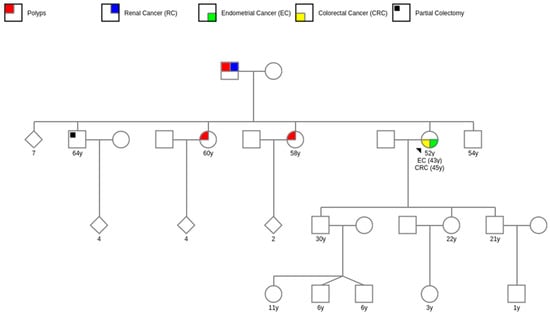
Figure 2.
Pedigree example of a proband with two primary cancers.
One proband met the Bethesda criteria for Lynch syndrome (LS), and six others had family members who also met the criteria. While the Bethesda criteria are more sensitive than the Amsterdam criteria, they are often used for LS screening [41,42]. However, up to 50% of individuals with LS do not meet the revised Bethesda criteria [19]. Moreover, since the research on the Bethesda criteria was primarily focused on colorectal cancer, their applicability to EC remains uncertain [43].
Regarding other families that met the criteria for any HNS, 22% (n = 8) of these were identified as families that met the criteria for Li Fraumeni Syndrome. All families that met the criteria for Li-Fraumeni corresponded to Li-Fraumeni-Like Type 1, as proposed by Eeles [44]. The average age of probands who met the Li-Fraumeni criteria was 61.3 years, which is considered late when compared to previous studies that estimate a cumulative risk of up to 90% for the development of a wide range of cancers before 45 years in this syndrome [45]. Our findings, however, are explained by Achatz [46], who discovered a particular mutation in the TP53 gene (R337H) in southern and southeastern Brazil, which was linked to a founder effect. This mutation has its unique features, such as lower penetrance when compared to conventional Li-Fraumeni syndrome; as a result, the diagnosis is delayed until later in life. The investigation of the variation in this study’s probands may help to explain this finding.
One noteworthy characteristic of our study population was the presence of 33% of participants diagnosed with EC before the age of 50. Typically, EC affects the majority of women over 50, with a mean age of about 60, and is rare in women under 40 [47,48]. Most studies have reported that younger EC patients tend to have lower-grade histological subtypes. In line with these previous findings, our investigation revealed a correlation between lower-grade cancer and staging in women diagnosed with EC before age 50 [48,49,50,51].
Individuals with suspected HNS were found to have a higher tumor grade compared to those with sporadic cases. These findings are consistent with previous studies, such as Carcangiu’s investigation in 2010, which reported that the proportion of grade 3 tumors was 46.1% among patients with HNS, compared to 11.3% among controls. Similar results have been observed in other syndromes, including Cowden syndrome, hereditary breast and ovarian cancer (HBOC), and Li-Fraumeni syndrome [52,53].
While FH of cancer may not meet the specific criteria for HNS in some cases, it may still warrant adjustments in cancer screening. In our study, 15% of families exhibited a cancer family cluster, which necessitates more vigilant monitoring than sporadic cancer cases, even if the individuals do not qualify for genetic testing for known susceptibility genes [54]. Moreover, family clusters often reflect non-genetic factors that are shared among family members, such as dietary habits, social and environmental exposures, as well as cultural beliefs and attitudes, that span multiple generations [24]. Hence, promoting health education and lifestyle interventions are crucial cancer prevention strategies for these families [55].
Our study found that several probands had a family history of colorectal and EC, with four having a first-degree relative with colorectal cancer, two having a first-degree relative with EC, and three having a second-degree relative with EC. Similar observations were reported by Cook [13], while Bharati [14] and Win [56] found that women with a first-degree relative diagnosed with colon or EC had a higher risk of developing EC themselves. These results suggest that EC may have a genetic component that runs in families, although environmental factors shared by family members may also play a role, either alone or in combination with genetic factors (gene-environment interaction) [56].
One potential limitation of our study is that it was conducted entirely over the phone. However, studies such as Campacci [10] have validated the use of the PSQ instrument to identify families at risk for HNS via telephone contact, finding no significant differences in applicability compared to in-person use. Similarly, Joseph [57] utilized a telephone survey to identify individuals who may benefit from genetic counseling. In fact, he found that telephone counseling can be used effectively to reduce geographic barriers and increase access to individuals without causing long-term negative psychosocial consequences. Therefore, while in-person counseling may be preferred in some cases, telephone counseling can be a useful alternative that enables increased access to care.
Our study provides important insights into the significance of collecting family history information in pMMR EC cases. The findings from our investigation, as well as other similar studies, highlight the critical role of FH in identifying individuals and families at increased risk of developing EC [12,13,14]. Our results also suggest that MMR gene mutations may account for only a small fraction of the overall familial risk of EC [13]. By identifying these high-risk patients and their families, our study highlights the importance of personalized cancer screening and prevention strategies, as well as tailored treatment options, including targeted therapies and prophylactic surgeries [3,4]. Overall, our study emphasizes the need for a comprehensive approach to the management of EC that incorporates family history data and individual risk assessments.
5. Conclusions
The results of this study demonstrate that, despite the advancements in genomic technologies and an improved understanding of genetic testing, FH remains a critical source of risk information for HNS, which extends beyond genetic susceptibility. Therefore, it is advisable that individuals and their families be evaluated based on their personal and familial history to identify potential HNS risks.
Identifying individuals with possible HNS enables healthcare professionals to implement preventive measures and intervene not only for an individual but also for the entire family. In addition, FH has been demonstrated to be a cost-effective and efficient technique for identifying individuals at an increased risk of developing cancer, particularly in Brazil where the majority of the population lacks access to health insurance, and genetic testing is financially unfeasible.
In order to enhance the prevention and treatment of EC in women pMMR, we suggest conducting additional studies that employ molecular and genetic approaches to investigate HNS. These advancements may lead to the identification of cost-effective techniques for identifying women at risk and improving the prevention and treatment of this neoplasm.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14111999/s1, Supplementary Material S1. Primary Screening Questionnaire (Translated from Brazilian Portuguese). Supplementary Material S2. Table S1—Criteria used to categorize the samples in the present study.
Author Contributions
Conceptualization, J.T.T.d.S. and R.C.A.R.; methodology, J.T.T.d.S. and M.F.; validation, J.T.T.d.S., A.L.E.P., A.V.A.-L. and B.T.B.; formal analysis, J.T.T.d.S., A.L.E.P. and A.V.A.-L.; investigation, J.T.T.d.S., R.C.A.R., V.E.d.F.F. and M.F.; resources, V.E.d.F.F. and M.F.; data curation, J.T.T.d.S., A.L.E.P. and M.F.; writing—original draft preparation, J.T.T.d.S. and M.F.; writing—review and editing, J.T.T.d.S., R.C.A.R., A.L.E.P., A.V.A.-L., B.T.B., V.E.d.F.F. and M.F.; visualization, J.T.T.d.S. and A.L.E.P.; supervision, M.F.; project administration, M.F.; funding acquisition, V.E.d.F.F. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded the São Paulo Research Foundation (FAPESP grant 2017/04283-8) and National Council for Scientific and Technological Development (CNPq grant 423007/2016-5).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the The Scientific and Research Committee of the Ribeirão Preto Medical School of the University of Sao Paulo, Brazil (HC-FMRP-USP), protocol number 1.578.206/2016).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in this study contain sensitive information, including identifiable sample information. As such, access to the data will only be provided through formal requests and with appropriate ethical approvals in place. Requests for access should be directed to thalitatargino@usp.br, and will be evaluated on a case-by-case basis to ensure that all necessary ethical and legal requirements are met.
Acknowledgments
We express our gratitude to all individuals and the sponsoring and funding institution whose contributions were fundamental in the successful execution of this study. We extend special thanks to the study participants and co-authors for their invaluable feedback, which greatly enhanced the quality of this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Casamassimi, A.; Federico, A.; Passariello, L.; Cioffi, M.; Molinari, A.M. Prevalence of Mutations in BRCA and MMR Genes in Patients Affected with Hereditary Endometrial Cancer. Med. Oncol. 2021, 38, 13. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Wu, W. Recent Advances in Lynch Syndrome. Exp. Hematol. Oncol. 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tsuji, K.; Banno, K.; Yanokura, M.; Kobayashi, Y.; Tominaga, E.; Aoki, D. Screening for Lynch Syndrome Using Risk Assessment Criteria in Patients with Ovarian Cancer. J. Gynecol. Oncol. 2018, 29, e29. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Guerini-Rocco, E.; Haricharan, S.; Fusco, N. Mismatch Repair-Deficient Hormone Receptor-Positive Breast Cancers: Biology and Pathological Characterization. Cancer Cell Int. 2021, 21, 266. [Google Scholar] [CrossRef] [PubMed]
- Manning-Geist, B.L.; Gatius, S.; Liu, Y.; Gil, M.; Da Cruz Paula, A.; Tuset, N.; Abu-Rustum, N.R.; Aghajanian, C.; Weigelt, B.; Matias-Guiu, X. Diagnosis and Management of an Endometrial Cancer Patient with Cowden Syndrome. Gynecol. Oncol. 2021, 163, 14–21. [Google Scholar] [CrossRef]
- Evans, D.G.; Woodward, E.R. New Surveillance Guidelines for Li-Fraumeni and Hereditary TP53 Related Cancer Syndrome: Implications for Germline TP53 Testing in Breast Cancer. Fam. Cancer 2021, 20, 1–7. [Google Scholar] [CrossRef]
- Zheng, G.; Sundquist, J.; Sundquist, K.; Ji, J. Family History of Breast Cancer as a Second Primary Malignancy in Relatives: A Nationwide Cohort Study. BMC Cancer 2021, 21, 1210. [Google Scholar] [CrossRef]
- Cleophat, J.E.; Nabi, H.; Pelletier, S.; Bouchard, K.; Dorval, M. What Characterizes Cancer Family History Collection Tools? A Critical Literature Review. Curr. Oncol. 2018, 25, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Fanale, D.; Corsini, L.R.; Brando, C.; Dimino, A.; Filorizzo, C.; Magrin, L.; Sciacchitano, R.; Fiorino, A.; Bazan Russo, T.D.; Calò, V.; et al. Impact of Different Selection Approaches for Identifying Lynch Syndrome-Related Colorectal Cancer Patients: Unity Is Strength. Front. Oncol. 2022, 12, 827822. [Google Scholar] [CrossRef] [PubMed]
- Campacci, N.; de Lima, J.O.; Carvalho, A.L.; Michelli, R.D.; Haikel, R.; Mauad, E.; Viana, D.V.; Melendez, M.E.; Vazquez, F.d.L.; Zanardo, C.; et al. Identification of Hereditary Cancer in the General Population: Development and Validation of a Screening Questionnaire for Obtaining the Family History of Cancer. Cancer Med. 2017, 6, 3014–3024. [Google Scholar] [CrossRef]
- Flória-Santos, M.; Lopes-Júnior, L.C.; Alvarenga, L.d.M.; Ribeiro, M.S.; Ferraz, V.E.d.F.; Nascimento, L.C.; Pereira-da-Silva, G. Self-Reported Cancer Family History Is a Useful Tool for Identification of Individuals at Risk of Hereditary Cancer Predisposition Syndrome at Primary Care Centers in Middle-Income Settings: A Longitudinal Study. Genet. Mol. Biol. 2016, 39, 178–183. [Google Scholar] [CrossRef]
- Bermejo, J.L.; Büchner, F.L.; Hemminki, K. Familial Risk of Endometrial Cancer after Exclusion of Families That Fulfilled Amsterdam, Japanese or Bethesda Criteria for HNPCC. Ann. Oncol. 2004, 15, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.S.; Nelson, H.E.; Stidley, C.A.; Dong, Y.; Round, P.J.; Amankwah, E.K.; Magliocco, A.M.; Friedenreich, C.M. Endometrial Cancer and a Family History of Cancer. Gynecol. Oncol. 2013, 130, 334–339. [Google Scholar] [CrossRef]
- Bharati, R.; Jenkins, M.A.; Lindor, N.M.; Le Marchand, L.; Gallinger, S.; Haile, R.W.; Newcomb, P.A.; Hopper, J.L.; Win, A.K. Does Risk of Endometrial Cancer for Women without a Germline Mutation in a DNA Mismatch Repair Gene Depend on Family History of Endometrial Cancer or Colorectal Cancer? Gynecol. Oncol. 2014, 133, 287–292. [Google Scholar] [CrossRef]
- Caycho-Rodríguez, T.; Noe-Grijalva, M.; Carbajal-León, C. Prevalence of Cancer Worry in Adults with a Family History. Enfermería Clínica (Engl. Ed.) 2021, 31, 258–260. [Google Scholar] [CrossRef]
- Rosa, R.C.A.; Santis, J.O.; Teixeira, L.A.; Molfetta, G.A.; dos Santos, J.T.T.; Ribeiro, V.d.S.; Chahud, F.; Ribeiro-Silva, A.; Brunaldi, M.O.; Silva, W.A., Jr.; et al. Lynch Syndrome Identification in a Brazilian Cohort of Endometrial Cancer Screened by a Universal Approach. Gynecol. Oncol. 2020, 159, 229–238. [Google Scholar] [CrossRef]
- Bennett, R.L. The Family Medical History as a Tool in Preconception Consultation. J. Community Genet. 2012, 3, 175–183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahon, S. The Three-Generation Pedigree: A Critical Tool in Cancer Genetics Care. Oncol. Nurs. Forum 2016, 43, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; Chapelle, A.d.l.; Ruschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. JNCI J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.M.; Corona, R.; Bodurtha, J.N.; Quillin, J.M. Family Ties: The Role of Family Context in Family Health History Communication about Cancer. J. Health Commun. 2016, 21, 346–355. [Google Scholar] [CrossRef]
- Choi, H.G.; Park, J.H.; Choi, Y.J.; Suh, Y.J. Association of Family History with the Development of Breast Cancer: A Cohort Study of 129,374 Women in KoGES Data. Int. J. Environ. Res. Public Health 2021, 18, 6409. [Google Scholar] [CrossRef]
- Lin, J.; Wolfe, I.; Ahsan, M.D.; Krinsky, H.; Lackner, A.I.; Pelt, J.; Bolouvi, K.; Gamble, C.; Thomas, C.; Christos, P.J.; et al. Room for Improvement in Capturing Cancer Family History in a Gynecologic Oncology Outpatient Setting. Gynecol. Oncol. Rep. 2022, 40, 100941. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-Y.; Sheng, J.-Q. Advances in the Study of Lynch Syndrome in China. World J. Gastroenterol. 2015, 21, 6861–6871. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.F.; Genkinger, J.M.; Farhat, M.; Wilson, A.; Gary-Webb, T.L.; Tehranifar, P. Residential Environment and Breast Cancer Incidence and Mortality: A Systematic Review and Meta-Analysis. BMC Cancer 2015, 15, 191. [Google Scholar] [CrossRef]
- Wood, M.E.; Rehman, H.t.; Bedrosian, I. Importance of Family History and Indications for Genetic Testing. Breast J. 2020, 26, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Martín-Morales, L.; Feldman, M.; Vershinin, Z.; Garre, P.; Caldés, T.; Levy, D. SETD6 Dominant Negative Mutation in Familial Colorectal Cancer Type X. Hum. Mol. Genet. 2017, 26, 4481–4493. [Google Scholar] [CrossRef]
- Yao, Z.-G.; Lv, B.-B.; Jing, H.-Y.; Su, W.-J.; Li, J.-M.; Fan, H.; Zhao, M.-Q.; Qin, Y.-J.; Sun, X.-C. A Practical Screening Strategy for Lynch Syndrome and Lynch Syndrome Mimics in Colorectal Cancer. J. Cancer Res. Ther. 2021, 17, 790. [Google Scholar] [CrossRef]
- Francisco, I.; Albuquerque, C.; Lage, P.; Belo, H.; Vitoriano, I.; Filipe, B.; Claro, I.; Ferreira, S.; Rodrigues, P.; Chaves, P.; et al. Familial Colorectal Cancer Type X Syndrome: Two Distinct Molecular Entities? Fam. Cancer 2011, 10, 623–631. [Google Scholar] [CrossRef]
- Koh, P.-K.; Kalady, M.; Skacel, M.; Fay, S.; McGannon, E.; Shenal, J.; Arroyo, L.; Toderick, K.; Church, J. Familial Colorectal Cancer Type X: Polyp Burden and Cancer Risk Stratification via a Family History Score. ANZ J. Surg. 2011, 81, 537–542. [Google Scholar] [CrossRef]
- Nejadtaghi, M.; Jafari, H.; Farrokhi, E.; Samani, K.G. Familial Colorectal Cancer Type X (FCCTX) and the Correlation with Various Genes—A Systematic Review. Curr. Probl. Cancer 2017, 41, 388–397. [Google Scholar] [CrossRef]
- Tanakaya, K. Current Clinical Topics of Lynch Syndrome. Int. J. Clin. Oncol. 2019, 24, 1013–1019. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Furukawa, Y.; Nakamura, Y.; Matsubara, N.; Ishikawa, H.; Arai, M.; Tomita, N.; Tamura, K.; Sugano, K.; Ishioka, C.; et al. Comparison of Clinical Features between Suspected Familial Colorectal Cancer Type X and Lynch Syndrome in Japanese Patients with Colorectal Cancer: A Cross-Sectional Study Conducted by the Japanese Society for Cancer of the Colon and Rectum. Jpn. J. Clin. Oncol. 2015, 45, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Peltomäki, P.; Olkinuora, A.; Nieminen, T.T. Updates in the Field of Hereditary Nonpolyposis Colorectal Cancer. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 707–720. [Google Scholar] [CrossRef]
- DeRycke, M.S.; Gunawardena, S.; Balcom, J.R.; Pickart, A.M.; Waltman, L.A.; French, A.J.; McDonnell, S.; Riska, S.M.; Fogarty, Z.C.; Larson, M.C.; et al. Targeted Sequencing of 36 Known or Putative Colorectal Cancer Susceptibility Genes. Mol. Genet. Genom. Med. 2017, 5, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Martín-Morales, L.; Garre, P.; Lorca, V.; Cazorla, M.; Llovet, P.; Bando, I.; García-Barberan, V.; González-Morales, M.L.; Esteban-Jurado, C.; de la Hoya, M.; et al. BRIP1, a Gene Potentially Implicated in Familial Colorectal Cancer Type X. Cancer Prev. Res. 2021, 14, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yean Cheah, P.; Hui Wong, Y.; Ping Chau, Y.; Loi, C.; Hon Lim, K.; Fong Lim, J.; Koon Koh, P.; Weng Eu, K. Germline Bone Morphogenesis Protein Receptor 1A Mutation Causes Colorectal Tumorigenesis in Hereditary Mixed Polyposis Syndrome. Am. J. Gastroenterol. 2009, 104, 3027–3033. [Google Scholar] [CrossRef]
- O’Riordan, J.M.; O’Donoghue, D.; Green, A.; Keegan, D.; Hawkes, L.A.; Payne, S.J.; Sheahan, K.; Winter, D.C. Hereditary Mixed Polyposis Syndrome Due to a BMPR1A Mutation. Color. Dis. 2009, 12, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, C.; Zhang, Y.; Guo, T.; Zhu, C.; Xu, Y.; Liu, F. Comparison Between Familial Colorectal Cancer Type X and Lynch Syndrome: Molecular, Clinical, and Pathological Characteristics and Pedigrees. Front. Oncol. 2020, 10, 1603. [Google Scholar] [CrossRef]
- Garre, P.; Martín, L.; Sanz, J.; Romero, A.; Tosar, A.; Bando, I.; Llovet, P.; Diaque, P.; García-Paredes, B.; Díaz-Rubio, E.; et al. BRCA2 Gene: A Candidate for Clinical Testing in Familial Colorectal Cancer Type X. Clin. Genet. 2015, 87, 582–587. [Google Scholar] [CrossRef]
- Terradas, M.; Capellá, G.; Valle, L. Dominantly Inherited Hereditary Nonpolyposis Colorectal Cancer Not Caused by MMR Genes. J. Clin. Med. 2020, 9, 1954. [Google Scholar] [CrossRef]
- Watkins, J.C.; Yang, E.J.; Muto, M.G.; Feltmate, C.M.; Berkowitz, R.S.; Horowitz, N.S.; Syngal, S.; Yurgelun, M.B.; Chittenden, A.; Hornick, J.L.; et al. Universal Screening for Mismatch-Repair Deficiency in Endometrial Cancers to Identify Patients With Lynch Syndrome and Lynch-like Syndrome. Int. J. Gynecol. Pathol. 2017, 36, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.B.; Kim, C.W.; Yoon, Y.S.; Park, I.J.; Lim, S.-B.; Yu, C.S.; Kim, J.C. Observational Study. Medicine 2016, 95, e2723. [Google Scholar] [CrossRef] [PubMed]
- Batte, B.A.L.; Bruegl, A.S.; Daniels, M.S.; Ring, K.L.; Dempsey, K.M.; Djordjevic, B.; Luthra, R.; Fellman, B.M.; Lu, K.H.; Broaddus, R.R. Consequences of Universal MSI/IHC in Screening ENDOMETRIAL Cancer Patients for Lynch Syndrome. Gynecol. Oncol. 2014, 134, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Eeles, R.A. Germline Mutations in the TP53 Gene. Cancer Surv. 1995, 25, 101–124. [Google Scholar]
- de Andrade, K.C.; Khincha, P.P.; Hatton, J.N.; Frone, M.N.; Wegman-Ostrosky, T.; Mai, P.L.; Best, A.F.; Savage, S.A. Cancer Incidence, Patterns, and Genotype–Phenotype Associations in Individuals with Pathogenic or Likely Pathogenic Germline TP53 Variants: An Observational Cohort Study. Lancet Oncol. 2021, 22, 1787–1798. [Google Scholar] [CrossRef]
- Achatz, M.I.W.; Olivier, M.; Calvez, F.L.; Martel-Planche, G.; Lopes, A.; Rossi, B.M.; Ashton-Prolla, P.; Giugliani, R.; Palmero, E.I.; Vargas, F.R.; et al. The TP53 Mutation, R337H, Is Associated with Li-Fraumeni and Li-Fraumeni-like Syndromes in Brazilian Families. Cancer Lett. 2007, 245, 96–102. [Google Scholar] [CrossRef]
- Binder, P.S.; Mutch, D.G. Update on Prognostic Markers for Endometrial Cancer. Women’s Health 2014, 10, 277–288. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Int. J. Gynecol. Cancer 2016, 26, 2–30. [Google Scholar] [CrossRef]
- Pellerin, G.P.; Finan, M.A. Endometrial Cancer in Women 45 Years of Age or Younger: A Clinicopathological Analysis. Am. J. Obstet. Gynecol. 2005, 193, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Soslow, R.A. Endometrial Carcinoma in Women Aged 40 Years and Younger. Arch. Pathol. Lab. Med. 2014, 138, 335–342. [Google Scholar] [CrossRef]
- Lau, H.-Y.; Chen, Y.-J.; Yen, M.-S.; Chao, K.-C.; Chen, R.-F.; Yeh, S.-O.; Twu, N.-F. Clinicopathological Features and Survival in Young Taiwanese Women With Endometrial Carcinoma. Int. J. Gynecol. Cancer 2014, 24, 1015–1020. [Google Scholar] [CrossRef]
- Mahdi, H.; Mester, J.L.; Nizialek, E.A.; Ngeow, J.; Michener, C.; Eng, C. Germline PTEN, SDHB-D, and KLLN Alterations in Endometrial Cancer Patients with Cowden and Cowden-like Syndromes: An International, Multicenter, Prospective Study. Cancer 2015, 121, 688–696. [Google Scholar] [CrossRef]
- Neto, N.; Cunha, T.M. Do Hereditary Syndrome-Related Gynecologic Cancers Have Any Specific Features? Insights Imaging 2015, 6, 545–552. [Google Scholar] [CrossRef]
- Lu, C.; Xie, M.; Wendl, M.C.; Wang, J.; McLellan, M.D.; Leiserson, M.D.M.; Huang, K.; Wyczalkowski, M.A.; Jayasinghe, R.; Banerjee, T.; et al. Patterns and Functional Implications of Rare Germline Variants across 12 Cancer Types. Nat. Commun. 2015, 6, 10086. [Google Scholar] [CrossRef]
- Koehly, L.M.; Morris, B.A.; Skapinsky, K.; Goergen, A.; Ludden, A. Evaluation of the Families SHARE Workbook: An Educational Tool Outlining Disease Risk and Healthy Guidelines to Reduce Risk of Heart Disease, Diabetes, Breast Cancer and Colorectal Cancer. BMC Public Health 2015, 15, 1120. [Google Scholar] [CrossRef]
- Win, A.K.; Reece, J.C.; Ryan, S. Family History and Risk of Endometrial Cancer. Obstet. Gynecol. 2015, 125, 89–98. [Google Scholar] [CrossRef]
- Joseph, G.; Kaplan, C.; Luce, J.; Lee, R.; Stewart, S.; Guerra, C.; Pasick, R. Efficient Identification and Referral of Low-Income Women at High Risk for Hereditary Breast Cancer: A Practice-Based Approach. Public Health Genom. 2012, 15, 172–180. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).